Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/077112
PCT/US2022/078944
MODIFIED ALGINATES AND METHODS OF MAKING AND USING THEREOF
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims benefit of U.S. Provisional Application No.
63/272,975, filed
October 28, 2021, and U.S. Provisional Application No. 63/288,906, filed
December 13, 2021,
each of which is hereby incorporated herein by reference in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
This invention was made with government support under Grant No. CA246414
awarded
by the National Institutes of' Health. The government has certain rights in
the invention.
BACKGROUND
Hydrogel biomaterials offer utility in biomedical applications due to their
stability,
tunable mechanics and degradation profiles, as well as hiocompatibility with
surrounding
tissues. Clinical and preclinical applications of hydrogel biomaterials
include tissue engineered
constructs, depots for drug and cell delivery, and cellular scaffolds used in
the study of
biological processes.
Among hydrogels, alginate is rapidly gaining attention because it gels under
neutral,
physiological conditions, exhibits good biocompatibility at tissue sites, and
has wide chemical
versatility. Alginate gelation utilizes ionic cross-links between the carboxyl
groups on alginate
and divalent cations. The calcium cross-linked hydrogels are injectable and
self-healing,
enabling facile injection into tissues. In vivo, calcium cross-linked alginate
hydrogels elicits low
levels of foreign body responses, very low toxicity and limited
immunogenicity. In addition,
alginate is generally recognized as safe (GRAS) by the FDA, which has
motivated alginates
preclinical testing to deliver drugs, biologicals, viruses and cells and
clinically as a dietary
supplement, material for wound dressings, sealant agent and as an injectable
implant.
The chemical versatility of alginate is of particular interest to researchers
trying to take
advantage of alginate's biocompatibility. Chemical modification of alginate
has been used to
decorate alginate polymers with small molecules for controlled and tunable
drug delivery and
with peptides and proteins to mediate cell attachment and signaling. More
recently, alginate
polymers have been conjugated to bioorthogonal "click" chemical motifs in
order to enhance or
¨ 1 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
enable alginate cross-linking, to expedite polymer modification, and to create
targetable drug
depots.
Although alginate is straightforward to chemically modify, modification
carries
undesired complications. Most frequently, alginate polymers are modified
through carbodiimide
coupling between the carboxyl group on alginate and nucleophiles (alcohols,
amines, and
others). Unfortunately, this chemical modification decreases polymer viscosity
and can inhibit
gelation. Indeed, alginate hydrogels modified to a high degree of substitution
(DS) suffer from
poor or nonexistent calcium cross-linking. What is needed are new methods of
modifying
alginate that do not suffer from the drawbacks currently experienced when
modifying alginate
to with a high DS.
SUMMARY
Disclosed are alginate strands with a high degree of substitution and methods
of making
the same.
In one aspect disclosed herein are substituted alginate strands comprising
alginate strands
coupled to a functional moiety (e.g., a click motif, such as, for example, an
azide) via a linker,
wherein the linker comprises a nucleophilic terminus (such as, for example,
amino, alcohol,
thiol) and additionally a carboxyl group attached to the linker; and wherein
the nucleophilic
terminus is coupled to a carboxyl group on the alginate strand. In some
aspects, the substituted
alginate strands can form polymer strands.
In one aspect, disclosed herein are methods of coupling an alginate strand to
an
functional moiety (e.g., a click motif, such as, for example, an azide) via
carbodiimide coupling
said method comprising conjugating the functional moiety to a linker
comprising a nucleophilic
terminus and a carboxyl group of the nucleophilic terminus of the linker (such
as, amine,
alcohol, thiol); wherein the functional moiety is chemically coupled to the
linker at the opposing
end to the nucleophilic terminus; and coupling the nucleophilic terminus of
the linker to the
alginate strand via carbodiimide coupling; wherein the modification allows for
synthesis of
highly substituted alginate; wherein the substitution does not disrupt the
integrity of the gel.
Also disclosed herein are methods of coupling an alginate strand to an
functional moiety
(e.g., a click motif, such as, for example, an azide) of any preceding aspect,
wherein the
nucleophilic terminus of the linker comprise a protecting group (such as, for
example, methyl,
ethyl, benzyl, benzyloxycarbonyl, s-Butyl,
OBO, silyl, photo-sensitive
group propyl, tert-butyl, and NVOC); and wherein the nucleophilic terminus is
deprotected prior
to being coupled to a sugar on the alginate strand.
¨ 2 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Also disclosed herein are methods of coupling an alginate strand to an
functional moiety
of any preceding aspect, wherein the carboxyl group on the linker is modified
with a protecting
group (such as, for example, methyl, ethyl, benzyl, benzyloxycarbonyl, s-
Butyl, 2-Alky1-1,3-
oxazoline, OBO, silyl, photo-sensitive group propyl, tert-butyl, and NVOC);
and wherein the
carboxyl group may be deprotected after the coupling to the alginate strand.
Also disclosed are modified alginates comprising one or more covalently
modified
monomers defined by Formula I
OOH
L2
OxX
yi
y2
Formula
wherein:
Xis 0, S, or NR3;
R3 is hydrogen, alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, or
heterocycloalkyl;
Li and L2 are each independently absent or represent a linking group;
A represents a functional moiety (e.g., a click motif or an active agent); and
Yi and Y2 independently are hydrogen or ¨PO(OR)2, or Y2 is absent, and Yi,
together
with the two oxygen atoms to which Yi and Y2 are attached form a cyclic
structure as shown
below
0 -OH
L1 L2
(XX
0
A-- 0
R5
R4
wherein
- 3 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
wherein R4 and R5 are, independently, hydrogen, alkyl, alkenyl, alkynyl, aryl,
heteroaryl,
cycloalkyl, heterocycloalkyl, alkoxy, aroxy, alkylthio, carbonyl, carboxyl,
amino, or amido; or
R4 and R5, together with the carbon atom to which they are attached, form a 3-
to 8-membered
unsubstituted or substituted carbocyclic or heterocyclic ring.
Also disclosed herein are hydrogel matrixes or scaffolds comprising polymer
strands of
the substituted alginate strand of any preceding aspect or the modified
alginates of any preceding
aspect, wherein the polymer strands are cross-linked to other alginate polymer
stands via a
divalent metal cation (e.g., via calcium crosslinking).
to BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part of
this
specification, illustrate several embodiments and together with the
description illustrate the
disclosed compositions and methods.
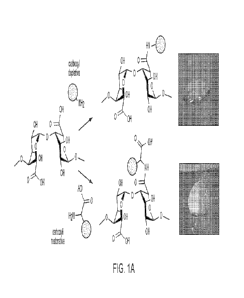
Figure lA shows an overview of depletive and restorative alginate
modifications.
Alginate can be modified with chemical groups (blue) through the carboxyl
groups (red), which
depletes the carboxyl groups, and destroys calcium cross-linking. In contrast,
if the
modifications restore carboxyl groups, alginate gels maintain calcium cross-
linking and gelation.
Figure 1B shows a representation of cross-linking that occurs in traditional
unmodified
alginate gels.
Figure 1C shows the effect of modification on alginate cross-linking.
Figure ID shows the present solution of introducing new carboxylic acid groups
and the
effect of said groups on cross-linking.
Figure 2 shows the synthetic approach to (S)-2-ammonio-6-(2-
azidoacetamido)hexanoate
(S4).
Figure 3 shows the coupling of azide-amine (depletive modification) and azide-
lysine
(restorative modification) to alginate.
Figures 4A, 4B, 4C, and 4D show restorative modifications at high degrees of
substitution maintain alginate gel mechanics. Figure 4A shows photos of
calcium cross-linked
alginate gels using depletive or restorative modifications at low- and high-DS
azide. Figure 4B
shows representative strain sweep of calcium cross-linked alginate gels with
restorative
modifications (linearity limit of yi._,=24%) showing viscoelastic behavior.
N=3 for each gel as
well as carboxyl-depleted and unmodified hydrogel can be found in Figure 5.
.5 % tolerance
range of deviation was used to select the plateau value of the linearity
limit. Figure 4C shows
representative frequency sweeps [1-100 Hz] within the LVE region showing the
gel-like
- 4 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
behavior and structural stability of calcium cross-linked gels modified with
high-DS restored
azide-alginate and high-DS depleted azide-alginate. Storage modulus (G') and
loss modulus
(G") measurements are shown. N=3 for each gel and low-DS gel mechanics can be
found in
Figure 6. Figure 4D shows cyclic strain time sweep theology showing self-
healing behavior of
calcium cross-linked alginate gels with restorative modifications demonstrated
by the recovery
after repeated deformation of high strain [500%[ followed by low strain [0.2%[
(frequency =
10Hz). N=3 for each gel type as well as unmodified and carboxyl-depleted gels
are shown in
Figure 8
Figure 5A, 5B, and 5C show the viscoelastic properties and linearity limits
shown by 10
Hz strain sweeps of calcium cross-linked alginate gels with (5A) restorative
modifications (N=3)
yi-Rm=26%, (5B) depletive modifications (N=3), and (.5C) unmodified (N=2) yL-
controt = 5%. 5
% tolerance range of deviation was used to select the plateau value of the
linearity limit.
Figures 6A and 6B show frequency Sweep [1-100 Hz] of calcium cross-linked gels
showing gel-like status and structural stability. Storage modulus (G') and
loss modulus (G")
measurements are shown. Figure 6A shows one sample from each gel group
including low-DS
gels and unmodified. Figure 6B shows all samples of all gel groups shown. N=3
Figure 7 shows rotational test showing injectability of unmodified alginate
(N=2) and
alginate with high degree of substitution using restorative modification
(N=3). Continuous shear
rate ramps from 50 to 0 s-1 for 2.5 minutes were performed to study the
continuous flow
behavior of the gel. Total of 74 data points with 20 data points per decade
was recorded.
Figures 8A, 8B, and 8C show the self-healing behavior of calcium cross-linked
alginate
gels demonstrated by the recovery after repeated deformation of high strain
[500%[ at 10 Hz
followed by low strain [0.2%1 Figure 8A shows calcium cross-linked alginate
with high azide
DS using restorative and depletive modification. N=3. Figure 8B shows one
sample of calcium
cross-linked alginate with high and low DS using restorative and depletive
modification. N=1.
Figure 8C shows calcium cross-linked unmodified alginate. N=1.
Figures 9A, 9B, 9C, 9D, and 9E show calcium cross-linked alginate gels with
restorative
azide modification reduce off-target gel migration. Figure 9A shows
representative images
(same BLI scale) and figure 9B shows quantitation of fluorescence signal after
intramuscular
injection of alginate hydrogels fluorescently conjugated to carboxyl-depletive
or -restorative
modifications over 2 weeks. Unmodified alginates with unconjugated fluorophore
served as
negative control. Figure 9C shows representative locations for quantified off-
target migration.
Quantitation of fluorescence signal in non-injected contralateral limb (9D)
and ankle (9E) over 2
weeks. Region of interest (R01) values were quantified as total radiance
efficiency in equivalent
-
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
sized regions. Figures show mean SEM. Statistical significance represented
as ***p <0.001,
and ****p < 0.0001 by two-way ANOVA followed by Tukey's multiple comparison
test as
compared to negative control. N=4. Full images of all mice can be found in
Figure 10.
Figure 10 shows calcium cross-linked alginate gels with restorative azide
modification
improve retention at injection sites as compared to alginate gels with no
modification. All mouse
images of fluorescence signal after intramuscular injection of alginate
hydrogels fluorescently
conjugated to carboxylate-depleting or -restoring modifications over 2 weeks.
Unmodified
alginates with unconjugated fluorophore served as negative control. Images
shown of each
group at the same scale. All fluorescent signal was measured using ICG/ICG
filter and shown as
radiance efficiency. N=4.
Figures 11A and 11B show restorative azide modification improves on-target
capture of
circulating DBCO fluorophores. Figure 11A shows representative images (same
BLI scale) and
11B shows quantitation of fluorescence at intramuscular sites from i.m.-
injected alginate
hydrogels cross-linked with calcium. DBCO-Cy7 was administered i.v. one week
following gel
injection. One week following i.v administration, mice were imaged to compare
the capture of
the fluorescent signal at the injected site. ROT values were quantified as
total radiance efficiency
in equivalent sized regions. Samples show mean + SEM. Statistical significance
represented as
**p <0.01, and ****p < 0.0001 by one-way ANOVA followed by Tukey's multiple
comparison
test of all groups. N=3.
DETAILED DESCRIPTION
Before the present compounds, compositions, articles, devices, and/or methods
are
disclosed and described, it is to be understood that they are not limited to
specific synthetic
methods or specific recombinant biotechnology methods unless otherwise
specified, or to
particular reagents unless otherwise specified, as such may, of course, vary.
It is also to be
understood that the terminology used herein is for the purpose of describing
particular
embodiments only and is not intended to be limiting.
Definitions
As used in the specification and the appended claims, the singular forms "a,"
"an" and
"the" include plural referents unless the context clearly dictates otherwise.
Thus, for example,
reference to "a pharmaceutical carrier- includes mixtures of two or more such
carriers, and the
like.
Ranges can be expressed herein as from "about" one particular value, and/or to
"about"
another particular value. When such a range is expressed, another embodiment
includes from
¨ 6 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
the one particular value and/or to the other particular value. Similarly, when
values are
expressed as approximations, by use of the antecedent -about," it will be
understood that the
particular value forms another embodiment. It will be further understood that
the endpoints of
each of the ranges are significant both in relation to the other endpoint, and
independently of the
other endpoint. It is also understood that there are a number of values
disclosed herein, and that
each value is also herein disclosed as "about" that particular value in
addition to the value itself.
For example, if the value "10" is disclosed, then "about 10" is also
disclosed. It is also
understood that when a value is disclosed that -less than or equal to" the
value, -greater than or
equal to the value" and possible ranges between values are also disclosed, as
appropriately
understood by the skilled artisan. For example, if the value "10" is disclosed
the "less than or
equal to 10"as well as "greater than or equal to 10" is also disclosed. It is
also understood that
the throughout the application, data is provided in a number of different
formats, and that this
data, represents endpoints and starting points, and ranges for any combination
of the data points.
For example, if a particular data point "10- and a particular data point 15
are disclosed, it is
understood that greater than, greater than or equal to, less than, less than
or equal to, and equal to
10 and 15 are considered disclosed as well as between 10 and 15. It is also
understood that each
unit between two particular units are also disclosed. For example, if 10 and
15 are disclosed,
then 11, 12, 13, and 14 are also disclosed.
In this specification and in the claims which follow, reference will be made
to a number
of terms which shall be defined to have the following meanings:
-Optional" or -optionally" means that the subsequently described event or
circumstance
may or may not occur, and that the description includes instances where said
event or
circumstance occurs and instances where it does not.
An "increase" can refer to any change that results in a greater amount of a
symptom,
disease, composition, condition or activity. An increase can be any
individual, median, or
average increase in a condition, symptom, activity, composition in a
statistically significant
amount. Thus, the increase can be a 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25,
30, 35, 40, 45, 50, 55,
60, 65, 70, 75, 80, 85, 90, 95, or 100% increase so long as the increase is
statistically significant.
A "decrease" can refer to any change that results in a smaller amount of a
symptom,
disease, composition, condition, or activity. A substance is also understood
to decrease the
genetic output of a gene when the genetic output of the gene product with the
substance is less
relative to the output of the gene product without the substance. Also for
example, a decrease
can be a change in the symptoms of a disorder such that the symptoms are less
than previously
observed. A decrease can be any individual, median, or average decrease in a
condition,
- 7 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
symptom, activity, composition in a statistically significant amount. Thus,
the decrease can be a
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70,
75, 80, 85, 90, 95, or
100% decrease so long as the decrease is statistically significant.
"Inhibit," "inhibiting," and "inhibition" mean to decrease an activity,
response, condition,
disease, or other biological parameter. This can include but is not limited to
the complete
ablation of the activity, response, condition, or disease. This may also
include, for example, a
10% reduction in the activity, response, condition, or disease as compared to
the native or
control level. Thus, the reduction can be a 10, 20, 30, 40, 50, 60, 70, 80,
90, 100%, or any
amount of reduction in between as compared to native or control levels.
By "reduce" or other forms of the word, such as "reducing" or "reduction," is
meant
lowering of an event or characteristic (e.g., tumor growth). It is understood
that this is typically
in relation to some standard or expected value, in other words it is relative,
but that it is not
always necessary for the standard or relative value to be referred to. For
example, -reduces
tumor growth- means reducing the rate of growth of a tumor relative to a
standard or a control.
By "prevent" or other forms of the word, such as "preventing" or "prevention,"
is meant
to stop a particular event or characteristic, to stabilize or delay the
development or progression
of a particular event or characteristic, or to minimize the chances that a
particular event or
characteristic will occur. Prevent does not require comparison to a control as
it is typically more
absolute than, for example, reduce. As used herein, something could be reduced
but not
prevented, but something that is reduced could also be prevented. Likewise,
something could be
prevented but not reduced, but something that is prevented could also be
reduced. It is
understood that where reduce or prevent are used, unless specifically
indicated otherwise, the
use of the other word is also expressly disclosed.
The term "subject- refers to any individual who is the target of
administration or
treatment. The subject can be a vertebrate, for example, a mammal. In one
aspect, the subject
can be human, non-human primate, bovine, equine, porcine, canine, or feline.
