Note: Descriptions are shown in the official language in which they were submitted.
CA 02506690 2005-06-O1
MEAL CARBON~1L VAPOUR .~E,~ffSITiON
,APPARATUS AND PROCESS
This invention relates to metal carbonyl vapour decomposition and metal
deposition processes for use in the manufacture of metal or metal coated
objects,
particularly nickel deposition, and more particularly to infrared heating in
said
processes; and to apparatus of use in said processes.
Chemical vapour deposition is a well-known method for depositing films or
coatings on substrates. One known chemical vapour used for depositing a nickel
film
or coating on a substrate is nickel carbonyl in the so-called Nickel Vapour
Deposition
proccss (NVD). Typically, the substrates to be'nickel coated are heated within
a
reaction or deposition chamber to a predetermined suitable reaction
temperature,
typically 1.75°C-180°C in an atmosphere of nickel carbonyl,
Ni(CO)4. The nickel
carbonyl decomposes at the surface of the heated substrate to deposit the Ni
film or
coating thereon.
Nickel carbonyl from a liquid supply tank flows through a vapourizer where it
is converted into a gas stream to which gaseous stream may be added a small
amount
of carrier gas, such as carbon monoxide.
Typically, nickel carbonyl vapour is continuously introduced to the deposition
chamber, wherein it reacts to produce elemental nickel and carbon monoxide by-
product. The spent gas is continuously purged from the chamber in order td
maintain
proper circulation of reactive nickel carbonyl across the surfaces of the
substrates.
The substrates may be heated according to well-known methods, such as heat
conduction, infrared radiation, inductance and the like.
1
CA 02506690 2005-06-O1
Infrared heating (IR) is required or otherwise desirable when the substrate to
be heated is not electrically conductive wherein the IR radiation is directed
into the
deposition chamber through infrared transparent windows to heat the substrate.
Preferably, the IR radiation selectively heats the substrate within the
chamber, not the
metal carbonyl gas or the 1R transparent window. If the metal carbonyl gas is
heated
above its decomposition temperature, it spontaneously decomposes. Similarly,
if the
IR transparent window is heated above the decomposition temperature of the
metal
carbonyl gas, the gaseous compound, undesirably, decomposes on the 1R
transparent
window, which essentially results in a stoppage of the carbonyl plating on the
substrate because the 1R radiation cannot effectively penetrate the fogged
windows.
To remove this problem, the windows must be removed, periodically, to be
cleaned or
replaced. The metal deposited on the window is also not infrared transparent,
which
causes increased infi~ared heating of the window.
One source of this problem is that infrared transparent windows constructed of
materials such as borosilicate glass, clear fused quartz, polyethylene,
terephthalate,
polytetrafluoroethylene, poly-tetrafluoroethylenepropylene and other materials
are not
perfectly transparent to inirrared radiation and,vin consequence, the window
becomes
hot enough to cause metal deposition thereon.
U.S. Patent No. 3,213,827 - Jerkin describes an air-cooled cooling duct for
cooling an infrared transparent chamber wall. However, due to the
inefficiencies of
air-cooling, it is believed that the design of Jerkin is insufficient to
effectively prevent
fogging.
European Patent Application No. 0424183A1 - Paserin and U.S. Patent No.
5,145,716 - Paserin, to prevent fogging of infrared windows in carbonyl
decomposition chambers, uses liquid coolant, which substantially infrared
transparent
for allowing infrared radiation through the infrared transparent window and
cooling
passage into the chamber. The laboratory test setup.utilized windows having
two
infrared transparent glass sheets sold under the trademark PYREX~spaced 6 mm
apart. The space between the PYREX~ sheets was filled with various fluids. The
tests
utilized PYREX~ sheets 4 and 5 mm in thickness. Spaced adjacent on one side of
the
2
CA 02506690 2005-06-O1
PYREX~ sheets and test fluid was a 140 volt, 440 watt infrared lamp. The
filament of
the infrared lamp was heated to a temperature of 1980 ° C, having a
peak wavelength
of 1.46 microns. Different coolants were used: water, ethylene glycol,
ethylene glycol
diacetate, tetrachlorethane and tetrachloroethylene.
However, there is still a need to provide an improved process of significantly
reducing or completely preventing deposition of metal on the window.
It is an object of this invention to effectively prevent fogging of the
windows
in carbonyl decomposition chambers without using any cooling systems for
windows.
Another object of this invention is to reduce power losses of the metal
carbonyl decomposition process.
The present invention provides an improved process and apparatus wherein
the deposition chamber window does not require cooling during the deposition
of
metal on a substrate.
