Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
PROTECTION CIRCUIT FOR IMPLANTABLE CARDIOVERTER
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to an implantable medical
device that delivers sufficient electrical energy to
cardiac tissue to terminate (cardiovert) tachycardias and
thus restore normal sinus rhythm. An improved protection
circuit prevents high voltage cardioversion pulses from
damaging the sensing circuit of the device.
2. Description of the Prior Art
Implantable medical devices for the therapeutic
stimulation of the heart are well known in the art from
U.S. Patent No. 3,478,746 issued to Wilson Greatbatch,
which discloses a demand pacemaker. The demand pacemaker
15 delivers electrical energy (5-25 microjoules) to the heart
to initiate the depolarization of cardiac tissue This
stimulating regime is used to treat heart block by
providing electrical stimulation in the absence of
naturally occurring spontaneous cardiac depolarizations.
Another form of implantable medical device for the
therapeutic stimulation of the heart is the automatic
implantable defibrillator (AID) described in U.S. Patent
Nos. Re. 27,652 and Re. 27,757 to Mirowski, et al and the
later U.S. Patent No. 4,030,509 to Heilman et al. These
25 AID devices deliver energy (40 joules) to the heart to
interrupt ventricular fibrillation of the heart. In
operation, the AID device detects the ventricular
fibrillation and delivers a nonsynchronous high-voltage
pulse to the heart through widely spaced electrodes
located outside of the heart, thus mimicking transthoracic
defibrillation. The Heilman et al technique requires both
a lim1ted thoracotomy to implant an electrode near the
apical tip of the heart and a pervenous electrode system
~.
.
.. .
p~
located in the superior vena cava of the heart. In practice, these
devices have received limited usage due to the complexity of their
implantation, their relatively large size and short operating life,
and to the small numbers of patients who might benefit from it.
Another example of a prior art implantable cardioverter
; includes a device which detects the onset of tachyarrhythmia and
includes means to monitor or detect the progression of the tachy- !
arrhythmia so that progressively greater energy levels may be applied
to the heart to interrupt the arrhythmia.
A further example is that of an external synchronized
cardioverter, described in Clinical Application of Cardioversion in
Cardiovascular Clinics, 1970,2, pp. 239-260 by Douglas P. Zipes.
This external device is described in synchronism with ventricular
depolarization to ensure that the cardioverting energy is not
delivered during the vulnerable T-wave portion of the cardiac cycle.
Still another example of a prior art implantable cardio-
verter includes the device disclosed in United States Patent
No. 4,384,585 to Douglas P. Zipes. This device includes sensing
circuitry to detect the intrinsic depolarizations of cardiac tissue
"nd includes pulse generator circuitry to deliver moderate energy
level stimuli (in the range of 0.1-10 joule) to the heart in syn-
chrony with the detected cardiac activity.
The functional objective of this stimulating regime is to
depolarize areas of the myocardium involved in the genesis and
maintenance of re-entrant or automatic tachyarrhythmias at lower
energy levels and with greater safety than is possible with non-
synchronous cardioversion. Nonsynchronous cardioversion always
incurs the risk of preclpitating ventricular fibrillation and
sudden death.
-
Synchronous cardioversion delivers the shock at a timewhen the bulk of cardiac tissue is already depolari~ed and
is in a refractory state.
It is expected that the safety inherent in the use of
lower energy levels, the reduced trauma to the myocardium,
and the smaller size of the implanted device will expand
the indications for use for this device beyond the patient
base of prior art automatic implantable defibrillators.
Since many episodes of ventricular fibrillation are
preceded by ventricular (and in some cases,
supraventricular) tachycardias, prompt termination of the
tachycardia may prevent ventricular fibrillation.
Typically, the electrical energy to power an
implantable cardiac pacemaker is supplied by a low
voltage~ low current, long-lived power source, such as a
lithium iodine pacemaker battery of the types manufactured
by Wilson Greatbatch Ltd. and Medtronic, Inc. While the
energy density of these power sources is relatively high,
they are not designed to be rapidly discharged at high
current drains, as would be necessary to directl~
cardiovert the heart with cardioversion energies in the
range of O.l-lO ~joules. Higher energy density battery
systems are known which can be more rapidly discharged,
such as lithium thionyl chloride power sources. ~owever,
none of the available implantable, hermetically sealed
power sources have the capacity to directly provide the
cardioversion energy necessary to deliver an impulse of
the aforesaid magnitude to the heart following the onset
of tachyarrhythmia.