The subject can
also be a guinea pig, rat, hamster, rabbit, mouse, or mole. Thus, the subject
can be a human or
veterinary patient. The term "patient" refers to a subject under the treatment
of a clinician, e.g.,
physician.
The term "therapeutically effective- refers to the amount of the composition
used is of
sufficient quantity to ameliorate one or more causes or symptoms of a disease
or disorder. Such
amelioration only requires a reduction or alteration, not necessarily
elimination.
The term "treatment" refers to the medical management of a patient with the
intent to
cure, ameliorate, stabilize, or prevent a disease, pathological condition, or
disorder. This term
- -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
includes active treatment, that is, treatment directed specifically toward the
improvement of a
disease, pathological condition, or disorder, and also includes causal
treatment, that is, treatment
directed toward removal of the cause of the associated disease, pathological
condition, or
disorder. In addition, this term includes palliative treatment, that is,
treatment designed for the
relief of symptoms rather than the curing of the disease, pathological
condition, or disorder;
preventative treatment, that is, treatment directed to minimizing or partially
or completely
inhibiting the development of the associated disease, pathological condition,
or disorder; and
supportive treatment, that is, treatment employed to supplement another
specific therapy
directed toward the improvement of the associated disease, pathological
condition, or disorder.
"Biocompatible" generally refers to a material and any metabolites or
degradation
products thereof that are generally non-toxic to the recipient and do not
cause significant adverse
effects to the subject.
"Comprising" is intended to mean that the compositions, methods, etc. include
the recited
elements, but do not exclude others. "Consisting essentially of' when used to
define
compositions and methods, shall mean including the recited elements, but
excluding other
elements of any essential significance to the combination. Thus, a composition
consisting
essentially of the elements as defined herein would not exclude trace
contaminants from the
isolation and purification method and pharmaceutically acceptable carriers,
such as phosphate
buffered saline, preservatives, and the like. "Consisting of' shall mean
excluding more than
trace elements of other ingredients and substantial method steps for
administering the
compositions provided and/or claimed in this disclosure. Embodiments defined
by each of these
transition terms are within the scope of this disclosure.
A "control" is an alternative subject or sample used in an experiment for
comparison
purposes. A control can be "positive" or "negative."
-Effective amount" of an agent refers to a sufficient amount of an agent to
provide a
desired effect. The amount of agent that is "effective" will vary from subject
to subject,
depending on many factors such as the age and general condition of the
subject, the particular
agent or agents, and the like. Thus, it is not always possible to specify a
quantified "effective
amount." However, an appropriate "effective amount" in any subject case may be
determined
by one of ordinary skill in the art using routine experimentation. Also, as
used herein, and
unless specifically stated otherwise, an -effective amount- of an agent can
also refer to an
amount covering both therapeutically effective amounts and prophylactically
effective amounts.
An "effective amount" of an agent necessary to achieve a therapeutic effect
may vary according
to factors such as the age, sex, and weight of the subject. Dosage regimens
can be adjusted to
- 9 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
provide the optimum therapeutic response. For example, several divided doses
may be
administered daily or the dose may be proportionally reduced as indicated by
the exigencies of
the therapeutic situation.
A "pharmaceutically acceptable" component can refer to a component that is not
biologically or otherwise undesirable, i.e., the component may be incorporated
into a
pharmaceutical formulation provided by the disclosure and administered to a
subject as
described herein without causing significant undesirable biological effects or
interacting in a
deleterious manner with any of the other components of the formulation in
which it is contained.
When used in reference to administration to a human, the term generally
implies the component
has met the required standards of toxicological and manufacturing testing or
that it is included
on the Inactive Ingredient Guide prepared by the U.S. Food and Drug
Administration.
"Pharmaceutically acceptable carrier" (sometimes referred to as a "carrier")
means a
carrier or excipient that is useful in preparing a pharmaceutical or
therapeutic composition that is
generally safe and non-toxic and includes a carrier that is acceptable for
veterinary and/or human
pharmaceutical or therapeutic use. The terms "carrier" or "pharmaceutically
acceptable carrier"
can include, but are not limited to, phosphate buffered saline solution,
water, emulsions (such as
an oil/water or water/oil emulsion) and/or various types of wetting agents. As
used herein, the
term "carrier" encompasses, but is not limited to, any excipient, diluent,
filler, salt, buffer,
stabilizer, solubilizer, lipid, stabilizer, or other material well known in
the art for use in
pharmaceutical formulations and as described further herein.
-Pharmacologically active" (or simply -active"), as in a -pharmacologically
active"
derivative or analog, can refer to a derivative or analog (e.g., a salt,
ester, amide, conjugate,
metabolite, isomer, fragment, etc.) having the same type of pharmacological
activity as the
parent compound and approximately equivalent in degree.
-Therapeutic agent" refers to any composition that has a beneficial biological
effect.
Beneficial biological effects include both therapeutic effects, e.g.,
treatment of a disorder or
other undesirable physiological condition, and prophylactic effects, e.g.,
prevention of a disorder
or other undesirable physiological condition (e.g., a non-immunogenic cancer).
The terms also
encompass pharmaceutically acceptable, pharmacologically active derivatives of
beneficial
agents specifically mentioned herein, including, but not limited to, salts,
esters, amides,
proagents, active metabolites, isomers, fragments, analogs, and the like. When
the terms
"therapeutic agent" is used, then, or when a particular agent is specifically
identified, it is to be
understood that the term includes the agent per se as well as pharmaceutically
acceptable,
¨ 10 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
pharmacologically active salts, esters, amides, proagents, conjugates, active
metabolites,
isomers, fragments, analogs, etc.
"Therapeutically effective amount" or "therapeutically effective dose" of a
composition
(e.g. a composition comprising an agent) refers to an amount that is effective
to achieve a
desired therapeutic result. In some embodiments, a desired therapeutic result
is the control of
type I diabetes. In some embodiments, a desired therapeutic result is the
control of obesity.
Therapeutically effective amounts of a given therapeutic agent will typically
vary with respect to
factors such as the type and severity of the disorder or disease being treated
and the age, gender,
and weight of the subject. The term can also refer to an amount of a
therapeutic agent, or a rate
of delivery of a therapeutic agent (e.g., amount over time), effective to
facilitate a desired
therapeutic effect, such as pain relief. The precise desired therapeutic
effect will vary according
to the condition to be treated, the tolerance of the subject, the agent and/or
agent formulation to
be administered (e.g., the potency of the therapeutic agent, the concentration
of agent in the
formulation, and the like), and a variety of other factors that are
appreciated by those of ordinary
skill in the art. In some instances, a desired biological or medical response
is achieved following
administration of multiple dosages of the composition to the subject over a
period of days,
weeks, or years.
Throughout this application, various publications are referenced. The
disclosures of
these publications in their entireties are hereby incorporated by reference
into this application in
order to more fully describe the state of the art to which this pertains. The
references disclosed
are also individually and specifically incorporated by reference herein for
the material contained
in them that is discussed in the sentence in which the reference is relied
upon.
Compositions and Methods
Disclosed are the components to be used to prepare the disclosed compositions
as well as
the compositions themselves to be used within the methods disclosed herein.
These and other
materials are disclosed herein, and it is understood that when combinations,
subsets, interactions,
groups, etc. of these materials are disclosed that while specific reference of
each various
individual and collective combinations and permutation of these compounds may
not be
explicitly disclosed, each is specifically contemplated and described herein.
For example, if a
particular substituted alginate strand or modified functional moiety is
disclosed and discussed
and a number of modifications that can be made to a number of molecules
including the
substituted alginate strand or modified functional moiety are discussed,
specifically
contemplated is each and every combination and permutation of substituted
alginate strand or
modified functional moiety and the modifications that are possible unless
specifically indicated
¨ 11 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
to the contrary. Thus, if a class of molecules A, B, and C are disclosed as
well as a class of
molecules D, E, and F and an example of a combination molecule, A-D is
disclosed, then even if
each is not individually recited each is individually and collectively
contemplated meaning
combinations, A-E, A-F, B-D, B-E, B-F, C-D, C-E, and C-F are considered
disclosed. Likewise,
any subset or combination of these is also disclosed. Thus, for example, the
sub-group of A-E,
B-F, and C-E would be considered disclosed. This concept applies to all
aspects of this
application including, but not limited to, steps in methods of making and
using the disclosed
compositions. Thus, if there are a variety of additional steps that can be
performed it is
understood that each of these additional steps can be performed with any
specific embodiment or
combination of embodiments of the disclosed methods.
Alginates are versatile polysaccharide based polymers that may be formulated
for
specific applications by controlling the molecular weight, rate of degradation
and method of
scaffold formation. Alginate molecules are comprised of (1-4)-linked I3-D-
mannuronic acid (M
units) and a L-guluronic acid (G units) monomers, which can vary in proportion
and sequential
distribution along the polymer chain. "Alginate', as used herein, is a
collective term used to
refer to linear polysaccharides formed from -D-mannuronate and L-guluronate in
any M/G ratio,
as well as salts and derivatives thereof The term "alginate", as used herein,
encompasses any
polymer having the structure shown below, as well as salts thereof
0 OH
OH
Imo; (-) 0-
o "
OH
OH -
"Mannuronate" and "Mannuronate Monomer", as used herein, refer to mannuronic
acid
monomers as well as salts thereof
0 OH
OH
0 __ c_OH 0
Cii) 0 0
7, 1ST HO
OH
"Gui uronate" and "Guluronate Monomer", as used heren_ refer to f2;11111rOlii
c acid
monomers' as well as salts thereof
¨ 12 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
_
OOH
css' OH
HO ThrL'N%,
OH
OII 01
"Chemically Modified Alginate' or "Modified Alginate, are used herein
interchangeably,
and refer to alginate polymers which contain one or more covalently modified
monomers.
"Covalently Modified Monomer', as used herein, refers to a monomer which is an
analog or
derivative of a mannuronate and/or guluronate monomer obtained from a
mannuronate and/or
guluronate monomer via a chemical process.
-Singularly Modified Alginate Polymer, as used herein, refers to modified
alginates that
contain one or more covalently modified monomers, wherein substantially all of
the covalently
modified monomers possess the same covalent modification (i.e., the polymer
contains one type
or species of covalently modified monomer). Singularly modified alginate
polymers include, for
example, modified alginate polymers wherein substantially all of the monomers
in
the modified alginate polymer are represented by mannuronate monomers,
guluronate
monomers, and a covalently modified monomer defined by Formula I described
below. Not all
of the monomers are necessarily covalently modified.
"Multiply Modified Alginate Polymer", as used herein, refers to modified
alginates that
contain covalently modified monomers, wherein substantially all of the
covalently modified monomers do not possess the same covalent modification
(i.e., the polymer
contains two or more different 'types' or species of covalently modified
monomers).
Multiply modified alginate polymers include, for example, modified alginate
polymers wherein
substantially all of the monomers in the modified alginate polymer are
represented by
mannuronate monomers, guluronate monomers, and two or more different types of
covalently modified monomers defined by Formula I. As used in this context, a
'type' or
'species' of covalently modified monomer refers to a covalent monomer defined
by Formula I,
wherein all possible variable positions are chemically defined. Not all the
monomers need be
covalently modified.
For clarity of discussion herein, singularly modified alginates are defined
using formulae
illustrating the structure of the covalently modified monomers incorporated in
the backbone and
omitting the mannuronate and guluronate monomers. For example, a
singularly modified alginate polymer composed of mannuronate monomers,
guluronate
monomers, and a covalently modified monomer defined by Formula I, wherein X is
NH, Li is
- 13 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
absent, L2 is ¨CH2-CH2-CH2-CH2-NH-CO-CH2¨, A is an azide, and Yi, and Y2 are
hydrogen,
is illustrated herein by the structure below.
OH
o Na
OxNH ______________________________ 0
__________________________________ 0 ,,, 0
y2
- n
"Substituted,- as used herein, refers to all permissible substituents of the
compounds or
functional groups described herein. In the broadest sense, the permissible
substituents include
acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic,
aromatic and
nonaromatic substituents of organic compounds. Illustrative substituents
include, but are not
limited to, halogens, hydroxyl groups, or any other organic groupings
containing any number of
carbon atoms, preferably 1-14 carbon atoms, and optionally include one or more
heteroatoms
such as oxygen, sulfur, or nitrogen grouping in linear, branched, or cyclic
structural formats.
Representative substituents include alkyl, substituted alkyl, alkenyl,
substituted alkenyl, alkynyl.
substituted alk-ynyl, phenyl, substituted phenyl, aryl, substituted aryl,
heteroaryl, substituted
heteroaryl, halo, hydroxyl, alkoxy, substituted alkoxy, phenoxy, substituted
phenoxy, aroxy,
substituted aroxy, alkylthio, substituted alkylthio, phenylthio, substituted
phenylthio, arylthio,
substituted arylthio, cyano, isocyano, substituted isocyano, carbonyl,
substituted carbonyl,
carboxyl, substituted carboxyl, amino, substituted amino, amido, substituted
amido, sulfonyl,
substituted sulfonyl, sulfonic acid, phosphoryl, substituted phosphoryl,
phosphonyl, substituted
phosphonyl, polyaryl, substituted polyaryl, C3-C20 cyclic, substituted C3-C20
cyclic, heterocyclic,
substituted heterocyclic, aminoacid, peptide, and polypeptide groups.
Heteroatoms such as nitrogen may have hydrogen substituents and/or any
permissible
substituents of organic compounds described herein which satisfy the valences
of the
heteroatoms. It is understood that "substitution- or "substituted" includes
the implicit proviso
that such substitution is in accordance with permitted valence of the
substituted atom and the
substituent, and that the substitution results in a stable compound, i.e. a
compound that does not
spontaneously undergo transformation such as by rearrangement, cyclization,
elimination, etc.
"Aryl," as used herein, refers to C5-C10-membered aromatic, heterocyclic,
fused
aromatic, fused heterocyclic, biaromatic, or bihetereocyclic ring systems.
Broadly defined,
¨ 14 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
"aryl,- as used herein, includes 5-, 6-, 7-, 8-, 9-, and 10-membered single-
ring aromatic groups
that may include from zero to four heteroatoms, for example, benzene, pyrrole,
furan, thiophene,
imidazole, oxazole, thiazole, triazole, pyrazole, pyridine, pyrazine,
pyridazine and pyrimidine,
and the like. Those aryl groups having heteroatoms in the ring structure may
also be referred to
as "aryl heterocycles" or "heteroaromatics." The aromatic ring can be
substituted at one or more
ring positions with one or more substituents including, but not limited to,
halogen, azide,
aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxyl, amino (or quatemized
amino), nitro,
sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl,
ether, alkylthio,
sulfonyl, sulfonamido, ketone, aldehyde, ester, heterocyclyl, aromatic or
heteroaromatic
moieties, ¨CF3, ¨CN; and combinations thereof
"Aryl" further encompasses polycyclic ring systems having two or more cyclic
rings in
which two or more carbons are common to two adjoining rings (i.e., -fused
rings") wherein at
least one of the rings is aromatic, e.g., the other cyclic ring or rings can
be cycloalkyls,
cycloalkenyls, cycloalkynyls, aryls and/or heterocycles. Examples of
heterocyclic rings include,
but are not limited to, benzimidazolyl, benzofuranyl, benzothiofuranyl,
benzothiophenyl,
benzoxazolyl, benzoxazolinyl, benzthiazolyl, benztriazolyl, benztetrazolyl,
benzisoxazolyl,
benzisothiazolyl, benzimidazolinyl, carbazolyl, 4aH carbazolyl, carbolinyl,
chromanyl,
chromenyl, cinnolinyl, decahydroquinolinyl, 2H,6H-1,5,2-dithiazinyl,
dihydrofuro[2,3b1tetrahydrofuran, furanyl, furazanyl, imidazolidinyl,
imidazolinyl, imidazolyl,
1H-indazolyl, indolenyl, indolinyl, indolizinyl, indolyl, 3H-indolyl,
isatinoyl, isobenzofuranyl,
isochromanyl, isoindazolyl, isoindolinyl, isoindolyl, isoquinolinyl,
isothiazolyl, isoxazolyl,
methylenedioxyphenyl, morpholinyl, naphthyridinyl, octahydroisoquinolinyl,
oxadiazolyl, 1,2,3-
oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl,
oxazolidinyl, oxazolyl,
oxindolyl, pyrimidinyl, phenanthridinyl, phenanthrolinyl, phenazinyl,
phenothiazinyl,
phenoxathinyl, phenoxazinyl, phthalazinyl, piperazinyl, piperidinyl,
piperidonyl, 4-piperidonyl,
piperonyl, pteridinyl, purinyl, pyranyl, pyrazinyl, pyrazolidinvl,
pyrazolinyl, pyrazolyl,
pyridazinyl, pyridooxazole, pyridoimidazole, pyridothiazole, pyridinyl,
pyridyl, pyrimidinyl,
pyrrolidinyl, pyrrolinyl, 2H-pyrrolyl, pyrrolyl, quinazolinyl, quinolinyl, 4H-
quinolizinyl,
quinoxalinyl, quinuclidinyl, tetrahydrofuranyl, tetrahydroisoquinolinyl,
tetrahydroquinolinyl,
tetrazolyl, 6H-1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl,
1,2,5-thiadiazolyl, 1,3,4-
thiadiazolyl, thianthrenyl, thiazolyl, thienyl, thienothiazolyl,
thienooxazolyl, thienoimidazolyl,
thiophenyl and xanthenyl. One or more of the rings can be substituted as
defined above for
"aryl."
