Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
`` NOVEL O,O'-, O,S'- OR
S,S'-DITHIODIALKYLENE-BIS(MONO- OR
DIHYDROCARBYL CARBAMOTHIOATES) AND
S,S'-DITMIODIALKYLENE-BIS(MONO-
OR DIHYDROCARBYL CARBAMODITHIOATES)
USEFUL AS FROTH FLOTATION COLLECTORS
This invention concerns novel compounds,
specifically O,O'-, O,S'- or S,S'-dithiodialkylene-bis~
(mono- or dihydrocarbyl carbamothioates) and S,S'-dithio-
dialkylene-~is(mono- or dihydrocarbyl carbamodithioates)
and their use as collectors in the recovery of sulfide
ores by froth flotation.
Flotation is a process of treating a mixture of
finely divided mineral solids, e.g., a pulverulent ore,
suspended in a liquid whereby a portion of such solids is
` ld separated from other finely divided mineral sollds, e.g.,
clays and the like materials present in the ore, by intro-
ducing a gas into the liquid (or providing a gas ln situ)
to produce a frothy mass containing certain of the solids
on the top of the liquid, and leaving suspended (unfrothed)
other solid components of the ore. Flotation is based on
31,361-F -1-
-2~ 3~ 6 Z
the principle that introducing a gas into a liquid
containing solid particles of different materials
suspended thereln causes adherence of some gas to
certain suspended solids and not to o-thers and makes
S the particles having the gas thus adhered thereto
lighter than the li~uid. Accordingly, these particles
rise to the top of the liquid to form a froth.
An understanding of the phenomena whic~ makes
flotation a particularly valuable industrial operation is
not essential to the practice of the present invention.
Such phenomena appear, however, to be largely associated
with selective affinity of the surface of particulated
solids, suspended in a liquid containing entrapped gas,
for the li~uid on one hand and the gas on the other.
.
The flotation principle is applied in.a number
of mineral separation processes among which is the
selective separation of such minerals as sul~ide copper
minerals, sulfide zinc minerals, sulfide molybdenum
minerals and others from sulfide iron minerals.
..
`~ 20 Various flotation agents have been admixed with
the suspension to improve the frothing process. Such
~; added agents are classed according to the function to be
performed: collectors, e.g., high carbon chain compounds
~ such as collectors for sulfide minerals including
; 25 xanthates, thionocarbamate, dithiophosphates, mercaptans,
and the like; frothers which impart the property of
forming a stable froth, e.g., natural oils such as pine
oil and eucalyptus oil; modifiers such as activators to
, induce flotation in the presence of a collector, e.g.,
copper sulfate; depressants, e.g., sodium cyanide, which
tend to prevent a collector from functioning as such on a
certain mineral which it is desired to retain in the
:. .
~ 31,361-F -2-
.,
;
.
.
:
.,
i;
-3~
liquid, and thereby discourage a substance ~rom being
carried up and forming a par-t of the froth; pH regulators
to produce optimum metallurgical results, e.g., lime, soda
ash and the like.
These foregoing flotation additaments are
selected for use according to th-e nature of the ore, the
mineral sought to be recovered, and the other additaments
which are to be used in combinat~on theréwith.
Xanthates and dithiophosphates are relatively
inexpensive collectors but have a comparatively low
activity as collectors, thus requiring larger concen-
trations than some other collectors to get satisfactory
activity. The thionocarbamates have good activity as
collectors but are relatively expensive to produce.
Further, in the preparation of thionocarbamates, salt and
odorous by-products are prepared. These by-products must
be removed from the thionocarbamates.
Although the following patents disclose
compounds similar to the compounds now claimed, the
patents concern other utilities and are structurally
distinguished.
U. S. Patent 3,579,516 discloses compounds of
the formula
.
Rl S S R
" "
R2 N-c-s-cH2-sx-cH2-s-c- N~R4
wherein R1 and R3 are alkyl or aryl; R2 and R~ are alkyl;
and R1 and R2 may form a ring, and R3 and R~ may form a
31,361-F . -3-
-4~
ring; and X is an integer of from 2 to 6. These compounds
are useful as vulcanizing agents.