Generally speaking, it is necessary to employ a DC-DC
converter to convert electrical energy from a low voltage,
low current power supply to a high voltage energy level
stored in a high ener~y storage capacitor. A typical form
of DC-DC converter is commonly referred to as a flyback
converter which ~mploys a transformer having a primary
winding in series with the primary power supply and a
secondary winding in series with the high energy
capacitor. An interrupting circuit or switch is placed in
series with the primary coil and battery. Charging of the
high energy capacitor is accomplished by inducing a
voltage in the primary winding o~ the trans~ormer creating
a magnetic field in the secondary winding. Wnen the
current in the primary winding is interrupted, the
collapsing field develops a current in the secondary
windin~ which is applied to the high energy capacitor to
charge it. The repeated interruption of the supply
current charges the high energy capacitor to a desired
level over time.
The delivery of the high energy cardioversion pulse
is controlled by a memory and logic circuit which responds
to a sensed tachycardiac according to programmable
detection criteria. The pulse is delivered through an
interface circuit which couples the pacing and
cardioversion lead electrodes to the sensing circuit, the
pacemaker output circuit and the high energy capacitor.
The delivery of the high energy pulse creates a load on
the sense amplifier due both to the energy of the pulse
and the polarization after potentials that develop as the
lead-tissue interface repolarizes following discharge.
; 25 The lead that is desirably employed in thi's system is
described in U.S. Patent No. 4,355,646. That lead has a
large surface area anode electrode locatable in the
superior vena cava and consisting of several ring
electrodes electrically connected together. A ring and
;~ 30 tip electrode are positioned at the distal portion of the
- lead adapted to be placed in the apex of the right
ventricle. During cardioversion~ the ring and tip
electrodes are to be electrically connected to~ether to
form a large surface area cathode electrode. At other
times the ring and tip electrodes are coupled to a sense
.
amplifier or sensing circuit or pacing circuit. The
present invention involves an improved circuit for
effecting the electrical connection to avoid circuit
damage and the elimination of polari~ation after
potentials which interfere with sensing after
cardioversion.
SUMMARY OF THE INVENTION
The implantable synchronous intracardiac cardioverter
of the present invention employs sensing means responsive
to cardiac depolarizations for producing a sense signal
indicative of naturally occurring cardiac activity, such
as ventricular R-waves; detection means responsive to the
sensing means for detecting cardiac tachyarrhythmias, such
as ventricular tachyarrhythmia, for producing a trigger
signal; pulse generator means responsive to the detection
of a tachyarrhythmia for delivering a cardioverting pulse
to cardiac tissue in response thereto; voltage conversion
means for providing a high energy power supply to said
~ pulse generator means; and output circuit means ~or
protecting the sensing means from damage done by the
cardioverting pulse.
Additional circuitry may be included to provide a
demand-pacing function in addition to the previous
described cardioverting output. It is also anticipated
that the amount of energy delivered can be controlled
(programmed) by an external unit to reduce the joules
delivered or increase the amount to a value that will be
capable of terminating ventricular fibrillation. Finally,
the device may be programmed to deliver the energy
automatically after sensing particular parameters of a
tachycardia or it can be programmed to deliver the energy
only when an external magnet is held in place over the
pulse generator.
-5a- 66742-255
Thus, in accordance with one broad aspect o-f the
invention there is provided an implantable medical device for
the electrical termination of an arrhythmic condition of the
heart comprising: first terminal means adapted to be coupled
to first electrode means positioned in relation to a first
portion of the heart; second and third terminal ~eans adapted
to be coupled to second and third electrode means, respect-
ively, positioned in relation to second and third portions,
respectively, of the heart; power source mean~ for providing
electrical power to said device; mean~ for detecting an
arrhythmia of the heart and providing a trigger signal in res-
ponse thereto, said detecting means coupled respectively to
said second and third terminal means; cardioversion energy
pulse generating means for providiny a cardioversion energy
pulse in response to a trigger signal; and protection circuit
means responsive to the generation of a cardioversion energy
pulse for electrically connecting said second and third term~
inal means together during delivery of a cardioversion energy
pulse and for applying said cardioversion energy pulse between
said first terminal means and said commonly connected second
and third terminal means.
In accordance with another broad aspect of the inven-
tion there is provided an implantable medical device for deliv~
ering cardioverting energy to cardiac tissue to terminate an
arrhythmic condition of the heart comprising: Eirst terminal
means adapted to be coupled to electrode means introduced into
the superior vena cava of the heart; second and third terminal
means adapted to be coupled to second and third electrode
means, respectively, positioned in the ventricle of the heart;
power source means for providing electrical power to said
a~
-5b- 66742-255
device; means for detecting an arrhythmia of the heart and pro~
viding a trigger signal in response thereto, said detecting
means coupled respectively to said second .~nd third terminal
means; cardioversion energy pulse generati:ng means for provid~
ing a cardioversion energy pulse in response to a trigger
signal; and protection circuit means responsive to the gener-
ation of a cardioversion energy pulse for electrically connect~
ing said second and third terminal means together during
delivery of a cardioversion energy pulse and for applying said
cardioversion energy pulse to said first terminal means.
