Une partie des informations de ce site Web a été fournie par des sources externes. Le gouvernement du Canada n'assume aucune responsabilité concernant la précision, l'actualité ou la fiabilité des informations fournies par les sources externes. Les utilisateurs qui désirent employer cette information devraient consulter directement la source des informations. Le contenu fourni par les sources externes n'est pas assujetti aux exigences sur les langues officielles, la protection des renseignements personnels et l'accessibilité.
L'apparition de différences dans le texte et l'image des Revendications et de l'Abrégé dépend du moment auquel le document est publié. Les textes des Revendications et de l'Abrégé sont affichés :
| (12) Brevet: | (11) CA 2711735 |
|---|---|
| (54) Titre français: | METHODE DE CONVERSION CONTINUE DE MATTE DE CUIVRE |
| (54) Titre anglais: | METHOD OF CONTINUOUS CONVERSION OF COPPER MATTE |
| Statut: | Périmé et au-delà du délai pour l’annulation |
| (51) Classification internationale des brevets (CIB): |
|
|---|---|
| (72) Inventeurs : |
|
| (73) Titulaires : |
|
| (71) Demandeurs : |
|
| (74) Agent: | BENOIT & COTE INC. |
| (74) Co-agent: | |
| (45) Délivré: | 2017-12-05 |
| (86) Date de dépôt PCT: | 2009-01-13 |
| (87) Mise à la disponibilité du public: | 2009-07-23 |
| Requête d'examen: | 2013-11-26 |
| Licence disponible: | S.O. |
| Cédé au domaine public: | S.O. |
| (25) Langue des documents déposés: | Anglais |
| Traité de coopération en matière de brevets (PCT): | Oui |
|---|---|
| (86) Numéro de la demande PCT: | PCT/IB2009/000039 |
| (87) Numéro de publication internationale PCT: | IB2009000039 |
| (85) Entrée nationale: | 2010-07-08 |
| (30) Données de priorité de la demande: | ||||||
|---|---|---|---|---|---|---|
|
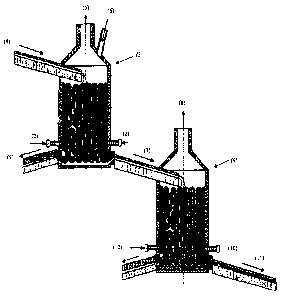
La práctica industrial de conversión de mata de cobre es la oxidación del sulfuro de hierro y posterior oxidación del sulfuro de cobre con formación de cobre blister, efectuada en convertidores Peirce-Smith u Hoboken en modo discontinuo. La presente invención resuelve dicha dificultad estableciendo continuidad operacional al proceso industrial. El método consiste en el uso de un flujo gravitacional continuo de mata de cobre a dos reactores conectados en serie por una canal, en donde la oxidación y escorifícación del hierro de la mata de cobre se efectúa en el primer reactor seguida por oxidación del sulfuro de cobre y formación de blister en el segundo reactor. Dicha operación intensiva de conversión de mata de cobre líquido ó liquido y sólido es continua utilizándose lechos empacados para incrementar la tasa de oxidación, en cada reactor, con menores tiempos de operación.
The copper matte conversion industrial practice consists of the oxidation of
iron sulfur, and subsequent oxidation of copper with formation of blister
copper in Peirce-Smith or Hoboken converters, in a discontinued mode. This
invention solves said difficulty by providing continuity to the industrial
process. The method consists of the usage of a copper matte continuous
gravitational flow to two reactors connected in series by a channel, where
the oxidation and slagging of the iron contained in the copper matte takes
place in the first reactor, and is followed by the oxidation of copper sulfur
and formation of blisters in the second reactor. Said intensive conversion of
liquid or liquid and solid copper matte is continuous, as packed beds are
used for increasing the oxidation rate in each reactor in a reduced operating
time.
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2024-08-01 : Dans le cadre de la transition vers les Brevets de nouvelle génération (BNG), la base de données sur les brevets canadiens (BDBC) contient désormais un Historique d'événement plus détaillé, qui reproduit le Journal des événements de notre nouvelle solution interne.
Veuillez noter que les événements débutant par « Inactive : » se réfèrent à des événements qui ne sont plus utilisés dans notre nouvelle solution interne.
Pour une meilleure compréhension de l'état de la demande ou brevet qui figure sur cette page, la rubrique Mise en garde , et les descriptions de Brevet , Historique d'événement , Taxes périodiques et Historique des paiements devraient être consultées.