Accordingly, in one aspect, the invention provides an improved process for the
production of metal or a metal-coated object by a metal carbonyl vapow
deposition
process, comprising
a) placing an object having a surface to be treated with metal carbonyl gas by
said metal vapour deposition process into a deposition chamber having a
partial IR
radiation transparent window;
b) feeding said gaseous metal carbonyl gas to said deposition chamber;
c) passing 1R radiation from an infrared source through said window for
radiant heating of said surface of said object to a temperature at which
decomposition
of said metal carbonyl gas occurs on said surface of said object, the
improvement
wherein
(a) said window is formed of a material having an effective transparency
to radiation of wavelengths in the range 0.2 Eun-2.SEun; and
(b) said infrared source provides said IR radiation to said window at a
power level intensity insufficient to heat said window material to a
temperatiue to
3
CA 02506690 2005-06-O1
effect metal deposition thereon under steady state conditions, but 'is
sufficient to heat
said object surface at a temperature sufficient to effect decomposition of
said metal
carbonyl on said object surface.
Preferably, the applied power level intensity should be insufficient to heat
the
window material to a temperatiue of at least 100°C.
The window material is preferably 90%, and more preferably 95% transparent
to the wavelength in the range 0.25Eun - 2.SEun.
The invention is most valuable in Ni(CO~ vapour decomposition to nickel and
carbon monoxide.
In a further aspect the invention provides an improved apparatus for the
production of metal or a metal-coated objet by a metal carbonyl vapour
deposition
process, comprising
(i) a metal carbonyl decomposition chamber for receiving said object
I S having a surface; said chamber having a partial IR radiation transparent
window and
adapted to receive metal carbonyl vapour;
(ii) an IR radiation source adjacent said chamber for operably projecting
IR radiation through said window into said chamber to heat said surface of
said
object; the improvement wherein
~ a) said window is formed of a material having an effective transparency to
radiation of wavelengths in the range of 0.2 dun-2.5 um; and
b) said 1R radiation source operably provides said IR radiation to said window
at a power level intensity insufficient to heat said window material to a
temperature to
effect metal deposition thereon under steady state conditions, but sufficient
to heat
said object surface at a temperature to effect decomposition of said metal
carbonyl on
said object surface.
The window is preferably transparent to at least 90%, and more preferably at.
least 95% to wavelength in the range 0.25 - 2.SEun.
4
CA 02506690 2005-06-O1
In order that the invention may be better understood, a prefen;ed embodiment
will now be described, by way of example only, with reference to the
accompanying
drawings, wherein
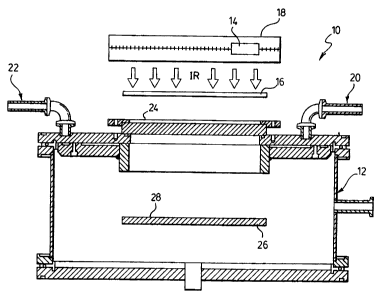
Fig. 1 is a diagrammatic layout of a process and apparatus according to the
invention;
Fig. 2 is a spectral radiation distribution of halogen lamp with filament
temperature 2400 K;
Fig. 3A and Fig. 3B are transmission curves for VICOR Glass (quartz type);
and
Fig. 4 is a transmission curve for BOROFLORAT'~ borosilicate.
DETAILED DESC$~"101~1 (,~ PREFERRED E~ODnVIENTS
With reference to Fig 1 this shows generally as 10 a metal deposition chamber
12 with associated 1R source 14, filter 16 and conveyance assembly 18 for IR
emitter
16.
In more detail, chamber 12 has a feed nickel carbonyl vapour inlet 20 and
spent carbon monoxide-containing exhaust gas outlet 22. Chamber 12 has an 1R
radiation transparent window 24.
Adjacent window 24 at an effective distance and parallel thereto is the 1R
radiation source 14, which in this embodiment has means 18 for transversely
conveying IR emitter 14 parallel to window 24 to provide uniform radiation
across
and through the full length of window 24.
In this preferred embodiment, filter 16 is located intermittent between 1R
source 14 and window 24.
Suitably disposed at a central location within chamber 12 is an object 26
having a surface 28 upon which the nickel carbonyl is decomposed and nickel
deposited.
5
CA 02506690 2005-06-O1
Window 24 (1.8 cm thick) is made preferably of fused quartz and more
preferably 7913 VYCORT"' glass (Coming) having very good transparency within
the
wavelength range 0.2-2.5 mICm.
A heat absorbing filter can be installed between the radiation source and the
window. The filter most preferably absorbs in the range between 2.0 lun to at
least,
5.0 lrm wavelength to decrease heat absorption by the window.
