Note: Descriptions are shown in the official language in which they were submitted.
CA 02711735 2010-07-08
METHOD OF CONTINUOUS CONVERSION OF COPPER MATTE
DESCRIPTIVE MEMORY
BACKGROUND
Smelting of copper concentrates produces matte and slag. Copper
matte is converted into blister copper in the Peirce-Smith or Hoboken
converters or, otherwise, in continuous conversion process such as the
Kennecott-Outokumpu, the Mitsubishi or the Noranda processes. Blister
copper is directed to fire refining process prior to the electro-refining.
The classic discontinuous conversion process of copper matte is
developed in a vascular furnace called Peirce-Smith converter or in a
vascular furnace with an off-gas siphon called Hoboken converter. The
classic process (batch) is discontinued and consists in two stages: iron
slagging and molding of blisters.
The first conversion stage aims at removing the FeS from the Cu2S-
FeS solution and the slagging of iron oxides by adding siliceous flux.
(FeS)matte 1,502+ Si02---4 (Fe2SiO4)siag 4- SO2
The Mitsubishi and Kennecott-Outokumpu continuous conversion
processes use limestone as flux, which forms calcium ferrite slag.
2(FeS)matte + 3,502 + (CaO Fe203)5la9 2S02
After removing the slag by blowing air or enriched air, it is conducted
to precipitation of metallic copper (blister copper).
(CU2S)matte + 02 --> 2(CU)blister + SO2
The classic conversion in a Peirce-Smith converter has the operational
flexibility of a typical discontinuous process, low energetic efficiency, high
labor requirements, and high emissions of sulfur dioxide and volatile
1
a = A.
CA 02711735 2010-07-08
impurities. The temperature fluctuation and the thermal impact shorten the
life of the refractory, especially in the tuyeres area.
The pyro-metallurgists' continuous conversion process idea
materialized in 1974 with the Mitsubishi process. Through it, high-grade
matte is continuously converted into blister copper through oxidation in
baths with enriched air injected through lances located in the ceiling of the
reactor. This is of a stationary vertical cylindrical type. Limestone is used
as
flux for iron slagging. The major problem faced by the Mitsubishi process is
the corrosion of the refractory due to the calcium ferrite slag with high
content of copper oxide. [(1) S. Okabe and E. Kimura, "Injection metallurgy
for continuous copper smelting and converting ¨ Fundamental aspects of
Mitsubishi process", The Howard Worner International Symposium on
Injection Metallurgy"; (2) M. Nilmani and T. Lehner, eds., TMS, 1996, 83-96.,
S.
Okabe and H. Sato, "Computer aided optimization of furnace design and
operating condition of Mitsubishi continuous copper converter, Sulfide
Smelting 98: Current and Future Practices, J.A. Asteljoki and R. L. Stephens,
eds., TMS, 1998, 607-618.; (3) H. Sato, F. Tanaka and S. Okabe, "Mechanism
of refractory wear by calcium ferrite slag", EPD Congress 1999, B. Mishra,
ed., TMS, 1999, 281-297.; (4) M. Goto and M. Hayashi, "The Mitsubishi
Continuous Process ¨ Metallurgical Commentary", Second Edition,
Mitsubishi Materials Corporation, June 2002.; (5) M. Goto and M. Hayashi,
"Recent advances in modern continuous converting", Yazawa International
Symposium, Metallurgical and Materials Processing; Principles and
Technologies, Vol. II ¨ High temperature metals production, F. Kongoli et al,
eds., TMS, 2003, 179-187.).
Outokumpu and Kennecott developed the continuous flash conversion
process. This process began to be industrially used in 1996 at the Kennecott
smelter. The process uses the Outokumpu flash furnace for oxidation of
high-grade powdered matte directly to blister copper. Limestone is used as
2
_ - -- -
CA 02711735 2010-07-08
flux agent, which produces a calcium ferrite slag with high copper oxide
content. The mayor advantage of the Kennecott-Outokumpu process is the
independence of the conversion process from the smelting of concentrates.