U. S. Patent 3,876,550 discloses lubrican-t
compositions having an aliphatic hydrocarbon substituted
succinic acid or derivatives thereof and an alkylene
~ithiocarbamate which corresponds to the formula
S S
RlR2N-C-S-X-S -C-NR3R4
wherein: R1, R2, R3 and R4 are a hydrogen or alkyl; or R
and R2 may be taken together to form a heterocyclic ring;
or R3 and ~4 may be taken together to form a heterocyclic
ring; and X is Cl 8 alkylene radical.
. There is needed a froth flotation collector
s, 15 which is relatively inexpensive to prepare which has a
high activity as a collector for sulfide ores. There is
furthex needed a collector which can be prepared by a
process that does not produce salt or odorous by-products.
i
The invention concerns novel 0,0'-, 0,S'- or
. 20 S,S'-dithiodialkylene-bis(mono- or dihydrocarbyl carbamo-
thioates) and S,S'-dithiodialkylene-bis(mono- or dihydro-
carbyl carbamodithioates).
Another aspect of this invention is a-process
for the preparation of 0,0'-, 0,S'- or S,S'-dithiodi-
alkylene-bis(mono- or dihydrocarbyl carbamothioates) and
, S,S'-dithiodialkylene-bis(mono- or dihydrocarbyl carbamo-
- thioates) which comprises
.
31,361-F -4-
.
: ~s
~,
. .
.. .
.
:~
.~!'
,:,
., '
. ~
~5- ~r~,6~
(1) contacting a 1,3-oxathiolane-2-thione or a
1,3-dithiolone-2-thione with a primary or secondary
amine in a nonpolar solvent under conditions such that
an S- or 0-(2~mercap-toalkyl~mono- or dihydrocarbyl
carbamothioate or an S-(2-mercaptoalkyl)mono- or
dihydrocarbyl carbamodithioate is formed; and
- - (2) addiny to the above reaction solution an
oxidant which is capable of oxidizing the mercapto
- moiety under cond.itions such that 0,0'-, 0,S'- or
S,S'-dithiodialkylene-bis(mono- or dihydrocarbyl
carbamothioate) or S,S'-dithiodialkylene-bis(mono-
or dihydrocarbyl carbamodithioate) is prepared.
A further aspect of this invention is a process
of concentrating sulfide ores by flotation, which comprises
subjecting the sulfide ore in the form of a pulp, to a
flotation process in the presence of a flotating amount of
a flotation collector for the sulfide comprising a 0,0'-,
0,S'- or S,S'-dithiodialkylene bis(mono- or dihydrocarbyl
carbamothioate) (hereinafter disulfide carbamothioates) or
S,S'-dithiodialkylene-bis(mono- or dihydrocarbyl carbamo-
dithioate) (hereinafter disulfide carbamodithioates), or
mixtures thereof.
The disulfide carbamothioates and disulfide
carbamodithioates of this invention have good activity as
collectors, and better activity than the xanthates and
- dithiophosphates, Further, the compounds of thi~ invention
are less expensive to prepare than the thionocarbamates.
Also, the process for the preparation of the compounds of
this invention does not result in the preparation of salt
or odorous by-products.
: `
~;
31,361-F -5~
.
~ 2
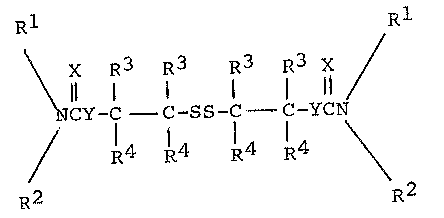
The invention includes O,O'-, O,S'- or S,S'-
-dithiodialkylene-bis(mono- or dihydrocarbyl carbamothio
ates) and S,S'-dithiodialkylene-bis(mono- or dihydrocarbyl
carbamodithloates) which correspond to the formula
Rl X R3 R3 R3 R3 X R1
~ 11 ~ I I 11 /
~ NCY-C -C-SS-C C-YCN'
R2 R4 R~ R4 R4 `~R2
~. . .
wherein
R1 is separately in each occurrence hydrogen
or C1 ~0 hydrocarbyl;
R is separately in each occurrence Cl 20
hydrocarbyl;
R3 is separately in each occurrence hydrogen
or C1_~0 hydrocarbyl;
R is separately in each occurrence hydrogen
or Cl 20 hydrocarbyli
X is separately in each occurrence O or S;
and
Y is separately in each occurrence O or S;
with the proviso that both X and Y cannot be oxygen and
with the further proviso that at least one R3 and one R4
on the same carbon atom on each alkylene moiety must be
hy~drogen.