Filter 16 is preferably formed of a borosilicate glass which is visible to
near IR
radiation (0.35-2.SmKm) but which is able to absorb radiation of than 2.Spm,
preferably when made of BOROFLORATT"~ borosilicate glass (Edmund Industrial
Optics, Barrington, New Jersey, U.S.A.). A typical filter has a diameter of
about 20cm
and a thickness of about 0.65cm.
The maximum radiation spectrum of the source is most preferably in the range
between W and Medium Infrared wavelengths, i.e. in the visible part of the
spectrum
to provide maximum transmission through the window and minimum absorption so
as
I S to keep the window temperature below which decomposition of the metal
carbonyl
gas occurs. Preferably, the window temperature does not reach 100°C in
the practice
of the invention. Preferred IR emitters are halogen lamps having a favourable
radiation emission wavelength range of 0.2-2.~pm with a maximum of about 1.1
to
1.4 mKm.
The 1R radiation emitter preferably is attaches to a traveling means to move
it
across transverse the,window evenly to provide uniform heating of the
substrate
surface. An ambient air ventilator can be installed between window and 1R
source to
prevent convection heat transfer, if the distance between the window and IR
source
cannot be optimized. It might be needed if the high density of the heat flow
is
requiral to heat a thick high thermal conductive object, an object with low
emissivity.
Selective halogen 1R radiation emitters can be supplied (Heraeus, Germany).
The most appropriate, is Short Wave IR emitter (1800°C -
2000°C filament
temperature; peak kWlm2). Halogen lamp short wave emitters are preferred
because
of their rigid canstrucdon and durability for the voltage variation
requirement.
It should be noted that the filter and window can be made of the same
material. In preferred embodiments thermal heat transfer from the filter to
the window
6
CA 02506690 2005-06-O1
may be reduced by means of ventilation or vacuum of the intervening space
between
them. Filament temperature of standard Halogen lamp of use in the practice of
the
invention is about 2100°C which provides a maximum wavelength~at about
l.3wm.
An alternative preferred IR radiation emitter is of the double carbon filament
type
provided with a gold reflector.
The purpose of the filter is to cut oil any long wavelength tail of the 1R
radiation, since quartz and VICORTM glass type start to absorb above about 2
iriKm.
EXAMPLES
It was found in several.test experiments that it was possible to use radiation
according to the invention to induce deposition of Ni on a substrate or object
surface
through the quartz window without metal deposition on the glass surface. A 1.5
kW
halogen lamp and a traveling system, to provide the uniformity of the growing
film
thickness by scanning the surface was used. It was found that the uniformity
of the
deposited Ni on an object having a 20 cm diameter silicon wafer surface was
0.025-
0.25mm, at a deposition rate in the various runs of from 0.1 to 0.4 mmlh.
The results showed that most favourably control of parameters, such as surface
and thickness of the substrate, flow of carbonyl and carrier gases and
radiation can be
achieved. The window is made of a material which is substantially transparent
in the
wavelength range between UV and Near Infrared wavelengths and is, thus, not
heated
so as to prevent the decomposition reaction of the metal carbonyl vapour on
the
window occurring.
Most satisfactory results were achieved with uniform deposition on flat thin
substrates made even from materials with very low thermal conductivity.
Expected
problems of uniform radiant heating, masking and separation of the metal, i.e.
in this
embodiment, nickel replica from the substrate after deposition were readily
addressed.
The preferred optimum distance between the IR lamp and the window is
chosen as to prevent heating of the glass by providing ambient air convection
or by
use of an air-moving ventilator. The surface of the window inside the chamber
is
cooled by the incoming mixture of precursor nickel carbonyl and carbon
monoxide
7
CA 02506690 2005-06-O1
carrier gases. The temperature of the incoming gases, clearly, has to be
higher than the
condensation temperature of the Ni(CO)4 precursor and significantly lower than
its
decomposition temperature. I have surprisingly found that this cooling effect
is
enough to keep the
temperature of the window below about 100°C and, thus, any deposition
temperature.
The nickel deposition on various substrates from different materials was
successfully done inside this chamber without fogging of the window. It was
found
out the certain advantages of the IR heating of the substrates:
As the deposited surface of the substrate is heated directly, the thermal
conductive
properties of the substrate material do not have a significant role and, in
principle, any
material can be used for the substrate. The only demand is that the material
withstand
the deposition temperature.
Although this disclosure has described and illustrated certain preferred
embodiments of the invention, it is to be understood that the invention is not
restricted
to those particular embodiments. Rather, the invention includes all
embodiments
which are functional or mechanical equivalence of the specific embodiments and
features that have been described and illustrated.
8