- 15 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
"Alkyl,- as used herein, refers to the radical of saturated or unsaturated
aliphatic groups,
including straight-chain alkyl, alkenyl, or alkynyl groups, branched-chain
alkyl, alkenyl, or
alkynyl groups, cycloalkyl, cycloalkenyl, or cycloalkynyl (alicyclic) groups,
alkyl substituted
cycloalkyl, cycloalkenyl, or cycloalkynyl groups, and cycloalkyl substituted
alkyl, alkenyl, or
alkynyl groups. Unless otherwise indicated, a straight chain or branched chain
alkyl has 30 or
fewer carbon atoms in its backbone (e.g., Ci-C3o for straight chain, C3-C3o
for branched chain),
preferably 20 or fewer, more preferably 10 or fewer, most preferably 6 or
fewer. If the alkyl is
unsaturated, the alkyl chain generally has from 2-30 carbons in the chain,
preferably from 2-20
carbons in the chain, more preferably from 2-10 carbons in the chain.
Likewise, preferred
cycloalkyls have from 3-20 carbon atoms in their ring structure, preferably
from 3-10 carbons
atoms in their ring structure, most preferably 5, 6 or 7 carbons in the ring
structure.
The terms "alkenyl" and "alkynyl" refer to unsaturated aliphatic groups
analogous in
length and possible substitution to the alkyls described above, but that
contain at least one
double or triple bond respectively.
"Alkyl" includes one or more substitutions at one or more carbon atoms of the
hydrocarbon radical as well as heteroalkyls. Suitable substituents include,
but are not limited to,
halogens, such as fluorine, chlorine, bromine, or iodine; hydroxyl;
_____________ NR1R2, wherein Ri and
R2 are independently hydrogen, alkyl, or aryl, and wherein the nitrogen atom
is optionally
quatemized; ¨SR, wherein R is hydrogen, alkyl, or aryl; ¨CN; ¨NO2; ¨COOH;
carboxylate;
¨COR, ¨COOR, or ¨CONR2, wherein R is hydrogen, alkyl, or aryl; azide, aralkyl,
alkoxyl,
imino, phosphonate, phosphinate, silyl, ether, sulfonyl, sulfonamido,
heterocyclyl, aromatic or
heteroaromatic moieties, ______ CF3; __ CN; __ NCOCOCH2CH2, __ NCOCOCHCH;
______ NCS; and
combinations thereof
"Amino- and "Amine,- as used herein, are art-recognized and refer to both
substituted
and unsubstituted amines, e.g., a moiety that can be represented by the
general formula:
R' R"
+
¨N or ¨N¨R'
wherein, R, R', and R" each independently represent a hydrogen, substituted or
unsubstituted
alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted
alkynyl, substituted or
unsubstituted carbonyl, ¨(CH2)m¨W", or R and R' taken together with the N atom
to which
they are attached complete a heterocycle having from 3 to 14 atoms in the ring
structure; R"
represents a hydroxy group, substituted or unsubstituted carbonyl group, an
aryl, a cycloalkyl
¨ 16 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
ring, a cycloalkenyl ring, a heterocycle, or a polycycle; and m is zero or an
integer ranging from
1 to 8. In preferred embodiments, only one of R and R' can be a carbonyl,
e.g., R and R' together
with the nitrogen do not form an imide. In preferred embodiments, R and R'
(and optionally R")
each independently represent a hydrogen atom, substituted or unsubstituted
alkyl, a substituted
or unsubstituted alkenyl, or __ (CH21 ,m R'". Thus, the term `alkylamine'
as used herein refers to
an amine group, as defined above, having a substituted or unsubstituted alkyl
attached thereto
(i.e. at least one of R, R', or R" is an alkyl group).
-Carbonyl," as used herein, is art-recognized and includes such moieties as
can be
represented by the general formula:
0 0
____________________________ X __ R or ___ X ______ R`
wherein X is a bond, or represents an oxygen or a sulfur, and R represents a
hydrogen, a
substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl,
substituted or
unsubstituted alkynyl, ¨(CH2)m¨R", or a pharmaceutical acceptable salt, R'
represents a
hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted
alkenyl, substituted or
unsubstituted alkynyl, or ¨(CH2)m¨R"; R" represents a hydroxy group,
substituted or
unsubstituted carbonyl group, an aryl, a cycloalkyl ring, a cycloalkenyl ring,
a heterocycle, or a
polycycle; and m is zero or an integer ranging from 1 to 8. Where X is oxygen
and R is defines
as above, the moiety is also referred to as a carboxyl group. When X is oxygen
and R is
hydrogen, the formula represents a 'carboxylic acid.' Where X is oxygen and R'
is hydrogen, the
formula represents a 'formate.' In general, where the oxygen atom of the above
formula is
replaced by a sulfur, the formula represents a `thiocarbonyr group. Where X is
sulfur and R or
R' is not hydrogen, the formula represents a `thioester.' Where X is sulfur
and R is hydrogen, the
formula represents a 'thiocarboxylic acid.' Where X is sulfur and R' is
hydrogen, the formula
represents a `thioformate.' Where X is a bond and R is not hydrogen, the above
formula
represents a 'ketone.' Where X is a bond and R is hydrogen, the above formula
represents an
'aldehyde.'
"Heteroalk-yl," as used herein, refers to straight or branched chain, or
cyclic carbon-
containing radicals, or combinations thereof, containing at least one
heteroatom. Suitable
heteroatoms include, but are not limited to, 0, N, Si, P and S, wherein the
nitrogen, phosphorous
and sulfur atoms are optionally oxidized, and the nitrogen heteroatom is
optionally quatemized.
Examples of saturated hydrocarbon radicals include, but are not limited to,
methyl, ethyl,
n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, cyclohexyl,
(cyclohexyl)methyl,
- 17 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
cyclopropylmethyl, and homologs and isomers of, for example, n-pentyl, n-
hexyl, n-heptyl, n-
octyl. Examples of unsaturated alkyl groups include, but are not limited to,
vinyl, 2-propenyl,
crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3-(1,4-pentadienyl),
ethynyl, 1- and 3-
propynyl, and 3-butynyl.
"Alkoxy," "alkylamino," and "alkylthio" are used herein in their conventional
sense, and
refer to those alkyl groups attached to the remainder of the molecule via an
oxygen atom, an
amino group, or a sulfur atom, respectively.
-Alkylaryl," as used herein, refers to an alkyl group substituted with an aryl
group (e.g.,
an aromatic or hetero aromatic group).
"Heterocycle" or "heterocyclic," as used herein, refers to a cyclic radical
attached via a
ring carbon or nitrogen of a monocyclic or bicyclic ring containing 3-10 ring
atoms, and
preferably from 5-6 ring atoms, consisting of carbon and one to four
heteroatoms each selected
from the group consisting of non-peroxide oxygen, sulfur, and N(Y) wherein Y
is absent or is H,
0, Ci-Cm) alkyl, phenyl or benzyl, and optionally containing 1-3 double bonds
and optionally
substituted with one or more substituents. Examples of heterocyclic ring
include, but are not
limited to, benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl,
benzoxazolyl,
benzoxazolinyl, benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl,
benzimidazolinyl, carbazolyl, 4aH-carbazolyl, carbolinyl, chromanyl,
chromenyl, cinnolinyl,
decahydroquinolinyl, 2H,6H-1,5,2-dithiazinyl, dihydrofuro12,3-
bitetrahydrofuran, furanyl,
furazanyl, imidazolidinyl, imidazolinyl, imidazolyl, 1H-indazolyl, indolenyl,
indolinyl,
indolizinyl, indolyl, 3H-indolyl, isatinoyl, isobenzofuranyl, isochromanyl,
isoindazolyl,
isoindolinyl, isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl,
methylenedioxyphenyl,
morpholinyl, naphthyridinyl, octahydroisoquinolinyl, oxadiazolyl, 1,2,3-
oxadiazolyl, 1,2,4-
oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl, oxazolidinyl, oxazolyl,
oxindolyl,
pyrimidinyl, phenanthridinyl, phenanthrolinyl, phenazinyl, phenothiazinyl,
phenoxathinyl,
phenoxazinyl, phthalazinyl, piperazinyl, piperidinyl, piperidonyl, 4-
piperidonyl, piperonyl,
pteridinyl, purinyl, pyranyl, pyrazinyl, pyrazolidinyl, pyrazolinyl,
pyrazolyl, pyridazinyl,
pyridooxazole, pyridoimidazole, pyridothiazole, pyridinyl, pyridyl,
pyrimidinyl, pyrrolidinyl,
pyn-olinyl, 2H-pyrrolyl, pyrrolyl, quinazolinyl, quinolinyl, 4H-quinolizinyl,
quinoxalinyl,
quinuclidinyl, tetrahydrofuranyl, tetrahydroisoquinolinyl,
tetrahydroquinolinyl, tetrazolyl, 6H-
1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl, 1,2,5-
thiadiazolyl, 1,3,4-thiadiazolyl,
thianthrenyl, thiazolyl, thienyl, thienothiazolyl, thienooxazolyl,
thienoimidazolyl, thiophenyl and
xanthenyl. Heterocyclic groups can optionally be substituted with one or more
substituents as
defined above for alkyl and aryl.
- 18 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
We and others have demonstrated that poor calcium cross-linking directly
caused by high
DS causes loss of gel stiffness, migration of implants away from desired
injection sites, and
enhanced calcium leaching, leading to an increased foreign body response to
the gel. One
approach to loss of calcium cross-linking at high DS is to use alternative
cross-linkers, but this
has several drawbacks. The addition of new chemical cross-linkers add
unnecessary
complication to the regulatory pathway for clinical use and can have
unexpected physiological
toxicity. Additionally, as alternative cross-links are often covalent, these
cross-linkers eliminate
two advantages of using alginate in the first place - alginate's ability to
self-heal and its shear-
thinning characteristic.
to Since the carboxyl groups in alginate strands are required for both
calcium cross-linking
and for carbodiimide coupling, we hypothesized that the depletion of the
carboxyl groups during
EDC coupling was responsible for the observed loss of calcium cross-linking
and further
increasing the DS on alginate worsens this affect. This is unfortunate as for
many applications,
including in refillable drug delivery depots, extremely high DS would be
beneficial for hydrogel
function.
To overcome the challenge of achieving high DS modification without
sacrificing
calcium cross-linking, we report a new strategy that uses modifications that
restore carboxyl
groups on the alginate. We propose that the groups that are coupled to
alginate carry their own
carboxyl groups so that carboxyl groups are replaced at every modified spot.
In this report we
demonstrate that alginate gels conjugated to azide groups that restore the
carboxyl groups
(restorative modifications) provide much improved calcium cross-linking and
gelation properties
as compared to the same degree of substitution of azide groups that deplete
the carboxyl groups
(depletive modifications). In addition, alginates with restored carboxyl
groups have improved
retention and drug capture at injected sites. Taken together, we believe that
restorative
modifications are a promising approach to highly modified calcium cross-linked
alginate
hydrogels.
Accordingly, in one aspect disclosed herein are substituted alginate strands
comprising
alginate strand coupled to an functional moiety (such as, for example, azide)
via a linker with a
nucleophilic terminus (such as, for example, carbamic acid, carbamate ester,
glycine, arginine,
or lysine), wherein the linker comprises a nucleophilic terminus and a
carboxyl group of the
nucleophilic terminus; and wherein the nucleophilic terminus is coupled to a
carboxyl group on
the alginate strand. In some aspects, the substituted alginate strands can
form polymer strands.
Alginate polysaccharides are polyelectrolyte systems which have a strong
affinity for
divalent cations (e.g., Ca+2, Mg+2, Ba+2) and form stable hydrogels when
exposed to these
- 19 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
molecules. See Martinsen A., et al., Biotech. & Bioeng., 33 (1989) 79-89.) For
example,
calcium cross-linked alginate hydrogels are useful for the methods described
herein. For
example, the polymers, e.g., alginates, of the hydrogel are 0-100%
crosslinked, e.g., at least 1%,
5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or more, crosslinked. In
other
embodiments, the polymers, e.g., alginates, of the hydrogel are not
crosslinked. In some
examples, the polymers, e.g., alginates, of the hydrogel contain less than
50%, e.g., less than
50%, 40%, 30%, 20%, 10%, 50%, 2%, 1%, or less, crosslinking. Accordingly, also
disclosed
herein are hydrogel matrixes or scaffolds comprising polymer strands of the
substituted alginate
strand disclosed herein, wherein the polymer strands are crosslinked to other
alginate polymer
stands via calcium crosslinking.
Alginate may be chemically modified to yield new properties. For example,
alginate may
be oxidized to increase the rate of biodegradation. Alternatively, alginate
may be reduced for
improved biocompatibility. Alginate can also be chemically modified to change
their
crosslinking behavior. For instance, alginate can be modified with
bioorthogonal click groups to
allow click crosslinking. In another example, alginate can be modified with
acrylic groups to
allow radical polymerization crosslinking. As another example, alginates can
be modified with
host-guest chemistries to allow host-guest crosslinking.
Coupling reactions can be used to covalently attach bioactive epitopes, such
as the cell
adhesion sequence RGD to the polymer backbone. Chemical functionalization with
small
molecules regulates immune and foreign body response, functionalization with
peptides
mediates cellular and tissue responses, and modification with reactive
chemical groups enables
new modes of drug delivery. Alginate polymers conjugated to bioorthogonal -
click" chemical
motifs (as functional moieties) enhance cross-linking and expedite polymer
modification.
Additionally, alginates modified with click motifs have been used as
targetable drug depots,
capable of repeatedly capturing and releasing drugs.
Click chemistry refers to a class chemical reaction between two click groups
that exhibit
good yields, wide functional group tolerance, and are highly selective even in
the presence of a
complex mixture of biological molecules. These characteristics allow the click
reactions to
proceed even in vivo. Example click motif pairs used as the first click motif
and the second
click motif include, but not limited to, azide with phosphine; azide with
cyclooctyne; nitrone
with cyclooctyne; nitrile oxide with norbornene; oxanorbomadiene with azide;
trans-cyclooctene
with s-tetrazine; quadricyclane with bis(dithiobentil)nickel(II).
In some embodiments, the second click motif comprises an alkene, e.g., a
cyclooctene,
e.g., a transcyclooctene (TCO) or norbomene (NOR), and the first click motif
comprises a
- 20 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
tetrazine (Tz). In other embodiments, the second click motif comprises an
alkyne, e.g., a
cyclooctyne such as dibenzocyclooctyne (DBCO), and the first click motif
comprises an azide
(Az). In some embodiments, the second click motif comprises a Tz, and the
first click motif
comprises an alkene such as transcyclooctene (TCO) or norbornene (NOR).
Alternatively or in
addition, the first click motif comprises an Az, and the second click motif
comprises a
cyclooctyne such as dibenzocyclooctyne (DBCO). TCO reacts specifically in a
click chemistry
reaction with a tetrazine (Tz) moiety. DBCO reacts specifically in a click
chemistry reaction
with an azide (Az) moiety. Norbornene reacts specifically in a click chemistry
reaction with a
tetrazine (Tz) moiety.
Exemplary click chemistry reactions (and by extension click motifs) are shown
below.
For example, copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC) comprises
using a
Copper (Cu) catalyst at room temperature. The Azide-Alkyne Cycloaddition is a
1,3-dipolar
cycloaddition between an azide and a terminal or internal alkyne to give a
1,2,3-triazole.
Another example of click chemistry includes Staudinger ligation, which is a
reaction that
is based on the classic Staudinger reaction of azides with triarylphosphines.
It launched the field
of bioorthogonal chemistry as the first reaction with completely abiotic
functional. The azide
acts as a soft electrophile that prefers soft nucleophiles such as phosphines.
This is in contrast to
most biological nucleophiles which are typically hard nucleophiles. The
reaction proceeds
selectively under water-tolerant conditions to produce a stable product.
Phosphines are
completely absent from living systems and do not reduce disulfide bonds
despite mild reduction
potential. Azides had been shown to be biocompatible in FDA-approved drugs
such as
azidothymidine and through other uses as cross linkers. Additionally, their
small size allows
them to be easily incorporated into biomolecules through cellular metabolic
pathways.
Copper-free click chemistry is a bioorthogonal reaction first developed by
Carolyn
Bertozzi as an activated variant of an azide alkyne cycloaddition. Unlike
CuAAC, Cu-free click
chemistry has been modified to be bioorthogonal by eliminating a cytotoxic
copper catalyst,
allowing reaction to proceed quickly and without live cell toxicity. Instead
of copper, the
reaction is a strain-promoted alkyne-azide cycloaddition (SPAAC). It was
developed as a faster
alternative to the Staudinger ligation, with the first generations reacting
over sixty times faster.
The incredible bioorthogonality of the reaction has allowed the Cu-free click
reaction to be
applied within cultured cells, live zebrafish, and mice. Cyclooctynes were
selected as the
smallest stable alkyne ring which increases reactivity through ring strain
which has calculated to
be 19.9 kcal/mol.
- 21 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Copper-free click chemistry also includes nitrone dipole cycloaddition. Copper-
free click
chemistry has been adapted to use nitrones as the 1,3-dipole rather than
azides and has been used
in the modification of peptides.