The energetic efficiency of the process is low due to the loss of heat by the
solidification of the matte, and the energy required for crushing and grinding
the matte. The major operational problem is the quick corrosion of the
refractory due to the calcium ferrite slag with a high content of copper
oxide,
and the generation of a large quantity of dust in the feeding duct, from 9% to
11%. [(1) D. B. George, R. J. Gottling and C. J. Newman, "Modernization of
Kennecott Utah copper smelter", COPPER 95 ¨ COPPER 95 International
Conference, Vol. IV ¨ Pyrometallurgy of Copper, W. J. (Pete) Chen et at.,
eds.,
The MetSoc of CIM, 1995, 41-52.; (2) C. J. Newman, D. N. Collins and A. J.
Weddick, "Recent operation and environmental control in the Kennecott Utah
copper smelter", Copper 99 ¨ Copper 99 International Conference, Vol. V ¨
Smelting Operations and Advances, D. B George et at, eds., TMS, 1999, 29-
45.; (3) C.J. Newman and M. M. Weaver, "Kennecott Flash Converting
Furnace design improvements ¨ 2-1", Sulfide Smelting 2002, R. L. Stephens
and H. Y. Sohn, eds. TMS, 2002, 317-328.; (4) D. B. George, "Continuous
copper Converting ¨ A perspective and view of the future", Sulfide Smelting
2002, R. L. Stephens and H. Y. Sohn, eds., TMS, 2002, 3-13.; (5) R. Walton, R.
Foster and D. George-Kennedy, "An update on flash converting at Kennecott
Utah Copper Corporation", 2005 TMS Annual Meeting. Converter and Fire
Refining Practices, A. Ross et al, eds.,TMS, 2005, 283-294.].
The other continuous conversion process was put into operation by
the Noranda company in 1997. The Noranda Continuous Conversion process
uses Noranda's reactor for continuous oxidation of the copper matte, by
maintaining three layers inside the reactor: one of semi-blister, one of white
metal and one of slag. Use of siliceous flux produces fayalite slag saturated
in magnetite. The process is not fully continuous. For obtaining blister
copper, final blowing must be performed the Peirce-Smith converter.
3
CA 02711735 2010-07-08
Refractory of reactor needs to be frequently repaired, particularly in the
tuyeres area. At present, the process is not in operation. [(1) P. J. Mackey,
C.
Harris and C. Levac, "Continuous converting of matte in the Noranda
Converter: Part I Overview and metallurgical background", COPPER 95 -
COPPER 95 International Conference, Vol. IV - Pyrometallurgy of Copper.
W.J. (Pete) Chen et al., eds., The MetSoc of CIM, 1995, 337-349.; (2) C. A.
Levac et al., "Design and construction of the Noranda Converter at the Home
Smelter", Sulfide Smelting 98, Current and Future Practices, J. A. Asteljoki
and R. L. Stephens, eds., TMS, 1998, 569-583.; (3) Y. Prevost, R. Lapointe, C.
A. Levac and D. Beaudoin, "First year of operation of the Noranda
continuous converter". Copper 99 - Copper 99 International Conference, Vol.
V - Smelting Operations and Advances, D. B. George et al, eds., TMS, 1999,
269-282,].
The Ausmelt continuous conversion process is still in the
development stage. The process takes place in the known vertical cylindrical
Ausmelt reactor with lances. Silica and limestone is used for slagging of iron
oxides, which produces an olivine-type slag. [(1) J. Sofra and R. Matusewics,
"Ausmelt technology - Flexible, low cost technology for copper production
in the 21st century", Yazawa International Symposium, Metallurgical and
Materials Processing: Principles and Technologies. Vol. II - High temperature
metals production, F. Kongoli et al, eds., TMS, 203, 211-226.; (2) J. Sofra
and
R. Matusewics, "Ausmelt technology - Copper production technology for the
21st. century". COPPER 2003 - COPPER 2003, Vol. IV - The Hermann -
Schwarze Symposium on Copper Pyrometallurgy. Book 1: Smelting
Operations, Ancillary Operations and Furnace Integrity, C. Diaz et al, eds.,
The MetSoc of CIM, 2003,157-172,].