A preferred class of compounds of the above
formula is the O,O'-S,S'-dithiodialkylene-bis(mono- or
dihydrocarbyl carbamothioates), such as the O,O'-disulfide
dialkylene-bis(mono- or dihydrocarbyl carbamothioates) of
31,361 F -6-
)
-7- .~
this invention which include those corresponding to the
formula
R1 S R3 R3 R3 R3 s R
~ "
~NCO-C- C-SS-C--C-OCN~
R R4 R4 R4 R4 \ R2
wherein R1, R2, R3 and R4 are as defined above.
The 0,S'-disulfide dialkylene-bis(mono- or
dihydrocarbyl carbamothioates) of this invention corre-
spond to the formula
R1 R3 R3 R3 R3 S R
" , , , . Il
`NCS-C--C-SS-C -C-OCN~
R2 / R4 R4 R4 R4 \ R2
wherein R1, R2, R3 and R4 are as defined above.
The S,S'-disulfide dialkylene-bis(mono- or
dihydrocarbyl carbamothioates) of this invention corre-
spond to the formula
Rl R3 R3 R3 R3 R
\ NCS-C-~C-SS-C - C-SCN /
` 25 R2 R4 R4 R4 R4 \ R2
wherein R1, R2, R3 and R4 are as defined above.
The S,S'-disulfide dialkylene-bis(mono- or
dihydrocarbyl carbamodithioates) of this invention include
those corresponding to the formula
i~ .
~ 31,361-F -7-
`
~`~?
.
``,~'`
''~'
~.,
`:
,
-8- .~
Rl S R3 R3 R3 R3 S R
~ " , , I 1 11 ~
"NCS-C--C-SS-C--C-SCN~
R2 R4 R4 R4 R~L ~R2
wherein R1, R2, R3 and R4 are as defined above.
Rl is preferably hydrogen or C1_20 alkyl and
most preferably hydrogen. R is preferably C1 20 alkyl
or phenyl; more preferably C2 10 alkyl, and most prefer-
ably C2 6 alkyl. R3 is preferably hydrogen or C1 20
alkyl, more preferably hydrogen or C1 4 alkyl, and most
preferably hydrogen. R4 is preferably C1 20 alkyl and
most preferably C1 4 alkyl.
In one preferred embodiment, the nitrogen atom
on the carbamate moiety is substituted with one hydro-
carbyl group. In another preferred embodiment, the
alkylene moiety has only one substituent.
C1 20 hydrocarbyl means herein an organic
radical containing between one and twenty carbon atoms
to which are bonded hydrogen atoms. Included are the
following groups: Cl_20 alkyl~ C1-20 alkenyl~ C1-20
alkynyl~ C3_20 cycloalkyl, C3_20 cycloalkenyl~ C6 20 aryl,
C7_20 alkaryl or C7 20 aralkyl.
.
The term aryl Eefers herein to biaryl, phenyl,
naphthyl, phenanthranyl and anthranyl. Alkaryl refers
herein to an alkyl-, alkenyl- or alkynyl-substituted aryl
substituent wherein aryl is as defined hereinbefore.
Aralkyl means herein an alkyl, alkenyl or alkynyl substit-
uent substituted with an aryl group, wherein aryl is as
defined hereinbefore.
i
31, 361~F -8-
_g~ $ ~
C3 20 cycloalkyl refers to an alkyl group
containing one, two, three or more cyclic rings. C3 20
cycloalkenyl refers to mono-, di- and polycycllc groups
containing one or more double bonds. C3 20 cycloalkenyl
also refers to the cyclo~alkenyl groups wherein two or more
double bonds are present.