This cycloaddition between a nitrone and a cyclooctyne forms N-alkylated
isoxazolines.
The reaction rate is enhanced by water and is extremely fast with second order
rate constants
ranging from 12 to 32 M's'. depending on the substitution of the nitrone.
Although the
reaction is extremely fast, incorporating the nitrone into biomolecules
through metabolic
labeling has only been achieved through post-translational peptide
modification.
Another example of click chemistry includes norbomene cycloaddition. 1,3
dipolar
cycloadditions have been developed as a bioorthogonal reaction using a nitrile
oxide as a 1,3-
dipole and a norbomene as a dipolarophile. Its primary use has been in
labeling DNA and RNA
in automated oligonucleotide synthesizers.
Norbomenes were selected as dipolarophiles due to their balance between strain-
promoted reactivity and stability. The drawbacks of this reaction include the
cross-reactivity of
the nitrile oxide due to strong electrophilicity and slow reaction kinetics.
Another example of click chemistry includes oxanorbomadiene cycloaddition. The
oxanorbomadiene cycloaddition is a 1,3-dipolar cycloaddition followed by a
retro-Diels Alder
reaction to generate a triazole-linked conjugate with the elimination of a
furan molecule. This
reaction is useful in peptide labeling experiments, and it has also been used
in the generation of
SPECT imaging compounds.
Ring strain and electron deficiency in the oxanorbomadiene increase reactivity
towards
the cycloaddition rate-limiting step. The retro-Diels Alder reaction occurs
quickly afterwards to
form the stable 1,2,3 triazole. Limitations of this reaction include poor
tolerance for substituents
which may change electronics of the oxanorbomadiene and low rates (second
order rate
constants on the order of 1(Y4).
Another example of click chemistry includes tetrazine ligation. The tetrazine
ligation is
the reaction of a trans-cyclooctene and an s-tetrazine in an inverse-demand
Diels Alder reaction
followed by a retro-Diels Alder reaction to eliminate nitrogen gas. The
reaction is extremely
rapid with a second order rate constant of 2000 M'-s' (in 9:1 methanol/water)
allowing
modifications of biomolecules at extremely low concentrations.
The highly strained trans-cyclooctene is used as a reactive dienophile. The
diene is a 3,6-
diaryl-s-tetrazine which has been substituted in order to resist immediate
reaction with water.
The reaction proceeds through an initial cycloaddition followed by a reverse
Diels Alder to
eliminate N2 and prevent reversibility of the reaction.
- 22 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Not only is the reaction tolerant of water, but it has been found that the
rate increases in
aqueous media. Reactions have also been performed using norbomenes as
dienophiles at second
order rates on the order of 1 M1-s'in aqueous media. The reaction has been
applied in labeling
live cells and polymer coupling.
Another example of click chemistry includes is [4+11 cycloaddition. This
isocyanide
click reaction is a [4+1] cycloaddition followed by a retro-Diels Alder
elimination of N2.
The reaction proceeds with an initial [4+1] cycloaddition followed by a
reversion to
eliminate a thermodynamic sink and prevent reversibility. This product is
stable if a tertiary
amine or isocyanopropanoate is used. If a secondary or primary isocyanide is
used, the produce
will form an imine which is quickly hydrolyzed.
Isocyanide is a favored chemical reporter due to its small size, stability,
non-toxicity, and
absence in mammalian systems. However, the reaction is slow, with second order
rate constants
on the order of 10-2M-1.s-1.
Another example of click chemistry includes quadricyclane ligation. The
quadricyclane
ligation utilizes a highly strained quadricyclane to undergo [2+2+2]
cycloaddition with 7E
systems.
Quadricyclane is abiotic, unreactive with biomolecules (due to complete
saturation),
relatively small, and highly strained (-80 kcal/mol). However, it is highly
stable at room
temperature and in aqueous conditions at physiological pH. It is selectively
able to react with
electron-poor it systems but not simple alkenes, alkynes, or cyclooctynes.
Bis(dithiobenzil)nickel(II) was chosen as a reaction partner out of a
candidate screen
based on reactivity. To prevent light-induced reversion to norbomadiene,
diethyldithiocarbamate
is added to chelate the nickel in the product.
These reactions are enhanced by aqueous conditions with a second order rate
constant of
0.25 M-1- s-1. Of particular interest is that it has been proven to be
bioorthogonal to both oxime
formation and copper-free click chemistry.
The exemplary click chemistry reactions have high specificity, efficient
kinetics, and
occur in vivo under physiological conditions. See, e.g., Baskin et al. Proc.
Natl. Acad.. Sci.
USA 104(2007):16793; Oneto et al. Acta biomaterilia (2014); Neves et al.
Bioconjugate
chemistry 24(2013):934; Koo et al. Angewandte Chemie 51(2012):11836; and
Rossin et
al. Angewandte Chemie 49(2010):3375. For a review of a wide variety of click
chemistry
reactions and their methodologies, see e.g., Nwe K and Brechbiel M W, 2009
Cancer
Biotherapy and Radiopharmaceuticals, 24(3): 289-302; Kolb H C et al., 2001
Angew. Chem. Int.
¨ 23 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Ed. 40: 2004-2021. The entire contents of each of the foregoing references are
incorporated
herein by reference.
Exemplary click motif pairs are shown in the table below.
Functional Paired Functional group/Click Motif
Reaction type
group/Click with
(Reference)
Motif
azide Phosphine Stauclinger
ligation
(Saxon et al. Science
287(2000):2007-10)
azide Cyclooctyne, e.g., dibenzocyclooctyne, one
of Copper-free click
the cyclooctynes shown below, or other similar chemistry (Jewett et al.
cyclooctyncs: J. Am.
Chem. Soc.
132.11(2010):3688-90;
= 1:
Sletten et a/. Organic
, .
OCT
Ha' txso 21ARAC Letters
10.14
1 r=P (2008):3097-
9; Lutz.
Angew. Chem., Int. Ed
AL:6
OPO
47.12(2008):2182)
; DII3AC
DMAC:
noo,4
nitrone Cyclooctyne Nitrone
Dipole
Cycloadclition (Ning et
al. Angew. Chem., Int.
Ed 49.17 (2010):3065)
Nitrile oxide Norbomene Norbornene
Cycloadclition
(Gutsmiecll et at.
Organic Letters
11.11(2009):2405-8)
oxanorbornadiene Azide
Oxanorbornadiene
Cycloadclition (Van
Berkel et al.
ChemBioChem
8.13(2007): 1504-8)
Trans- s-tetrazine Tetrazine
ligation
cyclooctene, (Hansel' et
al. J Am.
norborncnc, or Chem. Soc.
other alkene
133.35(20H):13828-31)
nitrilc 1,2,4,54ctrazinc [4+1]
cycloaddition
(Stockman etal.
Organic and Biomol.
Chem. 9.21(2011):7303)
quadricyclane Bis(dithiobenzil)nickel(lI)
Quadricyclane Ligation
(Sletten et al. J. Am.
Chem. Soc.
133.44(2011):17570-3)
Ketone or Hydrazines, hydrazones, oximes, amines.
Non-aldol carbonyl
aldehyde ureas, thioureas, etc. chemistry
(Khomyakova EA, et al.
Nucleosides Nucleotides
Nucleic Acids. 30(7-8)
(2011) 577-84
¨ 24 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Thiol Maleimide Michael
addition
(Zhou et al. Bioconjug
Chem 2007 18(2):323-
32.)
Dienes Dienophiles Diets Alder
(Rossin et
al. Nucl Med. (2013)
54(11):1989-95)
Tetrazine norbomene, propene, trans-cyclooctene, other
strained alkenes.
Other suitable include the motifs can be found, for example, in Patterson,
D.M., et al.
"Finding the Right (Bioorthogonal) Chemistry,- ACS Chem. Biol., 2014, 9(3):
592-605; Akgun,
B., et al. "Synergic "Click" Boronate/Thiosemicarbazone System for Fast and
Irreversible
Bioorthogonal Conjugation in Live Cells," I Am. Chem. Soc., 2017, 139(40):
14285-14291; and
Akgun, B. and Hall, D.G. -Fast and Tight Boronate Formation for Click
Bioorthogonal
Conjugation,"Angew. Chem., Int. Ed. 2016, 55(12): 3909-3913, each of which is
hereby
incorporated by reference in its entirety
Alginate polymers are formed into a variety of hydrogel types. Alginate
hydrogels can
be formed from low molecular weight (MW) alginate or from high MW alginate.
Differences in
hydrogel formulation control the kinetics of hydrogel degradation. Release
rates of
pharmaceutical compositions, e.g., small molecules, morphogens, or other
bioactive substances,
from alginate hydrogels is controlled by hydrogel formulation to present the
pharmaceutical
compositions in a spatially and temporally controlled manner. This controlled
release eliminates
systemic side effects and the need for multiple injections.
Mannuronate and guluronate monomers contain a carboxylic acid moiety which can
serve as a point of covalent modification. In preferred embodiments, the
carboxylic acid moiety
present on one or more mannuronate and/or guluronate residues can be
covalently modified via
amidation. However, as noted herein, amidation of alginate typically causes
loss of carboxyl
residues which in turn results in destructive effects on the ability of
alginate polymers to
crosslink via Calcium crosslinking. The result is a loss of many of the
advantageous properties
of the alginate polymers. Accordingly, disclosed herein are substituted
alginate strands that do
not suffer from these problems and methods for achieving said modified
alginate strands. In one
aspect, disclosed herein are methods of coupling an alginate strand to a
functional moiety (e.g., a
click motif discussed above, such as, for example, an azide) via carbodiimide
coupling said
method comprising conjugating the functional moiety to a linker comprising an
nucleophilic
terminus and a carboxyl group of the nucleophilic terminus of the linker (such
as, for example,
carbamic acid, carbamate ester, glycine, arginine, or lysine); wherein the
functional moiety is
¨ 25 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
chemically coupled to the linker at the opposing end to the nucleophilic
terminus; and coupling
the nucleophilic terminus of the linker to the alginate strand via
carbodiimide coupling; wherein
the modification allows for synthesis of highly substituted alginate; wherein
the substitution
does not disrupt the integrity of the gel.
To prevent the amino terminal end of the linker binding to the carboxyl group
of the
linker prior to amidation to the alginate strand, the carboxyl group on the
linker can be blocked
with a protecting group. Any protecting group known and used in the art can be
used for this
purpose, including, but not limited to methyl, ethyl, benzyl,
benzyloxycarbonyl, s-Butyl, 2-
Alky1-1,3-oxazoline, OBO, silyl, photo-sensitive group propyl, tert-butyl, and
NVOC.
Also disclosed herein are methods of coupling an alginate strand to a
functional moiety,
wherein the nucleophilic terminus of the linker comprises a protecting group
(such as, for
example, tert-butyloxycarbonyl, oc-boc,p-methoxybenzyl carbonyl,
carbobenzyloxy, acetyl,
benzoyl, benzyl, carbamate, p-methoyxybenzyl, 3,4-dimethoxybenyl,p-
methoxyphyl,
trichloroethyl chloroformate, or tosyl); and wherein the nucleophilic terminus
is deprotected
prior to being coupled to the alginate strand.
Also provided here are modified alginates. The modified alginates include
alginate
monomers that have been covalently modified to facilitate crosslinking while
without sacrificing
calcium cross-linking. The modified alginates can comprise one or more
covalently modified
monomers defined by Formula I below
OOH
L2
OxX
0
OMOA
yi
y2
Formula I
wherein:
Xis 0, S, or NR3;
R3 is hydrogen, alkyl, alkenyl, alkynyl, aryl, heteroaryl, cyoloalkyl, or
heterocycloalkyl;
Li and L2 are each independently absent or represent a linking group;
A represents a functional moiety; and
- 26 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Yi and Y2 independently are hydrogen or ¨PO(OR)2, or Y2 is absent, and Yi,
together
with the two oxygen atoms to which Yi and Y2 are attached form a cyclic
structure as shown
below
OOH
Li L2
0 X
0õ X"" 0
R5
R4
wherein
wherein R4 and R5 are, independently, hydrogen, alkyl, alkenyl, alkynyl, aryl,
heteroaryl,
cycloalkyl, heterocycloalkyl, alkoxy, aroxy, alkylthio, carbonyl, carboxyl,
amino, or amido; or
R4 and R5, together with the carbon atom to which they are attached, form a 3-
to 8-membered
unsubstituted or substituted carbocyclic or heterocyclic ring.
In certain embodiments, Yi and Y2 are both H.
In certain embodiments, X is NH.
In some embodiments, A can comprise a click motif Examples of suitable click
motifs
are described above. For example, in some embodiments, A can comprise an
azide, a phosphine,
a cyclooctene (e.g., a transcyclooctene (TCO)), a norbornene (NOR), a
tetrazine (Tz), an alkyne,
a cyclooctyne such as dibenzocyclooctyne (DSCO), or a quadricyclane. In
certain embodiments,
the functional moiety comprises an active agent, as described below.
In some embodiments, both Li and L2 are present. In some embodiments, Li is
absent
and L2 is present. In some embodiments, Li is present and L2 is absent. In
some embodiments,
both Li and L2 are absent.
When present, the linking group can be any suitable group or moiety which is
at
minimum bivalent, and connects the two radical moieties to which the linking
group is attached
in the monomers of Formula I. The linking group can be composed of any
assembly of atoms,
including oligomeric and polymeric chains. In some cases, the total number of
atoms in the
linking group can be from 3 to 200 atoms (e.g., from 3 to 150 atoms, from 3 to
100 atoms, from
3 and 50 atoms, from 3 to 25 atoms, from 3 to 15 atoms, or from 3 to 10
atoms).
- 27 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
In some embodiments, the linking group can be, for example, an alkyl, alkoxy,
alkylaryl,
alkylheteroaiyl, alkylcycloalkyl, alkylheterocycloalkyl, alkylthio,
alkylsulfinyl, alkylsulfonyl,
alkylamino, dialkylamino, alkylcarbonyl, alkoxycarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl, or polyamino group. In some embodiments, the linking
group can
comprises one of the groups above joined to one or both of the moieties to
which it is attached
by a functional group. Examples of suitable functional groups include, for
example, secondary
amides (-CONH-), tertiary amides (-CONR-), secondary carbamates (-000NH-; -
NHC00-),
tertiary carbamates (-000NR-; -NRC00-), ureas (-NHCONH-; -NRCONH-; -NHCONR-,
or -NRCONR-), carbinols ( -CHOH-, -CROH-), ethers (-0-), and esters (-000-,
¨CH202C-,
CHRO2C-), wherein R is an alkyl group, an aryl group, or a heterocyclic group.
For example, in
some embodiments, the linking group can comprise an alkyl group (e.g., a Ci-
C12 alkyl group, a
C1-C8 alkyl group, or a C1-C6 alkyl group) bound to one or both of the
moieties to which it is
attached via an ester (-000-, ¨CH202C-, CHRO2C-), a secondary amide (-CONH-),
or a tertiary
amide (-CONR-), wherein R is an alkyl group, an aryl group, or a heterocyclic
group. In certain
embodiments, the linking group can be chosen from one of the following:
R1 R1 R1
rn f T1 fll f f I f I
0 0
R1
0(1r-0,1_1-.0,1r\ 0 N 0
rn I
" m
0 0 m m R1 R1
R1 R1 R1 I I I =-t,
I I IrS
F1 R1 R1 R1 R1,zar 0 y N N
y 0
N N N
m m 0 0 0 0
R1 R1 Ri
N
y
1r
N
0 0 0 0 or m m
where m is an integer from 1 to 12 and R1 is, independently for each
occurrence, hydrogen, an
alkyl group, an aryl group, or a heterocyclic group.
If desired, the linker can serve to modify the solubility of the compounds
described
herein. In some embodiments, the linker is hydrophilic. In some embodiments,
the linker can be
an alkyl group, an alkylaryl group, an oligo- or polyalkylene oxide chain
(e.g., an oligo- or
polyethylene glycol chain), or an oligo- or poly(amino acid) chain.
¨ 28 ¨
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Modified alginate polymers can be of any desired molecular weight. The weight
average
molecular weight of the alginates is preferably between 1,000 and 1,000,000
Daltons, more
preferably between 10,000 and 500,000 Daltons as determined by gel permeation
chromatography.
Modified alginate polymers can contain any ratio of mannuronate monomers,
guluronate
monomers, and covalently modified monomers. In some embodiments, greater than
2.5%, 5%,
7.5%, 10%, 12%, 14%, 15%, 16%, 18%, 20%, 22%, 24%, 25%, 26%, 28%, 30%, 32.5%,
35%,
37.5%, 40%, 45%, 50%, 55%, or 60% of the monomers in the modified alginate
polymer are
covalently modified monomers. Preferably greater than 10%, more preferably
greater than 20%,
and most preferably greater than 30% of the monomers in the modified alginate
polymer are
coval ently modified monomers.
Active Agents
The term "Active Agent", as used herein, refers to a physiologically or
pharmacologically active substance that acts locally and/or systemically in
the body. An active
agent is a substance that is administered to a patient for the treatment (e.g,
therapeutic agent),
prevention (e.g., prophylactic agent), or diagnosis (e.g., diagnostic agent)
of a disease or
disorder.