BRIEF DESCRIPTION OF THE DRAWIN~S
Other features and advantages of the present invention
will become apparent from the following description of the
invention and the drawings wherein:
Fig. 1 is a block diagram showing the functional
organization of the synchronous intracardiac
cardioverter;
Fig. 2A and 2B are alternative timing diaqrams
illustrating the tachycardia detection criteria;
Fia. 3 is a timing diagram of the delivery o~ a high
energy cardioversion pulse to the heart;
Fig. 4 is a circuit diagram of the DC-DC converter
usable in the cardioverter; and
Fig. 5 is a circuit diagram of the improved output
circuit.
r
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
As described hereinbefore, implantable cardioverters
designed to treat ventricular tachyarrhythmias are
generally known as described in the a~orementioned U.S.
Patent No. 4,3S~,585. The presen~ invention is embodied
in a form of an implantable cardioversion system includina
a pulse generator and a lead, preferably a transvenous
lead. The pulse generator preferably includes the
components necessary to accomplish at least ventricular
demand pacing at ordinary pacing energies and rates and
circuitry for providing the cardioversion function. The
lead preferably includes ring and tip electrodes located
near one another in the distal portion of the lead adapted
to be placed in the apex of the right ventricle and large
surface area ring electrodes located more proximally along
the lead body to be positioned in or near the superior
vena cava as previously described. Very generally
speaking, sensing and demand pacing is accomplished
through ~he ventricular ring and tip electrodes, and
cardioversion is accomplished between the ring and tip
-- 7 --
electrodes as a single indifferent or cathode electrode, and the superior
vena cava electrode acting as the anode electrode.
The sense amplifier is connected between the ring and tip elec-
trodes and, with appropriate logic, is capable of detecting normal R-waves
to inhibit the operation of the pacing pulse generator and with appropriate
detection logic, is capable of detecting abnormally high rate R-waves and
operating the high energy cardioverter.
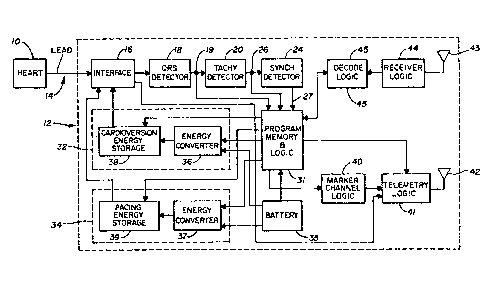
Referring ]IOW to Figure 1, there is shown a block diagram
similar to Figure 2 of United States Patent No. 4,3~,585 in which the
present invention may be practiced. In Figure 1, the patient's heart 10
is coupled to the synchronous :intracardiac carclioverter 12 through an
appropr:iato lead 1~ ot tho type descrlbed above and lead interface lG. 'I'he
lead interface 16 includes circuitry to isolate the QRS detector 18 from
the high voltage present during the delivery of a cardioverting pulse.
Very generally the ring and tip electrodes are coupled together, in accor-
dance wi~h the present invention, by lead interface 16 during delivery of
a cardioverting pulse.
Depolarization signals :Erom the heart are communicated to the
sensing amplifier or QRS detector 18 where they are detected. Such sense
amplifiers are known in the art from United States Patent No. 4,379,459.
A subsequent tachyarrhythmia detector section 20 is coupled to the QRS
detector through connection 19 to detect tachyarrhythmia based upon the
electrogram information producing a tachy detect signal.
The synchrony detector 2~ receives the tachy detect signal through
connection 26. The output 27 of the synchrony detector is communicated
to an appropriate logic section 31 which controls the cardioversion pulse
generator circuitry 32 and triggers the delivery of the
--8--
cardioverting pulse in response to the detected
tachyarrhythmia. The synchrony detector 24 insures that
the cardioverting pulse is delivered to the cardiac tissue
concurrent with a detected ventricular depolarization of
cardiac tissue. Circuit 32 includes a high energy
converter 36 which converts low battery voltage into
current and charges a capacitor in the high energy
cardioversion energy storage circuit 38.
In operation, the electrogram information from the
heart lO is processed by the device which detects
depolarizations of cardiac tissue and produces a sense
signal indicative of this fact. This sense amplifier
output is processed by a tachyarrhythmia detector 20 to
determine the presence or absence of a tachyarrhythmia in
a manner described hereafter.