4
CA 2711735 2017-03-21
BRIEF DESCRIPTION OF THE DRAWING
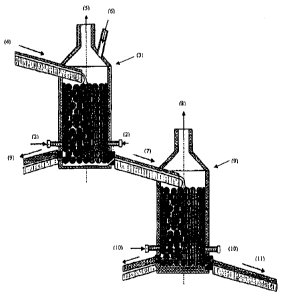
Figure 1 is a schematic diagram showing the side view, elevation and profile
of
the intensive pyrometallurgical method of continuous conversion of copper
matte in
two cascade packed-bed reactors.
DETAILED DESCRIPTION
This invention refers to a pyrometallurgical method for the continuous
conversion
of copper matte by using a flow of gravitational liquid matte in two reactors
installed in
series.
Accordingly, the present invention provides a continuous intensive
pyrometallurgical method for converting copper matte in two reactors,
comprising the
following successive stages:
a. continuous feeding of copper matte into a first oxidation reactor, which
has
a refractory chamber for containing said matte; wherein said refractory
chamber contains a packed bed of ceramic grains or other chemically
neutral grains over which said matte disperses and gravitationally flows
through said packed bed;
b. simultaneous supply of gases containing air or oxygen-rich air through
said
packed bed, in countercurrent to the liquid matte, for oxidation of iron
sulfur;
c. simultaneous supply of a flux of melted siliceous material, limestone or
a
mixture thereof for slagging iron oxides and impurities, with formation of a
conversion olivine-type slag (CaO-Si02-Fe0-Fe203), whiCh gravitationally
flows through the packed bed;
CA 02711735 2010-07-08
Slag formation:
Ca0 + Si02¨+ (CaSiO
3)slag
2(Fe0)s0lid Si02 (Fe2SiO4)slag
2(Fe304)solid (FeS)matte Si02 3(Fe2SiO4)slag 502
(Fe304)5011d Ca() ¨ (CaaFe203)slag FeO
Slag and white metal separation on bottom of the reactor;
Conversion slag continuous extraction through a tapping hole (1) and
white metal continuous extraction through a siphon or inclined hole;
Recycle of conversion slag to the melting furnace or to the slag-
cleaning furnace;
Continuous transfer of white metal (copper sulfur) through a channel
(7) to a second reactor of copper sulfide oxidation (9);
Dispersion and gravitational flow of white metal through a ceramic
grain packed bed;
Injection of air or oxygen-rich air through tuyeres (10);
Oxidation of white metal with molding of blister copper
(Cu2S)matte 4" 02 ---+ 2(CU)blister
Transfer of blister copper (11) through a channel to fire refining;
Evacuation of the off gases of the iron oxidation reactors (5) and of
copper mold (8) to the general system of gas cleaning of the smelter and to
the sulfuric acid plant;
6
- - -
CA 02711735 2010-07-08
The process' principle is schematically illustrated in Figure 1. The
copper matte (4) dispersed on the surface of the ceramic bed, flows
downwards in form of small drops and veins that get in contact with the
countercurrent flow of hot gas containing oxygen. An extremely high ratio of
liquid matte surface area (4) in relation to its volume results in a high rate
of
oxidation. Iron oxidation produces iron oxides that combine with the flux and
form the slag. The oxidation parameters, quantity of oxygen and temperature
can be precisely controlled by the flow of rich air blown through the tuyeres
(2). Similarly, the dispersion of the white metal (7) over the ceramic grain
packed bed of the second reactor increases the reaction surface area, which
in combination with the oxygen injected through the tuyeres (10) in
countercurrent to the liquid flow, results in a very high rate of copper
sulfide
oxidation, and forms blister copper. The temperature of the reactor can be
precisely controlled by the flow of injected air.