The active agent can be a small molecule, or a biologic. A biologic is a
medicinal
product manufactured in, extracted from, or semi-synthesized from biological
sources which is
different from chemically synthesized pharmaceuticals. In some embodiments,
biologics used as
the active agent can include, for example, antibodies, blood components,
allergenics, gene
therapies, and recombinant therapeutic proteins. Biologics can comprise, for
example, sugars,
proteins, or nucleic acids, and they can be isolated from natural sources such
as human, animal,
or microorganism.
In some embodiments, the active agent can comprise an anti-cancer drug, a drug
that
promotes wound healing, a drug that treats or prevents infection, or a drug
that promotes
vascularization. For example, the active agent can comprise an anti-cancer
drug, such as a
chemotherapeutic or a cancer vaccine. The anti-cancer drug can include a small
molecule, a
peptide or polypeptide, a protein or fragment thereof (e.g., an antibody or
fragment thereof), or a
nucleic acid.
Exemplary anti-cancer drugs can include, but are not limited to, Abiraterone
Acetate,
Abitrexate (Methotrexate), Abraxane (Paclitaxel Albumin-stabilized
Nanoparticle Formulation),
ABVD, ABVE, ABVE-PC, AC, AC-T, Adcetris (Brentuximab Vedotin), ADE, Ado-
Trastuzumab Emtansine, Adriamycin (Doxorubicin Hydrochloride), Adrucil
(Fluorouracil),
- 29 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Afatinib Dimaleate, Afinitor (Everolimus), Aldara (Imiquimod), Aldesleukin,
Alemtuzumab,
Alimta (Pemetrexed Disodium), Aloxi (Palonosetron Hydrochloride), Ambochlorin
(Chlorambucil), Aminolevulinic Acid, Anastrozole, Aprepitant, Aredia
(Pamidronate
Disodium), Arimidex (Anastrozole), Aromasin (Exemestane), Arranon
(Nelarabine), Arsenic
Trioxide, Arzerra (Ofatumumab), Asparaginase Erwinia chrysanthemi, Avastin
(Bevacizumab),
Axitinib, Azacitidine, BEACOPP, Bendamustine Hydrochloride, BEP, Bevacizumab,
Bexarotene, Bexxar (Tositumomab and I 131 Iodine Tositumomab), Bicalutamide,
Bleomycin,
Bortezomib, Bosulif (Bosutinib), Bosutinib, Brentuximab Vedotin, Busulfan,
Busulfex
(Busulfan), Cabazitaxel, Cabozantinib-S-Malate, CAF, Campath (Alemtuzumab),
Camptosar
(Irinotecan Hydrochloride), Capecitabine, CAPDX, Carboplatin, Carboplatin-
Taxol,
Carfilzomib, Casodex (Bicalutamide), CeeNU (Lomustine), Cerubidine
(Daunorubicin
Hydrochloride), Cervarix (Recombinant HPV Bivalent Vaccine), Cetuximab,
Chlorambucil,
Chlorambucil-Prednisone, CHOP, Cisplatin, Clafen (Cyclophosphamide),
Clofarabine, Clofarex
(Clofarabine), Clolar (Clofarabine), CMF, Cometriq (Cabozantinib-S-Malate),
COPP, COPP-
ABV, Cosmegen (Dactinomycin), Crizotinib, CVP, Cyclophosphamide, Cyfos
(Ifosfamide),
Cytarabine, Cytarabine (Liposomal), Cytosar-U (Cytarabine), Cytoxan
(Cyclophosphamide),
Dabrafenib, Dacarbazine, Dacogen (Decitabine), Dactinomycin, Dasatinib,
Daunorubicin
Hydrochloride, Decitabine, Degarelix, Denileukin Diftitox, Denosumab, DepoCyt
(Liposomal
Cytarabine), DepoFoam (Liposomal Cytarabine), Dexrazoxane Hydrochloride,
Docetaxel, Doxil
(Doxorubicin Hydrochloride Liposome), Doxorubicin Hydrochloride, Doxorubicin
Hydrochloride Liposome, Dox-SL (Doxorubicin Hydrochloride Liposome), DTIC-Dome
(Dacarbazine), Efudex (Fluorouracil), Elitek (Rasburicase), Ellence
(Epirubicin Hydrochloride),
Eloxatin (Oxaliplatin), Eltrombopag Olamine, Emend (Aprepitant), Enzalutamide,
Epirubicin
Hydrochloride, EPOCH, Erbitux (Cetuximab), Eribulin Mesylate, Erivedge
(Vismodegib),
Erlotinib Hydrochloride, Erwinaze (Asparaginase Erwinia chrysanthemi),
Etopophos (Etoposide
Phosphate), Etoposide, Etoposide Phosphate, Evacet (Doxorubicin Hydrochloride
Liposome),
Everolimus, Evista (Raloxifene Hydrochloride), Exemestane, Fareston
(Toremifene), Faslodex
(Fulvestrant), FEC, Femara (Letrozole), Filgrastim, Fludara (Fludarabine
Phosphate),
Fludarabine Phosphate, Fluoroplex (Fluorouracil), Fluorouracil, Folex
(Methotrexate), Folex
PFS (Methotrexate), Folfiri, Folfiri-Bevacizumab, Folfiri-Cetuximab,
Folfirinox, Folfox,
Folotyn (Pralatrexate), FU-LV, Fulvestrant, Gardasil (Recombinant HPV
Quadrivalent
Vaccine), Gazyva (Obinutuzumab), Gefitinib, Gemcitabine Hydrochloride,
Gemcitabine-
Cisplatin, Gemcitabine-Oxaliplatin, Gemtuzumab Ozogamicin, Gemzar (Gemcitabine
Hydrochloride), Gilotrif (Afatinib Dimaleate), Gleevec (Imatinib Mesylate),
Glucarpidase,
¨ 30 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Goserelin Acetate, Halaven (Eribulin Mesylate), Herceptin (Trastuzumab), HPV
Bivalent
Vaccine (Recombinant), HPV Quadrivalent Vaccine (Recombinant), Hycamtin
(Topotecan
Hydrochloride), Hyper-CVAD, Ibritumomab Tiuxetan, Ibrutinib, ICE, Iclusig
(Ponatinib
Hydrochloride). Ifex (Ifosfamide), Ifosfamide, Ifosfamidum (Ifosfamide),
Imatinib Mesylate,
Imbruvica (Ibrutinib), Imiquimod, Inlyta (Axitinib), Intron A (Recombinant
Interferon Alfa-2b),
Iodine 131 Tositumomab and Tositumomab, Ipilimumab, Iressa (Gefitinib),
Irinotecan
Hydrochloride, Istodax (Romidepsin), Ixabepilone, Ixempra (Ixabepilone),
Jakafi (Ruxolitinib
Phosphate), J evtana (Cabazitaxel), Kadcyla (Ado-I rastuzumab Emtansine),
Keoxifene
(Raloxifene Hydrochloride), Kepivance (Palifermin), Kyprolis (Carfilzomib),
Lapatinib
Ditosylate, Lenalidomide, Letrozole, Leucovorin Calcium, Leukeran
(Chlorambucil), Leuprolide
Acetate, Levulan (Aminolevulinic Acid), Linfolizin (Chlorambucil), LipoDox
(DoNorubicin
Hydrochloride Liposome), Liposomal Cytarabine, Lomustine, Lupron (Leuprolide
Acetate),
Lupron Depot (Leuprolide Acetate), Lupron Depot-Ped (Leuprolide Acetate),
Lupron Depot-3
Month (Leuprolide Acetate), Lupron Depot-4 Month (Leuprolide Acetate), Marqibo
(Vincristine
Sulfate Liposome), Matulane (Procarbazine Hydrochloride), Mechlorethamine
Hydrochloride,
Megace (Megestrol Acetate), Megestrol Acetate, Mekinist (Trametinib),
Mercaptopurine,
Mesna, Mesnex (Mesna), Methazolastone (Temozolomide), Methotrexate,
Methotrexate LPF
(Methotrexate), Mexate (Methotrexate), Mexate-AQ (Methotrexate), Mitomycin C,
Mitozytrex
(Mitomycin C), MOPP, Mozobil (Plerixafor), Mustargen (Mechlorethamine
Hydrochloride),
Mutamycin (Mitomycin C), Myleran (Busulfan), Mylosar (Azacitidine), Mylotarg
(Gemtuzumab Ozogamicin), Nanoparticle Paclitaxel (Paclitaxel Albumin-
stabilized
Nanoparticle Formulation), Navelbine (Vinorelbine Tartrate), Nelarabine,
Neosar
(Cyclophosphamide), Neupogen (Filgrastim), Nexavar (Sorafenib Tosylate),
Nilotinib,
Nolvadex (Tamoxifen Citrate), Npl ate (Romiplostim), Obinutuzumab, Ofatumumab,
Omacetaxine Mepesuccinate, Oncaspar (Pegaspargase), Ontak (Denileukin
Diftitox), OEPA,
OPPA, Oxaliplatin, Paclitaxel, Paclitaxel Albumin-stabilized Nanoparticle
Formulation,
Palifermin, Palonosetron Hydrochloride, Pamidronate Disodium, Panitumumab,
Paraplat
(Carboplatin), Paraplatin (Carboplatin), Pazopanib Hydrochloride,
Pegaspargase, Peginterferon
Alfa-2b, PEG-Intron (Peginterferon Alfa-2b), Pemetrexed Disodium, Pe eta
(Pertuzumab),
Pertuzumab, Platinol (Cisplatin), Platinol-AQ (Cisplatin), Plerixafor,
Pomalidomide, Pomalyst
(Pomalidomide), Ponatinib Hydrochloride, Pralatrexate, Prednisone,
Procarbazine
Hydrochloride, Proleukin (Aldesleukin), Prolia (Denosumab), Promacta
(Eltrombopag
Olamine), Provenge (Sipuleucel-T), Purinethol (Mercaptopurine), Radium 223
Dichloride,
Raloxifene Hydrochloride, Rasburicase, R-CHOP, R-CVP, Recombinant HPV Bivalent
¨ 31 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Vaccine, Recombinant HPV Quadrivalent Vaccine, Recombinant Interferon Alfa-2b,
Regorafenib, Revlimid (Lenalidomide), Rheumatrex (Methotrexate), Rituxan
(Rituximab),
Rituximab, Romidepsin, Romiplostim, Rubidomycin (Daunorubicin Hydrochloride),
Ruxolitinib
Phosphate, Sclerosol Intrapleural Aerosol (Talc). Sipuleucel-T, Sorafenib
Tosylate, Sprycel
(Dasatinib), Stanford V, Sterile Talc Powder (Talc), Steritalc (Talc),
Stivarga (Regorafenib),
Sunitinib Malate, Sutent (Sunitinib Malate), Sylatron (Peginterferon Alfa-2b),
Synovir
(Thalidomide), Synribo (Omacetaxine Mepesuccinate), Tafinlar (Dabrafenib),
Talc, Tamoxifen
Citrate, Tarabine PFS (Cytarabine), Tarceva (Erlotinib Hydrochloride),
Targretin (Bexarotene),
Tasigna (Nilotinib), Taxol (Paclitaxel), Taxotere (Docetaxel), Temodar
(Temozolomide),
Temozolomide, Temsirolimus, Thalidomide, Thalomid (Thalidomide), Toposar
(Etoposide),
Topotecan Hydrochloride, Toremifene, Torisel (Temsirolimus), Tositumomab and
In' Iodine
Tositumomab, Totect (Dexrazoxane Hydrochloride), Trametinib, Trastuzumab,
Treanda
(Bendamustine Hydrochloride), Trisenox (Arsenic Trioxide), Tykerb (Lapatinib
Ditosylate),
Vandetanib, VAMP, Vectibix (Panitumumab), VeIP, Velban (Vinblastine Sulfate),
Velcade
(Bortezomib), Velsar (Vinblastine Sulfate), Vemurafenib, VePesid (Etoposide),
Viadur
(Leuprolide Acetate), Vidaza (Azacitidine), Vinblastine Sulfate, Vincasar PFS
(Vincristine
Sulfate), Vincristine Sulfate, Vincristine Sulfate Liposome, Vinorelbine
Tartrate, Vismodegib,
Voraxaze (Glucarpidase), Vorinostat, Votrient (Pazopanib Hydrochloride),
Wellcovorin
(Leucovorin Calcium), Xalkori (Crizotinib), Xeloda (Capecitabine), XELOX,
Xgeva
(Denosumab), Xofigo (Radium 223 Dichloride), Xtandi (Enzalutamide), Yervoy
(Ipilimumab),
Zaltrap (Ziv-Aflibercept), Zelboraf (Vemurafenib), Zevalin (Ibritumomab
Tiuxetan), Zinecard
(Dexrazoxane Hydrochloride), Ziv-Aflibercept, Zoladex (Goserelin Acetate),
Zoledronic Acid,
Zolinza (Vorinostat), Zometa (Zoledronic Acid), and Zytiga (Abiraterone
Acetate).
In some embodiments, the active agent can comprise a drug that promotes wound
healing
or vascularization. In some embodiments, the active agent can comprise a drug
that reduces
ischemia, e.g., due to peripheral artery disease (PAD) or damaged myocardial
tissues due to
myocardial infarction. For example, the drug can comprise a protein or
fragment thereof, e.g., a
growth factor or angiogenic factor, such as vascular endothelial growth factor
(VEGF), e.g.,
VEGFA, VEGFB, VEGFC, or VEGFD, and/or IGF, e.g., IGF-1, fibroblast growth
factor (FGF),
angiopoietin (ANG) (e.g., Angl or Ang2), matrix metalloproteinase (MMP), delta-
like ligand 4
(DLL4), paclitaxel, or combinations thereof Drugs that promote wound healing
or
vascularization are non-limiting, as the skilled artisan would be able to
readily identify other
drugs that promote wound healing or vascularization.
- 32 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
In some embodiments, the active agent can comprise an anti-proliferative drug,
e.g.,
mycophenolate mofetil (MMF), azathioprine, sirolimus, tacrolimus, paclitaxel,
biolimus A9,
novolimus, myolimus, zotarolimus, everolimus, or tranilast. These anti-
proliferative drugs are
non-limiting, as the skilled artisan would be able to readily identify other
anti-proliferative
drugs.
In some embodiments, the active agent can comprise an anti-inflammatory drug,
e.g.,
corticosteroid anti-inflammatory drugs (e.g., beclomethasone, beclometasone,
budesonide,
flunisolide, fluticasone propionate, triamcinolone, methylprednisolone,
prednisolone, or
preclnisone); or non-steroidal anti-inflammatory drugs (NSAIDs) (e.g.,
acetylsalicylic acid,
diflunisal, salsalate, choline magnesium trisalicylate, ibuprofen,
dexibuprofen, naproxen,
fenoprofen, ketoprofen, dexketoprofen, fl uri bi pro fen , oxaprozin,
loxoprofen, indomethacin,
tolmetin, sulindac, etodolac, ketorolac, diclofenac, aceclofenac, nabumetone,
piroxicam,
meloxicam, tenoxicam, droxicam, lornoxicam, isoxicam, mefenamic acid,
meclofenamic acid,
flufenamic acid, tolfenamic acid, celecoxib, rofecoxib, valdecoxib, parecoxib,
lumiracoxib,
etoricoxib, firocoxib, nimesulide, licofelone, H-harpaide, or lysine
clonixinate). These anti-
inflammatory drugs are non-limiting, as the skilled artisan would be able to
readily identify
other anti-inflammatory drugs.
In some embodiments, the active agent can comprise a drug that prevents or
reduces
transplant rejection, e.g., an immunosuppressant. Exemplary immunosuppressants
include
calcineurin inhibitors (e.g., cyclosporine, Tacrolimus (FK506)); mammalian
target of rapamycin
(mTOR) inhibitors (e.g., rapamycin, also known as Sirolimus);
antiproliferative agents (e.g.,
azathioprine, mycophenolate mofetil, mycophenolate sodium); antibodies (e.g.,
basiliximab,
daclizumab, muromonab); corticosteroids (e.g., prednisone). These drugs that
prevent or reduce
transplant rejection are non-limiting, as the skilled artisan would be able to
readily identify other
drugs that prevent or reduce transplant rejection.
In some embodiments, the active agent can comprise an anti-thrombotic drug,
e.g., an
anti-platelet drug, an anticoagulant drug, or a thrombolytic drug.
Exemplary anti-platelet drugs include an irreversible cyclooxygenase inhibitor
(e.g.,
aspirin or triflusal); an adenosine diphosphate (ADP) receptor inhibitor
(e.g., ticlopidine,
clopidogrel, prasugrel, or tricagrelor); a phosphodiesterase inhibitor (e.g.,
cilostazol); a
glycoprotein IIB/IIIA inhibitor (e.g., abciximab, eptifibatide, or tirofiban);
an adenosine
reuptake inhibitor (e.g., dipyridamole); or a thromboxane inhibitor (e.g.,
thromboxane synthase
inhibitor, a thromboxane receptor inhibitor, such as terutroban). These anti-
platelet drugs are
non-limiting, as the skilled artisan would be able to readily identify other
anti-platelet drugs.