If a tachyarrhythmia is detected~ the logic section
31 will initiate a discharge of the energy storage section
38 to produce a cardioverting output. The synchrony
detector 24 will insure that the energy is delivered to
the heart lO concurrently with a detected ventricular
; depolarization. The synchrony detector 24 may comprise
combinatorial logic to activate the cardioverting pulse
generator circuit 32 only when a tachy detect signal has
been produced by the tachyarrhythmia detection circuitry.
After the delivery of the cardioverting energy, the device
will monitor the heart activity to determine if the
arrhythmia has been terminated. If the arrhythmia is
continuing, then additional cardioYerting pulses will be
delivered to the heart. These may be of the same or
greater energy.
~` The synchronous intracardiac cardioverter is shown
combined with demand pacing energy pulse generator
- circuitry 34 coupled to the lead interface l6. This pulse
generator comprises a low ener~y converter 37 for charging
the pacing energy storage circuitry 39 from the battery
35. In operation> logic 31 receives the signal from the QRS detector
18 and resets an escape interval timing system. If no cardiac depolariza-
tion is detec-ted within the escape interval, a pacing pulse will be
delivered to the heart. The integrated demand pacer with the synchronous
cardioverter permits the device to initiate a cardiac depolarization if
a previously delivered cardioverting pulse has prevented or slowed the
re-establishment of a normal sinus rhythm.
The power source 35 preferably consists of two lithium thionyl
chloride cells, connected in series which produce an open circuit voltage
of 7.33 volts to supply the carclioversion power circuit 32 and the backup
pacing circuit 34 and one lithium iodine ccll to provide the power Eor the
remainillg sellsing and control c;rcuits.
In addition, the preferred embodlment would be programmable and
possess telemetry as shown, for example, in United States Patent Nos.
4,401,120 and 4,324,382, respectively. Among the programmable character-
istics would be the mode (V00, VVI and VVI with cardioversion), pacing
rate, sensitivity, pulse width, tachy trigger interval, number oE intervals
to trigger, detection of interval change threshold, cardioversion pulse
energy, patient therapy record and inhibit function. The receiving antenna
43, receive logic 44 and decode logic 45 operate to effect programming
of memory registers within program memory and logic 31 in the manner des-
cribed in United States Patent No. 4,401,120.
Among the telemetry functions, digital data, such as the
device model identification, programmed parameter settings, cardioverter
battery status, programming confirmation, the electrogram from the
bipolar/vcntricular lead, the marker channel exhibiting the amplifier
sense after refractory period, the amplifier sense within tachy
trigger interval, the ventricular pace pulse and the
i ~D
-10 -
cardioversion pulse would all be transmitted out on
command. The marker channel telemetry would be similar to
that shown in U.S. Patent No. ~37~,382 and is depicted as
including marker channel logic 40, telemetry logic 41 and
transmitting antenna 42. (In practice~ the transmitt;ng
antenna 42 and receiving antenna 43 may be a single
antenna.) The pacing components, with the telemetry and
marker channel features3 preferably employ circuits
disclosed in the aforementioned prior Medtronic patents
and used in the prior Medtronic Spectrax-SXT ventricular
pulse generator. In addition, it is contemplated that the
DDD pacing components described in U.S. Patent No.
4,39~,020 could be 1ncorporated into the cardioversion
system to effect atrial and/or ventricular multimode
pacing and/or cardioversion.
The additional programmable features of the preferred
embodiment o~ the present invention, that dif~er from the
prior patents and device mentioned above, comprises the
additional cardioversion operating mode and the additional
tachyarrhythmia recognition and cardioversion pulse energy
parameters. These modes and parameters are stored in
program memory and logic circuit 31 and directed to the
tachy detector 20~ and the cardioversion circuit 32.
; The cardioversion output circuit 32 can only be
activated when a tachycardia has been detected.
Tachycardia recognition can be based on a sudden increase
in heart rate combined with high rate or on high rate
alone. The first method is called "the acceleration plus
interval mode"~ and the second is called "interval mode".
The "tachy trigger interval" ~TTI) is an interval
programmed into the pulse generator which recognizes
sensed intervals between consecutive R-waves as indicative
of a tachycardia if the sense intervals are shorter than
this programmed tachy trigger interval. The "number of
intervals to trigger" (NlT) is defined as that number o~
consecutive intervals shorter than the tachy trigger
interval which will initiate a cardioversion. The
"interval change threshold" (ICT) is defined as the number
of milliseconds by which an interval has to be shorter
than its predecessor in order to activate the tachy
trigger interval and number of intervals to trigger
criteria for tachycardia recognition. In order to detect
such changes, the pulse generator continuously measures
the difference between the last and second to last
interval. If the interval change threshold is Programmed
equal to zero, the tachycardia recognition depends on the
tachy trigger interval and number oF intervals to trigger
alone. Each of these factors, the TTI, NIT and ICT are
programmable and are stored in memory and logic c~rcuit
3l.