The O,O'-, O,S'- or S,S'-dithiodialk~lene-bis-
(mono- or dihydrocarbyl carbamothioates) are prepared by
reacting a primary or secondary amine with a 1,3~oxa-
1~ thiolane-2-thione in a suitable solvent to prepare a
S-(2-mercaptoalkyl)mono- or dihydrocarbyl carbamothioate
(hereinafter S-mercapto carbamothioate), 0-(2-mercapto-
alkyl)mono- or dihydrocarbyl carbamothioate (hereinafter
O-mercapto carbamothioate),- or mixtures thereo~. In order
to get high yields of the 0-(2-mercaptoalkyl)mono- or
di~ydrocarbyl carbamothioate, a nonpolar solvent should be
used. The 0-(2-mercaptoalkyl) carbamothioate, the S-(2-
-mercaptoalkyl) carbamothioate, or mixtures thereof are
then contacted with an oxidation agent to prepare the
2~ ~,O'-, O,S'- or S,S'-dithiodialkylene-bis(mono- or dihydro-
carbyl carbamothioate) or~mixtures thereof.
The dithiodialkylene~bis(mono- or dihydrocarbyl
carbamodithioates) are prepared by contac-ting a 1,3-
-dithiolane-2-thione with a primary or secondary amine to
~S prepare a (2-mercaptoalkyl)mono- or dihydrocarbyl carbamo-
dithioate ~hereinafter referred to as mercapto carbamodi-
thioate). The mercapto carbamodithioate is then contacted
with an oxidation agent to prepare the dithiodialkylene-
-bis(mono- or dihydrocarbyl carbamodithioates).
Amines useful in this process include those
which correspond to the formula HNRlR2 wherein R1 and R2
are as defined hereinbefore.
31,361-F -g-
t,~ J
--10--
Specific illustra-tive examples of the amines
contemplated herein are shown by the following:
(1) monoalkylamines including, for example,
methyl~mine, ethylamine, propylamine, isopropylamine,
n-butylamine, sec-butylamine, isobutylamine, pentyl-
~ amines, hexylamines, cyclohexylamines, heptylamines,
octylamines, dodecylamines, octadecylamines, eicosyl-
` amines, triacontanylamines, benzylamine, chloro-
~enzylamine, nitrobenzylamine, 2-ethoxyethylamine,
4-carbomethoxyhexylamine;
(2~ dialkylamines including, for example,
dimethylamine, diethylamine, di-n-propylamine,
diisopropylamine, di-,n-butylamine, di-sec-butylamine,
diisobutylamine, di-tert-butylamine, dipentylamines,
dihexylamines, dioctylamines, ditriacontanylamine,
N-methylethylamine, N-methylpropylamine, N-methyl-
octadecylamine, N-ethylhexylamine, N-ethyldodecyl-
amine, N-propyldodecylamine;
(3) heterocyclic aliphatic secondary amines
including, ~or example, piperazine, pyrrole,
imidazoline, pyrazole, piperazine;
(4) arylamines including, for example, aniline,
toluidine, anisidine, nitroaniline, bromoaniline,
xylidines, 4-ethylaniline, naphthylamine;
(5) diarylamines including, for example,
diphenylamine, N-phenyl-2-naphthylamine, N-phenyl-
naphthylamine;
(6) alkylarylamines having from 1 to about
30 carbon atoms in the alkyl group attached either to
the nitrogen atom or to the aryl group including, for
example, N-ethylaniline, N-methyl-o-toluidine,
N-methyl-p-toluidine, p-chloro-N~methylaniline, N,N'-
-dimethylphenylenediamine, 4-ethylaniline, 4-propyl-
aniline, 4-bu-tylaniline, 4-decylaniline; and
31,361-F -10-
(7) aminoalkyl-substituted amines including,
for example, ethylenediamine, diethylenetriamine,
triethylenetetramine, 1,3-propylenediamine, di-1,3-
-propylenetriamine, 1,6,11,16-tetraa~ahexadecane.
The 1,3-ox~athiolane-2-thiones useful in this
invention include those corresponding to the f~rmula
S .. ...