| Description | Date |
|---|---|
| Le délai pour l'annulation est expiré | 2023-07-13 |
| Lettre envoyée | 2023-01-13 |
| Lettre envoyée | 2022-07-13 |
| Lettre envoyée | 2022-01-13 |
| Requête pour le changement d'adresse ou de mode de correspondance reçue | 2020-11-18 |
| Requête pour le changement d'adresse ou de mode de correspondance reçue | 2020-05-25 |
| Représentant commun nommé | 2019-10-30 |
| Représentant commun nommé | 2019-10-30 |
| Accordé par délivrance | 2017-12-05 |
| Inactive : Page couverture publiée | 2017-12-04 |
| Préoctroi | 2017-10-20 |
| Inactive : Taxe finale reçue | 2017-10-20 |
| Un avis d'acceptation est envoyé | 2017-04-21 |
| Lettre envoyée | 2017-04-21 |
| Un avis d'acceptation est envoyé | 2017-04-21 |
| Inactive : Approuvée aux fins d'acceptation (AFA) | 2017-04-11 |
| Inactive : QS réussi | 2017-04-11 |
| Modification reçue - modification volontaire | 2017-03-21 |
| Entrevue menée par l'examinateur | 2017-03-09 |
| Retirer de l'acceptation | 2017-03-08 |
| Inactive : Demande ad hoc documentée | 2017-03-05 |
| Inactive : Approuvée aux fins d'acceptation (AFA) | 2017-03-03 |
| Inactive : Q2 réussi | 2017-03-03 |
| Modification reçue - modification volontaire | 2016-12-28 |
| Inactive : Dem. de l'examinateur par.30(2) Règles | 2016-07-22 |
| Inactive : Rapport - Aucun CQ | 2016-07-21 |
| Modification reçue - modification volontaire | 2016-06-02 |
| Inactive : Dem. de l'examinateur par.30(2) Règles | 2015-12-02 |
| Inactive : Rapport - Aucun CQ | 2015-11-30 |
| Modification reçue - modification volontaire | 2015-10-02 |
| Inactive : Dem. de l'examinateur par.30(2) Règles | 2015-04-02 |
| Inactive : Rapport - CQ échoué - Majeur | 2015-03-24 |
| Lettre envoyée | 2013-12-06 |
| Requête visant le maintien en état reçue | 2013-12-03 |
| Requête d'examen reçue | 2013-11-26 |
| Exigences pour une requête d'examen - jugée conforme | 2013-11-26 |
| Toutes les exigences pour l'examen - jugée conforme | 2013-11-26 |
| Requête visant le maintien en état reçue | 2012-11-09 |
| Lettre envoyée | 2011-08-23 |
| Inactive : Transfert individuel | 2011-07-28 |
| Inactive : Supprimer l'abandon | 2011-06-16 |
| Inactive : Supprimer l'abandon | 2011-06-13 |
| Inactive : Abandon. - Aucune rép. à dem. art.37 Règles | 2011-04-18 |
| Réputée abandonnée - omission de répondre à un avis exigeant une traduction | 2011-04-14 |
| Inactive : Déclaration des droits - PCT | 2011-04-07 |
| Inactive : Demande sous art.37 Règles - PCT | 2011-01-17 |
| Inactive : Lettre pour demande PCT incomplète | 2011-01-14 |
| Inactive : Page couverture publiée | 2010-10-08 |
| Inactive : Lettre de courtoisie - PCT | 2010-09-08 |
| Inactive : Notice - Entrée phase nat. - Pas de RE | 2010-09-08 |
| Inactive : CIB en 1re position | 2010-09-07 |
| Inactive : CIB attribuée | 2010-09-07 |
| Demande reçue - PCT | 2010-09-07 |
| Exigences pour l'entrée dans la phase nationale - jugée conforme | 2010-07-08 |
| Demande publiée (accessible au public) | 2009-07-23 |
| Date d'abandonnement | Raison | Date de rétablissement |
|---|---|---|
| 2011-04-14 |
Le dernier paiement a été reçu le 2016-12-13
Avis : Si le paiement en totalité n'a pas été reçu au plus tard à la date indiquée, une taxe supplémentaire peut être imposée, soit une des taxes suivantes :
Les taxes sur les brevets sont ajustées au 1er janvier de chaque année. Les montants ci-dessus sont les montants actuels s'ils sont reçus au plus tard le 31 décembre de l'année en cours.
Veuillez vous référer à la page web des
taxes sur les brevets
de l'OPIC pour voir tous les montants actuels des taxes.
| Type de taxes | Anniversaire | Échéance | Date payée |
|---|---|---|---|
| TM (demande, 2e anniv.) - générale | 02 | 2011-01-13 | 2010-07-08 |
| Taxe nationale de base - générale | 2010-07-08 | ||
| Enregistrement d'un document | 2011-07-28 | ||
| TM (demande, 3e anniv.) - générale | 03 | 2012-01-13 | 2011-11-22 |
| TM (demande, 4e anniv.) - générale | 04 | 2013-01-14 | 2012-11-09 |
| Requête d'examen - générale | 2013-11-26 | ||
| TM (demande, 5e anniv.) - générale | 05 | 2014-01-13 | 2013-12-03 |
| TM (demande, 6e anniv.) - générale | 06 | 2015-01-13 | 2015-01-12 |
| TM (demande, 7e anniv.) - générale | 07 | 2016-01-13 | 2015-12-18 |
| TM (demande, 8e anniv.) - générale | 08 | 2017-01-13 | 2016-12-13 |
| Taxe finale - générale | 2017-10-20 | ||
| TM (brevet, 9e anniv.) - générale | 2018-01-15 | 2017-12-13 | |
| TM (brevet, 10e anniv.) - générale | 2019-01-14 | 2018-11-28 | |
| TM (brevet, 11e anniv.) - générale | 2020-01-13 | 2019-11-11 | |
| TM (brevet, 12e anniv.) - générale | 2021-01-13 | 2021-01-11 |
Les titulaires actuels et antérieures au dossier sont affichés en ordre alphabétique.
| Titulaires actuels au dossier |
|---|
| UNIVERSIDAD DE CHILE |
| EMPRESA NACIONAL DE MINERIA |
| Titulaires antérieures au dossier |
|---|
| ALBERTO ARTURO TAPIA SANCHEZ |
| ANDRZEJ WARCZOK |
| ARIEL BALOCCHI VENTURELLI |
| DANIEL SMITH CRUZAT |
| GABRIEL ANGEL RIVEROS URZUA |
| IVAN ANDRES VARGAS DARUICH |
| JOSE TAPIA LUNA |
| PATRICIO ROJAS VERAZAY |
| RICARDO PONCE HERRERA |
| ROBERTO SAEZ SOLIS |
| TANAI LERAC MARIN ALVARADO |
| TORSTEIN ARFINN UTIGARD |