In the acceleration plus interval modeg tachycardia
is recognized if the interval change exceeds the interval
change threshold while the succeeding intervals are also
shorter than the selected tachy trigger interval for the
selected number of consecutive intervals to tri~qer. In
interval mode, tachycardia is recognized if the detected
intervals are shorter than the selected tachy tri~ger
interval for the selected consecutive number of intervals
to trigger.
Thus the tachy detector 20 comprises a logic circuit
having counters for timing out the intervals between the
successive R-waves and comparing them to the proqrammed
tachy triqger interval, interval chanoe threshold, and
number of intervals to trigger according to the
acceleration and interval mode or the interval mode alone.
Fig. 2A depicts the timing of a sequence o~ R-waves
where the tachy trigger interval is selected to be 400
milliseconds, the number of consecutive intervals to
~rigger equals 4 and the interval change threshold is
programmed to zero. Thus, very simply, if four
-12-
consecutive R-waves are sensed, each havin~ an R-R
interval less than 400 milliseconds, the tachyarrhyt~mia
detection criteria are satisfied and a tachy detected
signal is applied through logic 3l to the energy converter
36.
Fig. 2B depicts the acceleration plus interval mode
tachyarrhythmia detection operation wherein the interval
change threshold is programmed to lO0 milliseconds. Thus,
if the sensed R-R interval decreases by more than lO0
milliseconds and the subsequent four R-R intervals are
less than 400 milliseconds, the tachy detection criteria
are satisfied and a tachy detected signal is again applied
by logic 31 to the energy converter 36.
When the selected tachycardia criteria has been
fulfilled, the chargin~ cycle for the cardioversion output
stage 32 is initiated and controlled by the hiqh energy
charging circuit of Fi~. 4.
Fig. 3 depicts a timing sequence of the detection and
delivery of a cardioversion pulse in synchrony with a
sensed R-wave. The refractory intervals and the
cardioversion sense ~eriod are depicted as they occur
following the delivery of a cardioversion impulse.
After charging to the programmed energy level, the
cardioversion sense period is initiated during which a
cardioversion pulse will be delivered in synchrony with a
detected depolarization as described more specifically in
U.S. Patent No. 4,384,585. If sensing does not occur
within the cardioversion sense period (l,OOn milliseconds)
following completion of capacitor charge period, the
~30 cardioversion pulse is aborted and the device reverts to
VVI pacing. The VVI mode and tachycardia detection
circuitry are also activated for 150 milliseconds after
delivery of a cardioversion pulse. The amplifier
refractory period will ~e 195 milliseconds in VVI mode and
tachycardia detection, but 320 milliseconds after the
, , , : ,.
,, . : '
-13-
charge period because of the switch on time. If an ~-wave
follows within the 320 milliseconds refractory period
after charge completion, refractory is extended an
additional 200 milliseconds. The cardioversion pulse is
delivered 7.8 milliseconds delayed on the next sensed
event still within the cardioversion sense period. If
cardioversion remains unsuccessful after five attempts,
the cardioversion will remain inactive until one of the
tachy detection criteria is not met or a back up pacing
pulse is delivered.
Referring our attention now to the char~ing circuit
34 of Fiq. 4, the two series connected lithium thionyl
chloride batteries 50 and 52 are shown connected to the
primary coil 54 of transformer 56 and to the power FET
transistor switch 60. The secondary coil 58 is connected
through diode 62 to the cardioversion energy storage
capacitor 64. Very generally, the flyback converter works
as Follows: When switch 60 ;s closed, current Ip
passing through the primary winding 54 increases linearly
as a function of the formula V=LpdI/dt. When FET 6~ is
opened, the flux in the core of the transformer 56 cannot
change instantaneously so a complimentary current Is
which is proportional to the number of windings of the
primary and secondary coils 54 and 58 respectively starts
to flow in the secondary winding 58 according to the
formula (Np/Ns)Ip. Simultaneously, voltage in the
secondary windin~ is developed according to the function
Vs=LsdIs/dt. The cardioversion energy storage
capacitor 6~ is charged thereby to the programmed
voltage.