O~ ~S
R3- C - C - R4
'4 '3
R R
wherein R3 and R4 are as defined hereinbefore, with the
proviso that the R3 and R4 attached to one of the carbon
atoms must be hydrogen, that is either the 4 carbon or the
5 carbon must be unsubstituted. Included are 1,3-oxa-
thiolane-2-thione, 5-methyl-1,3-oxathiolane-2-thione,
5-ethyl-1,3-oxathiolane-2-thione, 5-propyl-1,3-oxathiolane-
-2-thione, 5-butyl-1,3-oxathiolane-2-thione, 5-pentyl-1,3-
-oxathiolane-2-thione, 5,5-dimethyl-1,3-oxathiolane-2-
-thione, 5,5-diethyl-1,3-oxathiolane-2-thione, 5,5-di-
propyl-1,3-oxathiolane-2-thione, 5,5-dibutyl-1,3-oxa-
thiolane-2-thione, 5,5-dipentyl-1,3-oxathiolane-2-thione,
.25 5-phenyl-1,3-oxathiolane-2-thione. The 1,3-oxathiolane-2-
; -thiones can be prepared by the method taught in U.S.
Patent 3,409,635.
The 1,3 dithiolane-2-thiones useful in this
invention correspond to the formula
31,361-F -11-
-12-
5R3~ C _.C -.R3
R4 R
wherein R3 and R4 are as defined above and with the proviso
that the R3 and R4 attached to one of the carbon atoms must
be hydrogen, that is either -the 4 carbon or the 5 carbon
must be unsubstituted. 1,3-Dithiolane-2-thiones are
prepared by contacting carbon disulfide with an alkylene
episulfide at a temperature of between 10C and 80C in
the presence of a catalyst comprising an alkali metal
halide, 2 to 12 weight percent water based on the alkali
-~ metal halide, and an alkylsulfonium halide or methanol.
\
The 0-(2-mercaptoalkyl)mono- or dihydrocarbyl
carbamothioates include those corresponding to the formula
; `
Rl S R3 R
20NCO-C C-SH
R2 R4 R4
:,
wherein Rl, R2, R3 and R4 are as defined hereinbefore.
.
25The S-(2-mercaptoalkyl)mono- or dihydrocarbyl
carbamothioates include those corresponding to the formula
Rl O R3 R3
\ "
' ,NCS-C--C-SE~
" 30 R R4 R4
..:
" 31,361-F -12-
,
;`
. .~.
:
:
,;,
::
. .,
wherein Rl, R2, R3 and R4 are as previously deflned.
The (2-mercaptoalkyl)mono- or dihydrocarbyl
carbamodithioates correspond -to the formula
Rl S R3 R3
~ ~ 11 1 1
NCS-C- C-SH
R4 R4
wherein Rl, R2, R3 and R4 are as previously defined.
In the preparation of a S- or 0-~2-mercapto-
alkyl~mono- or dihydrocarbyl carbamothioate or (2-mercapto-
alkyl)mono- or dihydrocarbyl carbamodithioate, an amine and
a l,3-oxathiolane-2-thione or l,3-dithiolane-2-thione are
contacted in a suitable solvent. Preferably in a molar
ratio of between about 0.95:l.0 to l.O:l.0 of l,3-oxa-
thiolane-2-thione or l,3-dithiolane-2-thione to amine,
more preferably in a l:l molar ratio. Although excesses
of either reagent are within the scope of this invention,
the reactants react in a stoichiometric manner such that
the use of such an excess provides no significant
advanta~e.
Suitable solvents include any inert solvent
which dissolves the reactants. When high yields of 0,01_
-dithiodialkylene-bis(mono- or dihydrocarbyl carbamo-
thioate) are desired, the solvent should be a nonpolarsolvent. Suitable nonpolar solvents include aromatlc
hydrocarbons, aliphatic hydrocarbons, chlorinated aromatic
hydrocarbons, aliphatic chlorinated hydrocarbons, cyclic
ethers and aliphatic ethers. Examples of aromatic solvents
include benzene, toluene, xylene, ethylbenzene and the.
like. Examples o~ aliphatic hydrocarbons include hexane,
~ .
`: :
; 31,~61-~ -13-
; :
i
. .