- 33 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Exemplary anticoagulant drugs include coumarins (e.g., warfarin,
acenocoumarol,
phenprocoumon, atromentin, brodifacoum, or phenindione); heparin and
derivatives thereof
(e.g., heparin, low molecular weight heparin, fondaparinux, or idraparintm);
factor Xa inhibitors
(e.g., rivaroxaban, apixaban, edoxaban, betrixaban, darexaban, letaxaban, or
eribaxaban):
thrombin inhibitors (e.g., hirudin, lepirudin, bivalirudin, argatroban, or
dabigatran); antithrombin
protein; batroxobin; hementin; and thrombomodulin. These anticoagulant drugs
are non-
limiting, as the skilled artisan would be able to readily identify other
anticoagulant drugs.
Exemplary thrombolytic drugs include tissue plasminogen activator (t-PA)
(e.g.,
alteplase, reteplase, or tenecteplase); anistreplase; streptokinase; or
urokinase.
1() In other embodiments, the active agent can comprise a drug that
prevents restenosis, e.g.,
an anti-proliferative drug, an anti-inflammatory drug, or an anti-thrombotic
drug. Exemplary
anti-proliferative drugs, anti-inflammatory drugs, and anti-thrombotic drugs
are described
herein.
In some embodiments, the active agent can comprise a drug that treats or
prevents
infection, e.g., an antibiotic. Suitable antibiotics include, but are not
limited to, beta-lactam
antibiotics (e.g., penicillins, cephalosporins, carbapenems), polymyxins,
rifamycins,
lipiarmycins, quinolones, sulfonamides, macrolides lincosamides,
tetracyclines,
aminoglycosides, cyclic lipopeptides (e.g., daptomycin), glycylcyclines (e.g.,
tigecy dine),
oxazonidinones (e.g., linezolid), and lipiarmycines (e.g., fidazomicin). For
example, antibiotics
include erythromycin, clindamycin, gentamycin, tetracycline, meclocycline,
(sodium)
sulfacetamide, benzoyl peroxide, and azelaic acid. Suitable penicillins
include amoxicillin,
ampicillin, bacampicillin, carbenicillin, cloxacillin, dicloxacillin,
flucloxacillin, mezlocillin,
nafcillin, oxacillin, penicillin g, penicillin v, piperacillin, pivampicillin,
pivmecillinam, and
ticarcillin. Exemplary cephalosporins include cefacetrile, cefadroxil,
cephalexin, cefaloglycin,
cefalonium, cefaloridine, cefalotin, cefapirin, cefatrizine, cefazaflur,
cefazedone, cefazolin,
cefradine, cefroxadine, ceftezole, cefaclor, cefamandole, cefmetazole,
cefonicid, cefotetan,
cefoxitin, cefprozil, cefuroxi me, cefuzonam, cfcapene, cefdaloxime, cefdinir,
cefditoren,
cefetamet, cefixime, cefmenoxime, cefodizime, cefotaxime, cefpimizole,
cefpodoxime,
cefteram, ceftibuten, ceftiofur, ceftiolene, ceftizoxime, ceftriaxone,
ceftazidime, cefclidine,
cefepime, ceflurprenam, cefoselis, cefozopran, cefpirome, cequinome,
ceftobiprole, ceftaroline,
cefaclomezine, cefaloram, cefaparole, cefcanel, cefedrlor, cefempidone,
cefetrizole, cefivitril,
cefmatilen, cefinepidium, cefovecin, cefoxazole, cefrotil, cefsumide,
cefuracetime, and
ceftioxide. Monobactams include aztreonam. Suitable carbapenems include
imipenem/cilastatin,
doripenem, meropenem, and ertapenem. Exemplary macrolides include
azithromycin,
- 34 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
erythromycin, larithromycin, dirithromycin, roxithromycin, and telithromycin.
Lincosamides
include clindamycin and lincomycin. Exemplary streptogramins include
pristinamycin and
quinupristin/dalfopristin. Suitable aminoglycoside antibiotics include
amikacin, gentamycin,
kanamycin, neomycin, netilmicin, paromomycin, streptomycin, and tobramycin.
Exemplary
quinolones include flumequine, nalidixic acid, oxolinic acid, piromidic acid,
pipemidic acid,
rosoxacin, ciprofloxacin, enoxacin, lomefloxacin, nadifloxacin, norfloxacin,
ofoxacin,
pefloxacin, rufloxacin, balofloxacin, gatifloxacin, repafloxacin,
levofloxacin, moxifloxacin,
pazufloxacin, sparfloxacin, temafloxacin, tosufloxacin, besifloxacin,
clinafoxacin, genatfloxacin,
sitafloxacin, trovafloxacin, and prulifloxacin. Suitable sulfonamides include
sulfamethizole,
sulfamethoxazole, and trimethoprim-sulfamethoxazone. Exemplary tetracyclines
include
demeclocycline, doxycycline, minocycline, oxytetracycline, tetracycline, and
tigecycline. Other
antibiotics include chloramphenicol, metronidazole, tinidazole,
nitrofurantoin, vancomycin,
teicoplanin, telavancin, linezolid, cycloserine, rifampin, rifabutin,
rifapentin, bacitracin,
polymyxin B, viomycin, and capreomycin. The skilled artisan could readily
identify other
antibiotics useful in the devices and methods described herein.
In some embodiments, the active agent can comprise a drug that reduces macular
degeneration. One common current treatment for macular degeneration involves
the injection of
anti-angiogenesis compounds intraocularly (Lucentis, Eylea). The repeated
intraocular injections
are sometimes poorly tolerated by patients, leading to low patient compliance.
As described
herein, the ability to noninvasively refill drug depots for macular
degeneration significantly
improves patient compliance and patient tolerance of disease, e.g., macular
degeneration,
treatment. Controlled, repeated release made possible by the methods described
herein allows
for fewer drug dosings and improved patient comfort.
In some embodiments, the active agent can comprise a drug that prevents
immunological
rejection. Prior to the invention described herein, to prevent immunological
rejection of cells,
tissues or whole organs, patients required lifelong therapy of systemic anti-
rejection drugs that
cause significant side effects and deplete the immune system, leaving patients
at greater risk for
infection and other complications. The ability to locally release anti-
rejection drugs and to
repeatedly load compound allows for more local anti-rejection therapy with
fewer systemic side
effects, improved tolerability and better efficacy.
In some embodiments, the active agent can comprise a drug that prevents
thrombosis.
Some vascular devices such as vascular grafts and coated stents suffer from
thrombosis, in
which the body mounts a thrombin-mediated response to the devices. Anti-
thrombotic drugs,
released from these devices, allows for temporary inhibition of the thrombosis
process, but the
- 35 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
devices have limited drugs and cannot prevent thrombosis once the drug supply
is exhausted.
Since these devices are implanted for long periods of time (potentially for
the entire lifetime of
the patient), temporary thrombosis inhibition is not sufficient. The ability
to repeatedly and
locally administer anti-thrombotic drugs and release the drug significantly
improves clinical
outcomes and allows for long-term thrombosis inhibition.
In some embodiments, the active agent can comprise a drug that treats
inflammation.
Chronic inflammation is characterized by persistent inflammation due to non-
degradable
pathogens, viral infections, or autoimmune reactions and can last years and
lead to tissue
destruction, fibrosis, and necrosis. In some cases, inflammation is a local
disease, but clinical
interventions are almost always systemic. Anti-inflammatory drugs given
systemically have
significant side-effects including gastrointestinal problems, cardiotoxicity,
high blood pressure
and kidney damage, allergic reactions, and possibly increased risk of
infection. The ability to
repeatedly and locally release anti-inflammatory drugs such as NSAIDs and COX-
2 inhibitors
could reduce these side effects. These methods can provide the ability to
deliver long term and
local anti-inflammatory care while avoiding systemic side effects.
Other suitable active agents include, for example, immunotherapeutics/
immunoadjuvants such as checkpoint inhibitors and STING agonists and agonists
for toll-like
receptors. Examples include STING ligands (e.g., natural cyclic dinucleotides,
cAIMP
dinucleotide, fluorine-containing cyclic dinulcoetides, phosphorothioate-
containing cyclic
clinucleotides, DMXAA); TLR2 ligands; TLR3 ligands (e.g., poly(I:C)); TLR4
ligands (e.g.,
lipopolysaccharides, monophosphoryl lipid A, CRX-527); TLR5 ligands; TLR7/8
ligands (e.g.,
gardiquimod, imiquimod, loxoribine, resiquimod, imidazoquinolines, adenine
base analogs,
benzoazepine analogs); TLR9 ligands (e.g., natural CpG ODNs, phosphorothioate
CpG ODNs);
TLR13 ligands (e.g., rRNA-derived ODNs); and NOD ligands (e.g., iE-DAP, meso-
lanthionine
tripeptide, D-gamma-Glu-mDAP, L-Ala-gamm-D-Glu-mDAP).
By way of example, representative active agents include doxorubicin,
paclitaxel,
gemcitabine, topotecan, tacrolimus, mycophenolic acid, rapamycin, tesiquimod,
erlotinib,
DMXAA, CdN, temozolomide, and docetaxel.
In some embodiments, the active agent can comprise an integrin-binding
peptide,
collagen-mimetic peptide, hydrazide, aldehyde, immunomodulating factor,
angiogenesis-
promoting factor, or cell-signaling factor.
- 36 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
EXAMPLES
The following examples are put forth so as to provide those of ordinary skill
in the art
with a complete disclosure and description of how the compounds, compositions,
articles,
devices and/or methods claimed herein are made and evaluated, and are intended
to be purely
exemplary and are not intended to limit the disclosure. Efforts have been made
to ensure
accuracy with respect to numbers (e.g., amounts, temperature, etc.), but some
errors and
deviations should be accounted for. Unless indicated otherwise, parts are
parts by weight,
temperature is in C or is at ambient temperature, and pressure is at or near
atmospheric.
Example 1
Synthesis of alginate polymers modified with depletive and restorative azide
modifications.
Alginate polymers modified with azide groups lose the ability to cross-link in
the
presence of calcium. In these previous alginate modifications, azide-PEG4-
amine (azide-amine)
was conjugated to alginate through carbodiimide coupling, replacing the
carboxyl group on the
alginate with an amide. Because these modifications deplete the available
carboxyl groups, we
name these modifications "depletive". We hypothesized that modifying alginate
polymers with
modifications that contain carboxyl groups would restore calcium cross-linking
and termed such
modifications "restorative".
To prepare alginate strands with restored carboxyl groups, we hypothesized
that this
could be accomplished by conjugating the carboxy-containing azide-lysine S4.
Azide-lysine was
prepared utilizing a modified three-step literature protocol starting from 2-
azi doacetic acid
(Figure 2). Synthesis of compound S4 began with NHS derivatization of 2-
azidoacetic acid Si
with N-hydroxysuccinimide. The azide-NHS was coupled to ct-BOC protected
lysine S2 to give
a-B0C-7-azido-lysine S3; which was deprotected W give compound the final azide-
lysine S4.
Preparation of alginate modified with depletive and restorative azide
modifications was
achieved through successive rounds of carbodiimide coupling. Each round
utilized N-Ethyl-N'-
(3-dimethylaminopropyl)carbodiimide (EDC) (2000 eq.), N-hydroxysuccinimide
(NHS) (1000
eq.), and azide (500 eq.) followed by dialysis, workup and measure of degree
of substitution.
Table 1 shows azide quantitation of alginates for each type of modification
after a single and
multiple rounds of coupling. The initial coupling of alginate to the carboxyl-
depletive
modification azide-amine (Figure 3) yielded a degree of substitution of 87
while coupling to the
carboxyl-restorative modification azide-lysine (S4) yielded a degree of
substitution of Si. These
¨ 37 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
low-DS materials were submitted to successive rounds of coupling, which
increased the DS for
both depletive (-179) and restorative (-145) modifications.
Table 1: Select information for modified alginate polymers used in this study.
Modification Alginate Azide Degree of Calculated DS
Classification
Type Type Material Substittuion Molecular
(azides/strand) Weight (kDa)
Restorative LF 20/40 Azide-Lysine 51 260 Low
Restorative LF 20/40 Azide-Lysine 145 278
High
Depletive LF 20/40 Azide-Amine 87 267 Low
Depletive LF 20/40 Azide-Ain ine 179 286
High
High DS was achieved through successive carbodiimide couplings using
restorative and
depletive modifications. Degree of substitution is defined as the number of
azides per alginate
strand.
Modification of alginate with carboxyl-restorative groups efficiently
maintains calcium cross-linking.
We next assessed whether carboxyl restoration also restores alginate viscosity
and
calcium cross-linked gel mechanics. General observations revealed that the
viscosity of 2%
(w/v) alginate solutions of low-DS carboxyl-depleted, low-DS carboxyl-restored
and high-DS
carboxyl-restored solutions were broadly similar. However, high-DS carboxyl-
depleted
materials demonstrated a marked decrease in solution viscosity. The 2%
alginate solutions were
then cross-linked with a 200 mM calcium sulfate solution. At low DS, both gel
modifications
permitted calcium cross-linking and gel formation. However, at high DS,
depletive modification
completely eradicated calcium cross-linking and alginate gelation (Fig. 4A).
In sharp contrast,
alginate gels modified with restorative modifications maintained gel
integrity, albeit with some
loss in gel stiffness.
Alginate hydrogels formed through vigorous mixing of alginate and calcium
sulfate were
subjected to -theological testing. Rheological testing confirmed the overall
observation that while
low-DS alginate with either modification form viscoelastic gels, only the
carboxyl-restoring
modifications permitted calcium cross-linking at high DS. Because the
advantage of restorative
modifications was most marked at high-DS, subsequent experiments compared high-
DS
carboxyl-depleted and -restored alginate gels.
Viscoelastic behaviors and injectability are crucial characteristics to
hydrogel function,
the restored alginate gels were submitted to strain sweep rheological testing.
Alginate with
¨ 38 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
restorative modifications showed gel-like behavior (storage modulus (G') >
loss modulus (G¨))
with a relatively strong three-dimensional (3D) network due to the appearance
of the G" peak
as shown in Fig. 4B). The linearity limiting value of the linear viscoelastic
(LVE) range for the
sample with restorative modifications was 26% as compared to 5% for unmodified
gels (Fig. 4B,
Fig. 5) indicating the modification indeed induced a relatively high degree of
cross-linking and
strengthened the structural organization of the sample. Frequency sweep tests
were performed
within the LVE region (Fig. 4C, Fig. 6). The figures showed that at low
frequency range,
G'>G" and a flat slope are found in the sample with restorative modifications,
whereas, the
sample using depletive modifications showed the opposite results. The results
confirmed that the
sample with restorative modifications (Fig. 4C, red) is in a gel-like state
and is relatively more
structurally stable when at rest in comparison to the sample using depletive
modifications (Fig.
4C, blue). In addition, a rotational test was performed to characterize
injectability. The
Increased shear rate induced a decrease of viscosity in the alginate gels with
restorative
modification (shear-thinning behavior) similarly to unmodified alginate (Fig.
7). Finally, self-
healing behavior was assessed through cyclic strain time sweep rheology
experiments, with
restorative samples showing excellent self-healing behavior (Fig. 4D, Fig. 8).
Taken together
these results demonstrate that in sharp contrast to modification that deplete
alginate carboxyl
groups, alginate polymers conjugated to restorative modifications maintain
calcium cross-
linking, gelation, injectability and self-healing properties.
Alginate conjugated to restorative modifications demonstrates improved gel
retention and systemic capture in vivo.
Hydrogel function requires gels to remain at injected sites. We found that
poorly cross-
linking hydrogels lose their ability to stay at the injection site and migrate
to other parts of the
body. We tested the ability of alginate carrying depletive and restorative
modifications to be
retained at target sites.
We tested in vivo gel retention of high-DS carboxyl-depleted and carboxyl-
restored
alginate gels after intramuscular (i.m.) injection into the hind limb. Azide-
modified alginate gels
were incubated with Cyanine7-dibenzocyclooctyne (Cy7-DBC0), which covalently
reacts with
azide groups to label the alginate. As a control, unmodified alginate, which
lacks the azide
modification, was also incubated with Cy7-DBCO. As expected, the unmodified
alginate control
quickly shed fluorescence signal owing to clearance of the unconjugated dye
from the gel (Fig.
9A-B). Both high-DS azide-alginates maintained signal at the injected site and
were statistically
significant compared to unmodified gels after the first day and through the
entire two-week
period.
¨ 39 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
The signal for carboxyl-depleted alginate gels did appear more spread out and
more
present at off-target locations, such as the ankle of the injected limb as
well as the rest of the
rodent's body. To quantify the off-target accumulation of alginate at
undesired sites, we
measured the fluorescence in the ankle of the injected limb and in the non-
injected contralateral
limb (Fig. 9C). We observed no significant off-target accumulation with the
restored alginate as
compared to unconjugated Cy7 release from unmodified alginate. However, we
found depleted
alginate to be significantly different from unmodified alginate after the
first day (ankle) and the
third day (contralateral limb) and through the entire two-week period (Fig. 9D-
E, Fig. 10).
Taken together, this data demonstrates that loss of hydrogel mechanical
strength leads to
migration of material from the injection site to undesired locations
throughout the body.
In previous reports, we demonstrated that azide-modified alginate captures
circulating
DBCO molecules from the blood for applications in ultra-specific drug
delivery. Therefore, we
tested whether restored alginate could also capture systemically-circulating
fluorescent DBCO
molecules at intramuscular sites. Alginate with restorative and depletive
modifications were
injected intramuscularly into the hind limb of outbred CD1 mice. One week
after i.m. injections,
DBCO-fluorophore was administered intravenously (i.v.), and fluorescence in
muscle was
measured over time. Unmodified alginate was used as a negative control. One
week after i.v.
injection, although both gels captured systemically circulating DBCO-Cy7, the
restored gel
showed significantly higher fluorescence capture as compared to the depleted
gel (Fig. 11A-B).