To simplify the drawin~s, optional connections have
been omitted from Fig. 4 which would be included to
connect the power source 50, 52 and the oscillator 66 to
the circuit 32 upon command of the program memory and
logic 3l. Such switchinq circuits could be at the output
:
~14-
of oscillator 66 and in the line between capacitor 55 and
resistor 132 in Fig. 4. In addition, the circuitry for
sensing the voltage on the capacitor 64, comparing it to
the programmed value and disconnecting the power source
means 509 52 and oscillator 66 is not shown. In general,
the voltage on capacitor 64 is reflected back throu~h the
transformer 56 and can be detected by comparison circuits
coupled to the input winding 5~ and the program memory and
logic 31 to disconnect the power source 50, 52 and
oscillator 66.
Each time the power FET 60 is switched into
conduction, the current in the primary winding 54 starts
to increase according to the precedinq formula. At the
moment that the power FET 60 is sw;tched off, the field of
the transformer 56 collapses and the secondary current
starts to charge the capacitor 64. If the power FET 60 is
switched on again before the secondary current has
decreased to zero, the primary current starts to increase
from a certain value (referred to as the pickup current)
which is determined by the secondary current and the
winding ratlo. If this happens for several consecutive
cycles, the primary current may go beyond the saturation
current of the transformer 56. To avoid this, current
through the power FET is monitored by sensing i5 drain
voltage (the on resistance of power FET 60 is relatively
constant) and power FET 60 is switched off when the
current reaches a certain value. In order to suppress
interference from this circuit, FET 60 is switched with a
constant frequency and a variable duty cycle. The
converter is driven at a frequency of 32 k2 developed by
osc;llator 66, powered by the lithium iodine battery,
which is driven by the crystal 68 and provides the basic
timing clock for all the pulse generator timing and loqic
circuits. The power FET 60 is driven on and off by the
the flip-flop 70 wnich is set at the leading edge of the
-15-
clock pulses developed by the oscillator 66 and reset by
an output signal from NOR gate 94. Flip-flop 70 has a
pair of output terminals Ql, Q2 Which go high
(ungrounded) when it is set and complementary outputs
5 ~15 ~2 Which go high when it is reset.
The low state of the outputs Ql~ Q2~
~2 may be at ground. Power FET 60 is driven into
conduction each time flip-flop 70 is set through the
operation of the voltage doubler circuit 72. The on time
10 of power FET 60 is governed by the time interval between
the setting and resetting of flip-flop 70 which in turn is
governed either by the current Ip flowing through the
primary winding 54 or as a function of a time limit
circuit, which contains further circuitry to vary the time
limit with battery impedance (represented schematically by
resistor 53).
The voltage doubler circuit 72 comprises a pair of
driving transistors 74 and 76, a diode 78, a capacitor 80,
and resistors 82, 84, 86. Assuming that flip-flop 70 is
set and FET 60 is conducting, the resetting of flip-flop
70 causes the gate voltage of FET 60 to discharge through
output Q2. Capacitor 80 charges to battery voltage
through diode 78 and output Ql. When flip-flop 70 is
again set, the battery ~olta~e and the capacitor 80
voltage are additively applied to the emitter of
transistor 76. Transistor 76 is biased to conduct by
resistors 82 and 84, and when it switches on (when
f lip-flop 70 is set) 2VBatt is applied to the qate
of FET 60. FET 60 is switched on until the flip-flop 70
is again reset in the manner described hereinafter.
If primary current would be the only criteria for
: switching off power FET 60, then a~ low battery voltage
:~ (high internal impedance 53) the current would be so
depleted that the saturation current leve1 would not be
reached before the next clock pulse. If that were to
. .
occur, power FET 60 would never be switched off ~nd the
flyback converter would cease functionin~.
To avoid this problem, the on-time or duty cycle of
power FET 60 is determined hy an OR function o~ a time
limit and a current limit. The current limit is
determined by the first comparator 90 which compares the
voltage drop across the source and drain of power FET 60
against a first voltage Vrefl. The signal is
applied through resistor 92 to one input of the comparator
90, and whenever that signal exceeds reference
Vrefl, the comparator 90 provides an output signal
to one input of the OR gate 94. The signal applied
to the OR gate 94 is passed through the reset input of
flip-flop 70 which switches the Ql and Q2 output low
and switches the transistors 74 and 76 off in the manner
previously described. During the time that the flip-flop
70 is reset, the ~2 utput of flip-flop 70 is
high and the transistor 9h is rendered conductive. Thus,
when the FET 6n drain voltage equals Vrefl,
comparator 90 resets flip-flop 70 via OR gate ~4 switching
power FET 60 off and the positive input of comparator gn
is grounded. When the flip-flop 70 is next set by a clock
pulse, the power FET 60 is switched on and the positive
input of comparator 90 is unqr~unded.