.
heptane, octane and the like. Examples of chlorinated
aromatic hydrocarbons include monochlorobenzenes, dichloro-
- benzenes, trichlorobenzenes, monochlorotoluene, monochloro-
e-thylbenzene and the like. Chlorinated aliphatic hydro-
carbons include chloromethane, dichloromethane, trichloro-
methane, tetrachloromethane, chloroethane, dichloroethane,
l,l,l-trichloroethane, viJnyl chloride, vinylidene chloride
and the like. Cyclic ethers include tetrahydrofuran and
' the''like. Aliphatic ethers include ethyl ether'and the
like.
Preferred solvents are cyclic ethers and
aliphatic ethers, with tetrahydrofuran most preferred.
The first step can be run at an~ temperature at
which the reaction rate-is reasonable and the product is
acceptable. Preferred temperatures are between about -40C
and 30C, with between about 0C and 20C more preferred
and between about 0C and 10C most preferred. Below -40C
the reaction rate is low, above 30C a siynificant amount
of dialkylthiourea by-products is prepared.
The reaction time is generally between about
1 minute and several hours with between about 30 and
120 minutes being preferred.
After the amine and 1,3-oxathiolane-2-thione or
1,3-dithiolane-2-thione have reacted for a sufficient ;time
to prepare the S-mercapto carbamothioate, 0-mercapto
carbamothioate or mercapto carbamodithioate, an oxidant is
added to the reaction solution to oxidize such compound to
the disulfide carbamothioates and disulfide carbamodi-
thioates claimed herein. Generally, a sufficient amount
of oxidant is added to oxidize all of the S-mercapto
31,361-F -14-
-15- ~
carbamothioate, 0-mercapto thioate or mercapto carbamodi-
thioate to the disulfide carbamothioates or disulfide
carbamodithioates. Preferably, between about 0.5 and
1.5 moles of oxidant per mole of swch compounds is used.
More preferably, as this oxidation process is a mole ratio
of 0.95:1 to 1.05:1 stoichiometric reaction, a 1:1 molar
ratio is most preferred.
Suitable oxidants are those which oxidize
mercaptans to disulfides. Examples of suitable oxidants
are hydrogen peroxide, bromine, chlorine or oxygen or
oxygen-containing gases in the presence of suitable
catalysts. Hydrogen peroxide is the preferred oxidant.
When bromine or chlorine is used as the oxidant, the
S,S'-dithiodialkylene bis(mono- or dihydrocarbyl carbamo-
thioates) are prepared.-
The oxidation can be run at any temperature atwhich the reaction rate is reasonable and product prepared
does not have an unacceptable amount of by~product in it.
Preferable temperatures are between about -10C and 50C,
more preferably between about 0C and 20C, and most
; preferably between about 0C and 10C. Below -10C the
reaction rate is slow, above 50C significant amounts of
unwanted by-products are prepared including dialkyl-
thioureas.
.
The disulfide carbamothioatés and disulfide
carbamodithioates are generally recovered by removing the
solvents, for example, by stripping off the solvents on a
rotary evaporator.
When a 1,3-oxathiolane-2-thione is the initial
starting material, the product generally comprises a
~. ~
, .. .
~' .
~ 31,361-F -15
;:
,.~;
.. . :
,~
,....
-16- ~
mixture of the O,O'-dithiodialkylene-bis(mono- or dihydro-
carbyl carbamothioate), O,S'-dithiodialkylene-bis(mono- or
dihydrocarbyl carbamo-thioates) and S,S'-dithiodialkylene-
-bis(mono- or dihydrocarby:L carbamothioates). The O,O'-
-dithiodialkylene-bis(mono- or dihydrocarbyl carbamothio-
ates) are preferred as they are better sulfide ore
collectors; The ratio of the O,O'-dithi-odialkylene-bis-
~mono- or dihydrocarbyl carbamothioates) to the S,S'-
-dithiodialkylene-bis(mono- or dihydroca~byl carbamothio-
ates) and O,S'-dithiodialkylene-bis(mono- or dihydrocarbyl
carbamothioates) can be increased by using a more nonpolar
solvent, running the reaction at lower temperatures and
using shorter reaction times. The O,O'-dithiodialkylene-
-bis(mono- or dihydrocarbyl carbamothioates) are thermally
less stable than the S,S'-dithiodialkylene-bis(mono- or
dihydrocarbyl carbamothioates) and at higher temperatures
undergo rearrangement to the latter compounds.