This invention has the following advantages as compared to the
traditional copper matte conversion methods:
Investment costs are significantly lower due to the small size of the
reactors required for the same production capacity;
Reduced labor requirements due to the totally continuous operation
mode;
Improved safety conditions for operators due to reduced work
exposed to high temperatures;
A more precise control of the process is achieved due to the reduced
inertia of the system. The grade of oxidation of the matte, and temperature of
7
CA 02711735 2010-07-08
the matte and slag can be precisely maintained within a narrow operating
range.
No liquid products need to be transported by crane, and no solid
products formation must be returned to the process;
The impurities removal ration is high due to the development of the
surface area, which allows obtaining blister copper of better quality.
Stationary condition of the reactors allows their easy pressurization,
and thereby fugitive emissions of sulfur dioxide and volatile impurities are
drastically reduced.
This invention has the following advantages as compared to the
copper matte continuous conversion existing methods:
Investment costs are significantly lower due to the small size of the
reactors required for the same production capacity;
Continuity of production can be assured with two parallel lines of
reactors, one in operation, the second in maintenance or on hold, thanks to
the low construction cost of the same;
Usage of MgO saturated olivine slag when using discard magnesite-
chrome bricks allows reducing corrosion of the reactor's refractory reactor.
The usage of tuyeres to inject oxygen-rich air directly into the porosity of
the
packed bed does not destroy the refractory in the tuyeres area;
A more precise control of the process is achieved due to the reduced
inertia of the system. The grade of oxidation of the matte, and temperature of
8
=
CA 02711735 2010-07-08
the matte and slag can be precisely maintained within a narrow operating
range.
EXAMPLE N 1
Copper matte with 73% - 75% of Cu continually flows through a
channel from the tapping hole of the Teniente Converter into the first
oxidation reactor (3) at a rate of 20 t/h. 3900 Nm3/h of air is blown and
injected through the tuyeres (2) inside the packed bed. Over it, 0.68 t/h of
quartz flux and 0.36 t/h of limestone flux are continuously charged. Off gases
containing 11% of SO2 and 5% of 02 are permanently transferred to the gas
cleaning system and to the acid plant. The slag (1) containing 6% of Cu, 40%
of Fe, 15% of CaO and 30% of Si02, is continuously tapped out at a rate of 2,4
t/h. White metal (7) flows from the siphon block at a rate of 18,3 t/h to a
channel of the second copper sulfide oxidation reactor (9). In the latter,
oxygen-rich air (24% of 02) is blown at 13,800 Nm3/h into the packed bed in
countercurrent to the white metal. Off gas (8), 17.470 Nm3/h, containing 17,3%
of SO2 and 5,2% of 02 is transferred to the gas cleaning system and to the
acid plant. The blister copper produced (11), containing 3000 ppm of 02 and
5000 ppm of S, flows through a channel of a siphon block to the copper fire-
refining furnace.
EXAMPLE N 2
Solid copper matte (73% - 75% of Cu) with a 20 ¨ 50 mm grain size is
fed over the packed bed surface of the oxidation reactor (3) at a rate of 20
t/h
together with the limestone flux (0,36 t/h) and siliceous flux (0,68 t/h) (6).
Oxygen-rich air (85% of 02) is blown at 2400 Nm3/h through the tuyeres to the
packed-bed. Off gases of this reactor (5) containing 80% of 02 and 4% of 02
are transferred to the gas cleaning system. Slag (1) containing 16% of Cu,
33% of Fe, 13% of CaO and 30% of Si02 is continuously extracted at a rate of
2,6 t/h. White metal and blister copper (7) flow at a rate of 16.1 t/h through
a
channel of the siphon block to a second reactor (9). In the latter, oxygen-
rich
9
. - -
CA 02711735 2010-07-08
air (24% of 02) is blown through the tuyeres (10) at 6750 Nm3/h into the
ceramic grain packed bed. Off gas (8), 8920 Nm3/h, containing 18,4% of SO2
and 5,3% of 02 is transferred to the gas cleaning system and to the acid
plant. Blister copper produced (11), containing 3000 ppm of 02 and 5000 ppm
of S, flows through a channel of the siphon block to the copper fire-refining
furnace.
=