Thus, by the means previously described, the duty
cycle of the power FET 60 is current limited to
efficiently operate the flyback converter as long as the
batteries 50 and 52 provide a su~iciently high volta~e
and current. However, as current is drained from the
batteries 50 and 52 to periodically recharge the high
energy capacitor 64, the internal impedance 53 of the
batteries will increase resulting in a lower available
current. As the current lowers, the duty cycle of power
FET 6~ will tend to increase. The danger that the
regulatin~ circuitry 90-96 will be unable to reset the
-17-
flip-flop 70 increases with time. The remaining circuitry
of Fig. 4 provides a backup time limit circuit to the duty
cycle while the batteries 50 and 52 exhibit normal voltage
and current output and further compensating means for
altering the time limit as the batteries 50 and ~2 exhibit
end-of-life voltage and impedance changes.
The time limit is determined by a one-shot circuit,
comprising resistor 102, capacitor 104, comparator 106,
flip-flop 108 and transistor 110. The time limit may be
varied by altering the value of resistor 102 at the time
of manufacture or through programming. The ti~e limit
interval is determined by the charge time oF capacitor 104
through resistor 102 in comp~r;son to a second reference
voltage Vref2. When volta~e on capac;tor ln4
equals the reference voltage Vref2, comparator 106
provides an output signal to the reset input of flip-flop
108 which provides a high output signal through coupling
capacitor 112 to a second input of OR gate 94. Ordinarily
it would be assumed that the time limit interval is longer
than the current level interval detected by comparator 90.
Therefore, the second input signal to the reset input of
flip-flop 7Q provided by the time limit interval circuit
would have no effect. If, however, the current limit
interval signal is delayed, then the time limit interval
signal would prov;de the proper reset input signal to
fliP-flop 70.
When the ~lip-flop 108 is reset, its ~ output
goes high, and the transistor 110 is switched into
conduction by the signal through resistor 111 to discharge
capacitor 104 and ready the timing circuit for ;ts next
timing cycte which commences upon the delivery of the next
clock impulse ~rom oscillator 66 to the set input terminal
of flip-flop 108. The time limit interval determining
circuit therefore provides a backup system for insuring
that the power FET 60 is turned off prior to the delivery
-18-
of the next clock signal. The remaining circuit
components are provided to modify the operation of the
time limit interval circuit when the batteries 50 and 52
exhibit end-of-life behavior.
As batteries 50 and 52 deplete, the internal
resistance 53 increases. Then as current is drawn from
the batteries 50, 52 (when power FET 60 is rendered
conductive), the voltage across the transformer 54 and
power FET 60 may be reduced by the internal resistance
related voltage drop of the batteries. Battery voltage
also becomes too low to maintain Vcc at its stabilized
voltage and thus too low to ensure proper action oF the
control circuit. Vcc Voltage is a re~ulated voltage
below battery voltage established by circuits not shown in
Fig. 4 to power certain control and logic circuits.
As long as battery voltage is sufficiently above
Vcc, collector current of transistor 126 is sufficient
to generate enough voltage across resistor 134 to keep
transistor 128 conducting. In turn, the emitter voltage
of transistor 124 is pulled low by the conduction of
transistor 128. Transistor 130 and resistor 136 tend to
bias transistor 124 to conduct, but it is prevented from
conductiny by transistor 128. Capacitor 104 is only
charged through resistor 102, and the time limit is
2~ established as described above.
When the voltage dif~erence between battery volta~e
an~ Vcc becomes lower, the collector current of
transistor 126 decreases until the voltage generated
across resistor 134 decreases and transistor 128 no longer
c~onducts. Then the collector current of transistor 124
takes on a value, determined by resistor 122, which also
charges capacitor 104 which is charged more quickly and
decreases the time limit.
~- Thus, the compensation circuitry as described
~hortens the time l1mit lnterval whenever the battery
,
::.
5~
-19-
voltage drops as a result of depletion and the consequent
increase in internal resistanceO The shortened interval
reduces the mean current drain on the power source ~0~ 52
to prevent the battery voltage from collapsing further.
The shortening of the time limit interval again provides
assurance that the duty cycle of the on time of the power
FET 60 is kept short enough to ensure that the Flyback
converter will work. This circuitry thus insures that the
high energy capacitor 64 can be charged reliably and
provide the energy necessary to provide the cardioversion
function previously described whenever the batteries 50,
52 are able to deliver the required enerqy.
Turning now to Fig. 5, the interface or output
circuit 16, the pacing energy storage circuit 39, the
cardioversion energy storage circuit 38 and triggering and
protection circuits 208, 210, 212, 215 and 217 of the
cardioverter are shown in greater detail. The superior
vena cava (SVC) electrode, the tip electrode and the ring
electrode are adapted to be connected to terminals 200,
202 and 204, respectively, through the lead system 14.