Taken together, the data demonstrates that the improvements in gelation
behavior
observed in vitro directly translate to in vivo behavior, highlighting the
importance of stabilizing
hydrogel mechanics for in vivo studies. Restorative modifications enable high
levels of
modification without sacrificing alginate gelation. The biomaterial change can
enable this highly
sought-after material to be used in drug depot and implant applications
including to capture
systemically circulating drug doses from the blood
Discussion
This paper reports that replacement of carboxyl groups during alginate
modification
preserves the calcium cross-linking of highly modified alginate polymers.
Carboxyl replacement
is achieved through the use of carboxyl-containing modification, instead of
the commonly used
amine groups. Carboxyl restoration allows increasing the degree of
substitution while
maintaining mechanical properties. In addition, carboxyl restoration improves
in vivo retention
of highly modified alginate gels at injection sites. Finally, carboxyl
restoration improved click-
specific targeting of small molecules to locally injected alginate hydrogels.
¨ 40 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
In previous work, we demonstrated protocols to increase alginate degree of
substitution
through repeated coupling. However, we and others have shown that alginate
gels with high DS
fail to cross-link. This observation forced us to resort to using click cross-
linkers. In this work,
in contrast, we show that click-based cross-linkers are not necessary and that
calcium cross-
linking can be recovered by restoring the carboxyl groups. Recovery of calcium
cross-linking is
beneficial because it modulates the self-healing and injectability behavior
observed for calcium
cross-linked alginate gels. Indeed, although modifications that depleted
carboxyl groups on
alginate decreased gel mechanics and prevented gelation, modifications that
restored carboxyl
groups demonstrated calcium cross-linking.
We observed that EDC/NHS-mediated coupling of azide-lysine to alginate showed
roughly similar yields to coupling of the azide-amine. The overall yields on
these reactions is
quite low, with 500 equivalents of azide yielding only 50-80 azide molecules
coupled to the
alginate. We propose that the poor yields derive from hydrolysis of alginate-
NHS esters as well
as steric hindrance preventing the amines from accessing the carbonyl of the
NHS ester when
attached to large alginate polymers. Some variability in the efficiency of
coupling was observed
for coupling of restorative and depletive modifications. This variability can
be attributed to
differences in efficiency of EDC coupling and nucleophilic character of the
amino groups.
Alternatively, the carboxyl groups on the lysine itself can divert EDC and NHS
from reacting
with alginate carboxyl groups.
Hydrogel retention at introduced sites is central to their use as tissue
engineering
scaffolds and as cell and drug delivery platforms. It was initially surprising
that alginate
hydrogels incorporating both restorative and depletive modifications were well
retained at the
injected tissue sites over two weeks, especially in light of previous
published results in which
poorly cross-linked alginate rapidly migrated away from the injection site.
Thus, despite the
complete loss of gelation behavior in vitro, some hydrogel coherence must have
remained.
However, calcium cross-linked alginate gels containing depletive modification
had significantly
increased accumulation at off-target sites, indicating that the loss of cross-
linking does lead to
polymer shedding over time. Lack of precise control over hydrogel localization
and off-target
accumulation can severely impede clinical translation so the restorative
modifications are highly
desirable in scaffold and drug delivery platforms that require high DS.
Alginate hydrogels using restorative modification of carboxyl groups
demonstrated
improved DBCO fluorescent capture from the blood at the site of the depot as
compared to
highly modified, poorly gelling hydrogels. In previous work, we demonstrated
that highly-
modified alginates lost their retention capacity after injection into tissues.
This difference is very
- 41 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
likely due to degradation of the poorly cross-linked hydrogels and their
migration away from
target sites.
Alginate hydrogels with restorative carboxyl groups improved small molecule
capture at
intramuscular sites. The amount of DBCO-fluorophore injected is still a small
percentage of the
theoretical number of available azides and we only expect 1-10% of the total
molecule reaching
the gel at any point, but the increased chance of an interaction between an
azide and a circulating
DBCO molecule increases the accumulation
Conclusions
Taken together, alginate modifications that restore carboxyl groups on the
polymer
backbone represent a significant improvement in hydrogel and depot stability
for in vivo
applications. Modifications that restore carboxyl groups make possible
mechanically robust,
highly modified alginate gels with superior calcium cross-linking behavior,
mechanical
properties, in vivo retention and depot capture compared to modifications that
deplete the
carboxyl group.
Materials and Methods
Reagents. Nutrition grade Alginate (Protanal LF 20/40, average MW = 300 kDa,
>60%
guluronic acid) was purchased from Dupont Nutrition and Health. Calcium
sulfate dihydrate
(C3771), MES (M3671), and N,N-Diisopropylethylamine (D125806) were purchased
from
Sigma-Aldrich. Azide-PEG4-amine (1868) was purchased from Lumiprobe
corporation. DBCO-
sulfo-amine (1227) and DBCO-Cy7 (1047) were purchased from Click Chemistry
Tools. 1-
Ethy1-3-[3-dimethylaminopropylicarbodiimide hydrochloride (EDC, 024810) was
purchased
from Oakwood Chemical. Azidoacetic acid (35109), N-Boc-L-lysine (02708) were
purchased
from Chem-Impex. N-hydroxysuccinimide was purchased from Chem-Impex
International
(00182) or Alfa Aesar (Al 0312). 1-(3-Dimethylaminopropy1)-3-ethylcarbodiimide
hydrochloride (A299) was purchased from AK Scientific. N,N-Dimethylformamide
(227056)
was purchased from Acros Organics. Trifluoroacetic Acid (04901-500), Methanol
(A433P-4),
Acetonitrile (A998-4) and Toluene (T290-4) were purchased from Fisher
Chemical.
Azide-lysine synthesis. (S)-2-ammonio-6-(2-azidoacetamido)hexanoate (S4).
Prepared
utilizing a modified literature known protocol. To a flame dried 250 mL round
bottom flask was
added N-hydroxysuccinimide (3.037 g, 26.39 mmol, 1.3 equiv.) and stir bar.
Septa was added
with an Argon balloon. 101 mL of anhydrous N,N-dimethylformamide (0.2 M) was
added and
reaction was set to stirring. Septa was removed and 1-(3-dimethylaminopropy1)-
3-
ethylcarbondiimide hydrochloride (5.06 g, 26.4 mmol, 1.3 equiv.) was added.
Septa was
replaced and the reaction was set to stirring at room temperature. Then 2-
azidoacetic acid (Sit)
¨ 42 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
(1.52 mL, 20.3 mmol 1 equiv.) was injected into reaction. Reaction was stirred
for 2 hours upon
which septa was removed and N-Boc-L-lysine (S2) (5.0 g, 20.3 mmol, 1 equiv.)
and N,N-
diisopropylethylamine (12.7 mL, 73.1 mmol, 3.6 equiv.) were added in that
order. Septa was
replaced and reaction was stirred for an additional 17 hours. Over the course
of 1 hour, the
reaction became an amber color and remained this color during the completion
of the reaction.
The crude reaction mixture was concentrated on a rotary evaporator followed by
high vacuum to
remove all DMF and N,N-diisopropylethylamine leaving an amber oil. Toluene was
added and
then evaporated by rotary evaporator and high vacuum to help remove any trace
DMF and A I ,A1-
cliisopropylethylamine. This crude oil was then taken forward to the next step
without
purification. To the crude N6-(2-azidoacety1)-N2-(tert-butoxycarbony1)-L-
lysine (S3) (6.69 g,
20.3 mmol, 1 equiv.), 44 mL of anhydrous dichloromethane and 22 mL of
trifluoroacetic acid
(2:1 ratio, 0.3 M reaction molarity) was added at room temperature. Reaction
was allowed to stir
for 4 h, upon which complete conversion was observed on LCMS. Reaction was
then
concentrated to volume on a rotary evaporator. Then 100 mL of acetonitrile was
added and this
was concentrated. Then 100 mL of toluene was added and this was also
concentrated_ This crude
oil was then triturated twice with a small volume of methanol followed by
addition of copious
amounts of diethyl ether providing (5)-2-ammonio-6-(2-azidoacetamido)hexanoate
(S4) (3.56 g,
15.5 mmol, 77%) as a light pink/brown solid with trace impurities. Isolated
solid was triturated
one time further obtaining (S)-2-ammonio-6-(2-azidoacetamido)hexanoate (S4)
(2.10 g, 9.16
mmol, 45%) pure as a light tan solid.
Azide conjugation of alginate. Nutrition grade, high guluronic acid content,
high
molecular weight (MW) alginate (PROTANAL LF 20/40) purchased from Dupont
Nutrition and
Health was first dissolved in DI water and charcoal was added (0.5 g of
charcoal per 1 g of
alginate), filtered, and then MES buffer was added to reach the desired 0.5%
weight per volume
(w/v) concentration (1 g of alginate in 200 mL, 20 uM, 1 eq.) (100 mM MES, 300
mM NaCl,
pH: 6.5). To begin the coupling reaction, EDC (40 mM, 2000 eq.) and NHS (20
mM, 1000 eq.)
was added to the alginate solution and allowed to stir for 5 minutes. Either
azide-lysine (10 mM,
500 eq.) or azide-amine (10 mM, 500 eq.) was added to the solution, and both
solutions stirred
overnight, covered, at room temperature. The solution was dialyzed against 4 L
of water with
successively lower salt content, changing solution 2-3 times per day for 4-5
days. Dialyzed
solutions were frozen and lyophilized under high vacuum. For additional
coupling, the samples
were dissolved in 0.5% w/v in 1X MES buffer solution (1 gin 200 mL) and the
coupling steps
were repeated as described.
¨ 43 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
Azide quantitation. The number of azides conjugated to the alginate was
quantified by
looking at the decrease in DBCO absorbance upon incubation of alginate azides
with a known
excess amount of DBCO. 0.1% w/v (2.5 mg in 2.5 ml) alginate solutions were
made in
phosphate buffer solution (PBS). 80 uM solutions of DBCO-amine (Click
Chemistry Tools-
1227) were made and three different amounts of alginate was added (400 pmol,
800 pmol and
1.6 nmol of alginate) to separate 80 uM solutions of DBCO-amine. A DBCO
negative control
solution was also tested along with a positive control consisting of DBCO
reacted with sodium
azide (1 umol, 2500 eq.). Spectrophotometry was performed using a UV/Vis
spectrophotometer
(Thermo Scientific Nanodrop 2000c) using a cuvette with a 1 cm pathlength.
Absorbance
changes were observed at 308 nm wavelength. This process was repeated with
replicates of the
0.1% alginate (alg) solutions and the absorbance values were averaged.
Decrease in absorbance
in alginate samples compared to DBCO negative control indicated the quantity
of azides that
reacted with DBCO.
DS = mol azide MW aly I mass azaig mol azide MW azide
Gel Formation. Alginate was dissolved in PBS (2% w/v, 20 mg in 1 mL). 10:1
ratio of
the 2% w/v alginate solution was mixed with a calcium sulfate solution (final
concentration of
18.2 mM) in a two syringe system while minimizing air. The solutions were
mixed by pushing
the syringe barrels back and forth 10 times. This system was used to create
gel sizes ranging
from 400 mt to 1 mL.
Rheology Testing. Alginate was cross-linked as described in section 5.4, with
1 mL gels
mixed and injected into a 24-well plate. The gel were allowed at least 24
hours to settle and then
collected for rheology testing. Rheology test of prepared gel was performed on
a Molecular
Compact Rheometer (MCR302, Anton Paar, Graz, Austria). A cone plate geometry
of a 50 mm
diameter and 1 degree (CP50-1/TG, Anton Paar, Graz_ Austria) was used in this
experiment.
After installing the geometry, zero force and zero gap with a truncation gap
of 0.1 mm was set.
Before analysis began, to calibrate the system, adjust measurement system
inertia and adjust
system tests were performed. After calibration was done, 1 mL of gel samples
(gently mashed
with a lab spatula) was placed on the preinstalled temperature-controlled
Peltier plate at 21 C.
Geometry cone was set at the truncation height and access of samples were
trimmed. Before
each test, cone geometry and Peltier plate were cleaned with distilled water
and absolute ethanol
and instrument was calibrated to ensure the consistency.
¨ 44 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
To determine the rheological characteristics of the gels, the following
simultaneous tests
were performed using the method of Chen et al. with slight modification.
Time sweep: Two minutes of time sweep was performed at 0.2% strain and a
frequency
of 10 Hz and a total 150 data points at a constant rate were collected before
and between each
test performed.
Amplitude sweep: Amplitude sweep test of the gel samples was performed on a
logarithmic ramp ranging from 0.01 to 500% at 10 Hz with 3 seconds of
conditioning and
sampling time, and 10 data points per decade were recorded.
Frequency sweep: Frequency sweep on a logarithmic ramp ranging from 0.01-100
Hz at
0.2% strain was performed with 3 seconds of sample conditioning and sampling
time, and 10
data points per decade were recorded.
Cyclic amplitude sweep: A continuous cyclic amplitude sweep test was performed
in five
intervals (1-5). Intervals 1, 3 and 5 were set at 0.2% strain, 10 Hz for 2
minutes, whereas
intervals 2 and 4 set at 500% strain, 10 Hz for one minute.
Shear rate ramp: Two continuous shear rate ramps from 0 to 50 s-1 and 50 to 0
s-1 for
2.5 minutes each were performed to study the continuous flow behavior of the
gel. Total of 74
data points with 20 data points per decade was recorded.
Gel retention of intramuscular implanted alginate gels. All animal work was
done in
compliance with institutional ethical use protocols, including the NIH Guide
for Care and Use of
Laboratory Animals. Unmodified and modified alginate were prepared as
described in section
5.4 but were instead dissolved in a 24 i_tM DBCO-Cy7 in PBS solution (2% w/v,
20 mg in 1 mL)
resulting in a final concentration of 21.81.IM after cross-linking the gel. 12-
week-old CD1 mice
(Charles River, 022) were injected intramuscularly in the left limb with 50
[IL of fluorescently
labeled unmodified alginate, depleted azide-alginate, or restored azide-
alginate (n=4). Cy7
fluorescence was monitored over two weeks using an 1VIS imager to obtain a
fluorescence
signal. ICG/ICG excitation and emission filters were used for all IVIS images
presented and no
image math in the Living Image software was performed. For all IVIS images,
only radiance
efficiency values were used to normalize the data over variable exposure
times. Regions of
Interest (ROIs) were used to sum the fluorescent signal associated with the
injected calf, the
injected ankle, and the contralateral calf
Systemic capture of fluorophore in intramuscular implanted alginate gels. All
animal work was done in compliance with institutional ethical use protocols,
including the NTH
Guide for Care and Use of Laboratory Animals. Unmodified and modified alginate
were
prepared as described in section 5.4. 12-week-old CD1 mice (Charles River,
022) were injected
¨ 45 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
intramuscularly in the left limb with 50 [IL of unmodified alginate, depleted
azide-alginate, or
restored azide-alginate (n=3). 5 g/L stock of DBCO-Cy7 in water was prepared
and diluted 100x
and sterile filtered. 1 week after injection, 100 p.1_, of the sterile 50 mg/L
DBCO-Cy7 solution
was injected retro-orbitally. Cy7 fluorescence was measured after 1 week using
an IVIS imager
to obtain a fluorescence signal. ICG/ICG excitation and emission filters were
used for all IVIS
images presented and no image math in the Living Image software was performed.
For all IVIS
images, only radiance efficiency values were used to normalize the data over
variable exposure
times. ROls were used to sum the fluorescent signal associated with the left
calf
References
A. Jull, N. Walker, V. Parag, P. Molan, A. Rodgers, Randomized clinical trial
of honey-
impregnated dressings for venous leg ulcers, Br. J. Surg. 95 (2008) 175-182.
A. Lueckgen, D.S. Garske, A. Ellinghaus, D.J. Mooney, G.N. Duda, A. Cipitria,
Dual alginate
crosslinking for local patterning of biophysical and biochemical properties,
Acta Biomater. 115
(2020) 185-196.
A. Lueckgen, D.S. Garske, A. Ellinghaus, R.M. Desai, A.G. Stafford, D.J.
Mooney, G.N. Duda,
A. Cipitria, Hydrolytically-degradable click-crosslinked alginate hydrogels,
Biomaterials. 181
(2018) 189-198.
A. Pal, B.L. Vernon, M. Nikkhah, Therapeutic neovascularization promoted by
injectable
hydrogels, Bioact Mater. 3 (2018) 389-400.
A.J. Vegas, 0. Veiseh, J.C. Doloff, M. Ma, H.H. Tam, K. Bratlie, J. Li, A.R.
Bader, E. Langan,
K. Olejnik, P. Fenton, J.W. Kang, J. Hollister-Locke, M.A. Bochenek, A. Chiu,
S. Siebert, K.
Tang, S. Jhunjhunvvala, S. Aresta-Dasilva, N. Dholakia, R. Thakrar, T. Vieth,
M. Chen, J. Cohen,
K. Siniakowicz, M. Qi, J. McGarrigle, S. Lyle, D.M. Harlan, D.L. Greiner, J.
Oberholzer, G.C.