The ring and tip electrode terminals 202 and 204 are
connected to the sense amplifier 18 and to the pacemaker
output circuit 39, the triac 206 and to the marker channel
logic 40. The high energy storage capacitor 64 is coupled
through power FET 208 across the SVC terminal 200 and the
series connected tip and ring terminals through the
operation of the triac protection circuit 210. The
further (optional) protection circuit 212 can be employed
; to provide a short circuit across the high energy
capacitor 64 under certain conditions.
Protection of the pacing clrcuit and sense amplifier
18 from dama~e by external high energy interference, e.g.
an external defibrillator, is accomplished by the standard
back-to-back zener diode 214. Protection of the
cardioversion circuit is effected by the breakdown voltage
4S~
-20-
of triac 216 (+500V) and, if this voltage is exceeded by
the zener diode 215 and diode 217. Positive voltage will
merely charge capacitor 64 via the reverse diode of FET
208 to the breakdown voltage of zener diode 215, negative
voltage will be short circuited by diode 217.
In reference to the cardioversion operation, the
capacitor 64 is char~ed during the charging cycle
previously described. The synch detector 24 triggers the
discharge of the capacitor by switching FET 208
conductive. The discharge is applied to the protection
circuit 210.
Protection circuit 210 cdmprises the series connected
triac 216 and the shunt triac 206~ together with the
biasing network 218, 220, 222 and 224. The initial
discharye produces a voltage drop across the blas
resistors which renders 216 and 206 conductive. In this
fashion, the ring and tip electrodes are electrically
connected in series just as the impulse is delivered. The
R-C timing network of capacitor 220 and resistors 218, 222
and 224 insures that the triacs 206 and 216 are edae
triggered by the voltage pulse. As current discharges
from capacitor 643 the voltage decays until FET 208
~ switches of~. Shortly thereafter, the current drops and
i triacs 206 and 216 switch off. In this way the protection
circuit prevents an imbalance in after-potential or
polarization from developing across the tip and ring
terminals 202, 204 which are also the input terminals to
the sense amplifier 18.
The pacing energy storage circuit 39 includes the
pacing energy storage capacitor 226 and load resistor 228
which are connected to the Vcc supply (developed by
~`~ batteries 50, 52) from which capacitor 226 is charged.
Capacitor 226 is dischar~ed across the tip and ring
terminals 202, 204 when transistor 230 is switched on in
.,
4rj~
- 21 -
response to a pacing command delivered by the program memory and logic
31 through resis-tors 232 and 234.
During the charging cycle, a "fast recharge" of the type describ-
ed in United States Patent No. 4~406,286 is accomp].ished by command from
the program memory and logic circuit 31. Very generally, for a short
period following discharge of capacitor 226, transistor 296 is switched
on to bypass load resistor 228 and accelerate the recharge time. Tran-
sistor 296 is switched on by the fast recharge signal applied to the base
of transistor 238 through resistors 240, 242. Conduction of transistor
238 changes the bias potential at the junction of resistors 244, 246
and switches transistor 296 conductive.
Thc signal at t:i.p clectrode 202 :is also applied by resistor
250 and capacitor 248 to the telemetry log:ic 41 :Eor trallsmissioll thro-l~h
antenna 42 to receiving equipment outside the patient's body.
Although not shown in Figure 5, it may be desirable to include
a further resistor of about 50K resistance across the tip and ring elec-
trodes 202 and 204 to reduce polarization effects during pacing.
The additional protection circuit 212 comprises power FET 254,
diode 256 and biasing resistor 258 and capacitor 260. This circuit
operates to redundantly protect from misfires caused by a defect in power
FET 208 or the synch detector 24.
Power FET 254 is kept conductive by a signal :Erom gate terminal
88 of FET 60 ~Figure 4) applied through diode 256 to the gate of FET 254
until the capacitor 64 is charged up and FET 60 is no longer switched on
and off. Each time FET 60 is switched on, current from circuit 72 also is
applied to capacitor 260. Capacitor 260 switches FET 254 on after it
charges to a certain voltage. Once circuit 72
-22-
stops supplying current (when capacitor 64 is charged Up)5
current from capacitor 260 discharges through resistor
258, and FET 254 is rendered nonconductive. Thus, if
power FET 208 were mistakenly fired prior to the complete
charging of transistor 64, then the energy would be
dissipated in power FET 254.
The invention described herein may advantageously be
implemented in external cardioverters but is preferably
employed in implantable cardioverters. The invention may
also be implemented in any suitable analog or digital
circuitry including software controlled custom or
conventional microprocessors. These and other equivalent
embodiments, modifications or uses of the invention will
be apparent to those skilled in the art.