Weir, R. Langer, D.G. Anderson, Erratum: Corrigendum: Combinatorial hydrogel
library enables
identification of materials that mitigate the foreign body response in
primates, Nat. Biotechnol. 34
(2016) 666-666.
AK. Vellimana, V.R. Recinos, L. Hwang, K.D. Fowers, K.W. Li, Y. Zhang, S.
Okonma, C.G.
Eberhart, H. Brem, B.M. Tyler, Combination of paclitaxel thermal gel depot
with temozolomide
and radiotherapy significantly prolongs survival in an experimental rodent
glioma model, J.
Neurooncol. 111 (2013) 229-236.
¨ 46 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
C. Bastiancich, J. Bianco, K. Vanvarenberg, B. Ucakar, N. Joudiou, B. Gallez,
G. Bastiat, F.
Lagarce, V. Preat, F. Danhier, Injectable nanomedicine hydrogel for local
chemotherapy of
glioblastoma after surgical resection, J. Control. Release. 264 (2017) 45-54.
C. Garcia-Astrain, L. Averous, Synthesis and evaluation of functional alginate
hydrogels based
on click chemistry for drug delivery applications, Carbohydr. Polym. 190
(2018) 271-280.
C. Wang, R.R. Varshney, D.-A. Wang, Therapeutic cell delivery and fate control
in hydrogels
and hydrogel hybrids, Adv. Drug Deliv. Rev. 62 (2010) 699-710.
C.A. DeForest, B.D. Polizzotti, K.S. Anseth, Sequential click reactions for
synthesizing and
patterning three-dimensional cell microenvironments, Nature Materials. 8
(2009) 659-664.
C.K. Kuo, P.X. Ma, Tonically crosslinked alginate hydrogels as scaffolds for
tissue engineering:
part 1. Structure, gelation rate and mechanical properties, Biomaterials. 22
(2001) 511-521.
C.T. Moody, S. Palvai, Y. Brudno, Click cross-linking improves retention and
targeting of
refillable alginate depots, Acta Biomater. 112 (2020) 112-121.
D. Jain, D. Bar-Shalom, Alginate drug delivery systems: application in context
of
pharmaceutical and biomedical research, Drug Dev. Ind. Pharm. 40 (2014) 1576-
1584.
D. Zeng, N.S. Lee, Y. Liu, D. Zhou, C.S. Dence, K.L. Wooley, J.A.
Katzenellenbogen, M.J.
Welch, 64Cu Core-labeled nanoparticles with high specific activity via metal-
free click
chemistry, ACS Nano. 6 (2012) 5209-5219.
E.A. Silva, D.J. Mooney, Spatiotemporal control of vascular endothelial growth
factor delivery
from injectable hydrogels enhances angiogenesis, Journal of Thrombosis and
Haemostasis. 5
(2007) 590-598.
F. de la Portilla, S. Dios-Barbeito, M.V. Maestre-Sanchez, J.M. Vazquez-
Monchul, A.M.
Garcia-Cabrera, I. Raman , M.L. Reyes-Diaz, Feasibility and safety of calcium
alginate
hydrogel sealant for the treatment of cryptoglandular fistula-in-ano: phase
I/IIa clinical trial,
Colorectal Dis. (2021).
G. Choe, J. Park, H. Park, J.Y. Lee, Hydrogel Biomaterials for Stem Cell
Microencapsulation,
Polymers. 10 (2018).
¨ 47 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
G. Eke, N. Mangir, N. Hasirci, S. MacNeil, V. Hasirci, Development of a UV
crosslinked
biodegradable hydrogel containing adipose derived stem cells to promote
vascularization for
skin wounds and tissue engineering, Biomaterials. 129 (2017) 188-198.
G. Orive, S.K. Tam, J.L. Pedraz, J.-P. Halle, Biocompatibility of
alginate¨poly-1-lysine
microcapsules for cell therapy. Biomaterials. 27 (2006) 3691-3700.
H. Wen, W. Xiao, S. Biswas, Z.-Q. Cong, X.-M. Liu, K.S. Lam, Y.-H. Liao, W.
Deng, Alginate
Hydrogel Modified with a Ligand Interacting with a3131 Integrin Receptor
Promotes the
Differentiation of 3D Neural Spheroids toward Oligodendrocytes in Vitro, ACS
Appl. Mater.
Interfaces. 11(2019) 5821-5833.
J. Li, D.J. Mooney, Designing hydrogels for controlled drug delivery, Nat Rev
Mater. 1 (2016).
J. Sayag, S. Lieaume, S. Bohbot, Healing properties of calcium alginate
dressings, J. Wound
Care. 5 (1996) 357-362.
J. Yang, Y. Liu, L. He, Q. Wang, L. Wang, T. Yuan, Y. Xiao, Y. Fan, X. Zhang,
Icariin
conjugated hyaluronic acid/collagen hydrogel for osteochondral interface
restoration, Ada
Biomater. 74 (2018) 156-167.
J.A. Johnson, J.M. Baskin, C.R. Bertozzi, J.T. Koberstein, N.J. Turro, Copper-
free click
chemistry for the in situ crosslinking of photodegradable star polymers, Chem.
Commun. .
(2008) 3064-3066.
J.A. Rowley, G. Madlambayan, D.J. Mooney, Alginate hydrogels as synthetic
extracellular
matrix materials, Biomaterials. 20 (1999) 45-53.
J.D. Medina, M. Alexander, M.D. Hunckler, M.A. Fernandez-Yagtie, M.M. Coronet,
A.M. Smink,
J.R. Lakey, P. de Vos, A.J. Garcia, Functionalization of alginate with
extracellular matrix peptides
enhances viability and function of encapsulated porcine islets, Adv. Healthc.
Mater. 9 (2020)
e2000102.
J.L. Madrigal, S. Shams, R.S. Stilhano, E.A. Silva, Characterizing the
encapsulation and release
of lentivectors and adeno-associated vectors from degradable alginate
hydrogels, Biomater Sci.
7 (2019) 645-656.
¨ 48 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
J.M. Mejia Oneto, I. Khan, L. Seebald, M. Royzen, In Vivo Bioorthogonal
Chemistry Enables
Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma, ACS Cent
Sci. 2 (2016)
476-482.
KM. Gattas-Asfura, C.L. Stabler, Chemoselective cross-linking and
functionalization of
alginate via Staudinger ligation, Biomacromolecules. 10 (2009) 3122-3129.
L. Yang, Y. Li, Y. Gou, X. Wang, X. Zhao, L. Tao, Improving tumor chemotherapy
effect using
an injectable self-healing hydrogel as drug carrier, Polym. Chem. 8 (2017)
5071-5076.
L. Zou, A.S. Braegelman, M.J. Webber, Spatially Defined Drug Targeting by in
Situ Host-Guest
Chemistry in a Living Animal, ACS Cent Sci. 5 (2019) 1035-1043.
M. Georg Jensen, M. Kristensen, A. Astrup, Effect of alginate supplementation
on weight loss in
obese subjects completing a 12-wk energy-restricted diet: a randomized
controlled trial, Am. J.
Clin. Nutr. 96 (2012) 5-13.
M. Gionet-Gonzales, A. Casella, D. Diloretto, C. Ginnell, K.H. Griffin, A.
Bigot, J.K. Leach,
Sulfated alginate hydrogels prolong the therapeutic potential of MSC spheroids
by sequestering
the secretome, Adv. Healthc. Mater. (2021) e2101048.
M. Menard, J. Dusseault, G. Langlois, W.E. Bailie, S.K. Tam, L. 'hocine Yahia,
X.X. Zhu, J.-P.
Halle, Role of protein contaminants in the immunogenicity of alginates, J.
Biomed. Mater. Res.
B Appl. Biomater. 93 (2010) 333-340.
M.A. Bochenek, 0. Veiseh, A.J. Vegas, J.J. McGarrigle, M. Qi, E. Marchese, M.
Omami, J.C.
Doloff, J. Mendoza-Elias, M. Nourmohammadzadeh, A. Khan, C.-C. Yeh, Y. Xing,
D. Isa, S.
Ghani, J. Li, C. Landry, A.R. Bader, K. Olejnik, M. Chen, J. Hollister-Lock,
Y. Wang, D.L.
Greiner, G.C. Weir, B.L. Strand, A.M.A. Rokstad, I. Lacik, R. Langer, D.G.
Anderson, J.
Oberholzer, Alginate encapsulation as long-term immune protection of
allogeneic pancreatic islet
cells transplanted into the omental bursa of macaques, Nat. Biomed. Eng. 2
(2018) 810-821.
M.H. Chen, L.L. Wang, J.J. Chung, Y.-H. Kim, P. Atluri, J.A. Burdick, Methods
To Assess
Shear-Thinning Hydrogels for Application As Injectable Biomaterials, ACS
Biomater Sci Eng. 3
(2017) 3146-3160.
¨ 49 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
M.H. Ghanian, H. Mirzadeh, H. Baharvand, In Situ Forming, Cytocompatible, and
Self-
Recoverable Tough Hydrogels Based on Dual Ionic and Click Cross-Linked
Alginate,
Biomacromolecules. 19 (2018) 1646-1662.
M.R. Adams, C.T. Moody, J.L. Sollinger, Y. Brudno, Extracellular-Matrix-
Anchored Click
Motifs for Specific Tissue Targeting, Mol. Pharm. 17 (2020) 392-403.
N. Xu, J. Xu, X. Zheng, J. Hui, Preparation of Injectable Composite Hydrogels
by Blending
Poloxamers with Calcium Carbonate-Crosslinked Sodium Alginate, ChemistrvOpen.
9 (2020)
451-458.
N.T.T. Uyen, Z.A.A. Hamid, N.X.T. Tram, N. Ahmad, Fabrication of alginate
microspheres for
drug delivery: A review, Int. J. Biol. Macromol. 153 (2020) 1035-1046.
0. Veiseh, J.C. Doloff, M. Ma, A.J. Vegas, H.H. Tam, A.R. Bader, J. Li, E.
Langan, J. Wyckoff,
W.S. Loo, S. Jhunjhunwala, A. Chiu, S. Siebert, K. Tang, H.-L. Jennifer, A.-D.
Stephanie, M.
Bochenek, M.-E. Joshua, Y. Wang, M. Qi, D.M. Lavin, M. Chen, N. Dholakia, R.
Thakrar, I.
Lacik, G.C. Weir, J. Oberholzer, Di. Greiner, R. Langer, D.G. Anderson, Size-
and shape-
dependent foreign body immune response to materials implanted in rodents and
non-human
primates, Nat. Mater. 14 (2015) 643-651.
P. Agarwalla, E.A. Ogunnaike, S. Ahn, F.S. Ligler, G. Dotti, Y. Brudno,
Scaffold-Mediated
Static Transduction of T Cells for CAR-T Cell Therapy, Adv. Healthc. Mater.
(2020) 2000275.
R.J. Lee, A. Hinson, R. Bauernschmitt, K. Matschke, Q. Fang, D.L. Mann, R.
Dowling, N.
Schiller, H.N. Sabbah, The feasibility and safety of Algisyl-LVRTm as a method
of left
ventricular augmentation in patients with dilated cardiomyopathy: Initial
first in man clinical
results, Int. J. Cardiol. 199 (2015) 18-24.
R.M. Desai, S.T. Koshy, S.A. Hilderbrand, D.J. Mooney, N.S. Joshi, Versatile
click alginate
hydrogels crosslinked via tetrazine¨norbornene chemistry, Biomaterials. 50
(2015) 30-37.
R.-S. Hsu, J.-H. Fang, W.-T. Shen, Y.-C. Sheu, C.-K. Su, W.-H. Chiang, S.-H.
Hu, Injectable
DNA-architected nanoraspberry depot-mediated on-demand programmable refilling
and release
drug delivery, Nanoscale. 12 (2020) 11153-11164.
¨ 50 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
R.S. Stilhano, J.L. Madrigal, K. Wong, P.A. Williams, P.K.M. Martin, F.S.M.
Yamaguchi, V.Y.
Samoto, S.W. Han, E.A. Silva, Injectable alginate hydrogel for enhanced
spatiotemporal control
of lentivector delivery in murine skeletal muscle, J. Control. Release. 237
(2016) 42-49.
S.B. Stephan, A.M. Taber, I. Jileaeva, E.P. Pegues, C.L. Sentman, M.T.
Stephan, Biopolymer
implants enhance the efficacy of adoptive T-cell therapy, Nat. Biotechnol. 33
(2015) 97-101.
S.D. Anker, A.J.S. Coats, G. Cristian, D. Dragomir, E. Pusineri, M. Piredda,
L. Bettari, R.
Dowling, M. Volterrani, B.-A. Kirwan, G. Filippatos, J.-L. Mas, N. Danchin,
S.D. Solomon, R.J.
Lee, F. Ahmann, A. Hinson, H.N. Sabbah, D.L. Mann, A prospective comparison of
alginate-
hydrogel with standard medical therapy to determine impact on functional
capacity and clinical
outcomes in patients with advanced heart failure (AUGMENT-HF trial), Eur.
Heart J. 36 (2015)
2297-2309.
S.H. Hong, Y. Li, J.B. Eom, Y. Choi, Responsive alginate-cisplatin nanogels
for selective
imaging and combined chemo/radio therapy of proliferating macrophages, Quant.
Imaging Med.
Surg. 8 (2018) 733-742.
S.J. Bidarra, C.C. Barrias, P.L. Granja, Injectable alginate hydrogels for
cell delivery in tissue
engineering, Acta Biomater. 10 (2014) 1646-1662.
S.M. Willerth, S.E. Sakiyama-Elbert, Combining stem cells and biomaterial
scaffolds for
constructing tissues and cell delivery, StemJournal. 1 (2019) 1-25.
S.N. Pawar, Chapter 8 - Chemical Modification of Alginate, in: J. Venkatesan,
S. Anil, S.-K.
Kim (Eds.), Seaweed Polysaccharides, Elsevier, 2017: pp. 111-155.
T. Joki, M. Machluf, A. Atala, J. Zhu, N.T. Seyfried, I.F. Dunn, T. Abe, R.S.
Carroll, P.M.
Black, Continuous release of endostatin from microencapsulated engineered
cells for tumor
therapy, Nat. Biotechnol. 19 (2001) 35-39.
T. Li, Z. Li, F. Nan, J. Dong, Y. Deng, Q. Yu, T. Zhang, Construction of a
novel inducing
system with multi-layered alginate microcapsules to regulate differentiation
of neural precursor
cells from bone mesenchymal stem cells, Medical Hypotheses. 85 (2015) 910-913.
¨ 51 -
CA 03236482 2024- 4- 26
WO 2023/077112
PCT/US2022/078944
T. Maguire, E. Novik, R. Schloss, M. Yarmush, Alginate-PLL microencapsulation:
Effect on the
differentiation of embryonic stem cells into hepatocytes, Biotechnology and
Bioengineering. 93
(2006) 581-591.
T.A. Read, D.R. Sorensen, R. Mahesparan, P.O. Enger, R. Timpl, B.R. Olsen,
M.H. Hjelstuen,
0. Haraldseth, R. Bjerkvig, Local endostatin treatment of gliomas administered
by
microencapsulated producer cells, Nat. Biotechnol. 19 (2001) 29-34.
T.A.S. Selmi, P. Verdonk, P. Chambat, F. Dubrana, J.-F. Potel, L. Barnouin, P.
Neyret,
Autologous chondrocyte implantation in a novel alginate-agarose hydrogel:
outcome at two
years, J. Bone Joint Surg. Br. 90 (2008) 597-604.
X. Chen, F. Li, Y.-W. Wu, Chemical labeling of intracellular proteins via
affinity conjugation
and strain-promoted cycloadditions in live cells, Chem. Commun. . 51 (2015)
16537-16540.
X. Liu, P. Gong, P. Song, F. Xie, A. Lee Miller II, S. Chen, L. Lu, Rapid
conjugation of
nanoparticles, proteins and siRNAs to microbubbles by strain-promoted click
chemistry for
ultrasound imaging and drug delivery, Polym. Chem. 10 (2019) 705-717.
X. Zhang, K. Wang, J. Hu, Y. Zhang, Y. Dai, F. Xia, Role of a high calcium ion
content in
extending the properties of alginate dual-crosslinked hydrogels. J. Mater.
Chem. A Mater.
Energy Sustain. 8 (2020) 25390-25401.
Y. Brudno, M.J. Pezone, T.K. Snyder, 0. Uzun, C.T. Moody, M. Aizenberg, D.J.
Mooney,
Replenishable drug depot to combat post-resection cancer recurrence,
Biomaterials. 178 (2018)
373-382.
Y. Brudno, R.M. Desai, B.J. Kwee, N.S. Joshi, M. Aizenberg, D.J. Mooney, In
vivo targeting
through click chemistry, ChemMedChem. 10 (2015) 617-620.
Y. Hui, Microorganisms and Microbially Derived Ingredients Used in Foods,
Handbook of
Plant-Based Fermented Food and Beverage Technology, Second Edition. (2012) 771-
790.
Y. Luo, K.R. Kirker, G.D. Prestwich, Cross-linked hyaluronic acid hydrogel
films: new
biomaterials for drug delivery, J. Control. Release. 69 (2000) 169-184.
¨ 52 -
CA 03236482 2024- 4- 26