In one preferred embodiment the process
described herein is performed as follows. The 1,3-oxa-
thiolane-2-thione is dissolved in a nonpolar solvent
(i.e., tetrahydrofuran, dichloromethane or toluene). The
solution is cooled to between 0C and 25C. A quanti-
tative amount of amine is added slowly, while the temper-
ature is maintained at between about 0C and 25C. After
sufficient time for the reaction to go to completion
(generally between 0.5 and 2.0 hours), a quantitative
amount of oxidant (hydrogen peroxide) is added slowly
while the temperature is maintained at between about 0C
and 25C. The reaction mixture is then allowed to warm to
room temperature and react for at least about 0.5 hour.
The solvent and water are removed to obtain the crude
product.
31,361-~ -16-
~.
p
- -17-
The O,O'-dithiodialk~lene-bis(mono- or dih~dro-
carbyl carbamothioates), O,S' dithiodialkylene-bis(mono-
or dihydrocarbyl carbamo-thioates) and S,S'-dithiodi-
alkylene-bis(mono- or dihydrocarbyl carhamothioa~es) are
useful as collectors for sulfide ores in ~roth flo~,ation
processes. Generally, the compounds are added to a
frothing aqueous sulfide ore pulp in which they aid the
sulfide ores in becoming attached to the air bubbles and
being carried with the bubbles Into the froth.
Ores for which these compounds are useful include
mineral ores containing copper, zinc, molybdenum, cobalt,
nickel, lead, arsenic, silver, chromium, gold, platinum,
uranium and mixtures thereof. It is preferable to use
the disulfide carbamothioates and disulfide carbamodithio-
ates as collectors for copper-containing sulfide minerals.
Examples of metal-containing sulfide minerals which may be
concentrated by froth ~lotation using the disulfide
carbamothioates and disulfide carbamodithioates of this
invention as collectors include copper-bearing minerals
such as, for example, covellite (CuS), chalcocite (Cu2S),
chalcopyrite (CuFeS2), bornite (Cu5FeS4), cubanite
(Cu2SFe4S5), vallerite (Cu2Fe4S7 or Cu3Fe~S7), enargite
[Cu3(AsSb)S4], tetrahedrite (Cu3SbS2), tennanti-te
(Cu12As4Sl3), cuprite (Cu20), tenorite (CuO), malachite
[Cu2~0H)2C03], azurite [Cu3(0H)2C03], antlerite
[Cu3S04(0H)4], brochantite [Cu4(0H)6S04], atacamite
" [Cu2Cl~OH)3], chrysocolla (CuSiO7), fama-tinite
[Cu3(SbAs)S4], and bournonite (PbCuSbS3); lead-bearing ~
minerals such as, for example, galena (PbS); an-timony-
-bearing minerals such as, for example, stibni-te (Sb2S4);
: zinc-bearing minerals such as, for example, sphalerite
(ZnS), zincite (ZnO), and smithsonite (ZnC03); silver-
` -bearing minerals such as, for example, argentite (Ag2S),
stephanite (Ag5SbS4), and hessite (AgTe2); chromium-bearing
31,361-F -17-
., ,
:.
;T~3~
minerals such as, for example, daubreelite (FeSCrS3) and
chromite (FeOCr2O3); gold-bearing minerals such as, for
example, sylvanite (AuAgTe2) and calaverite (AuTe),
pla-tinum-bearing ores such as, for example, cooperite
[Pt(AsS)2] and sperrylite (PtAs2); and uranium-bearing
ores such as, for example, pitchblende [U2O5(U3O8)] and
g~mmite (U~3nH2O).
The amount of the disulfide carbamothioate or
disulfide carbamodithioate used for froth flotation depends
upon the type of ore used, the grade of the ore, the size
of the ore particles and the particular compound used.
Generally, that amount which separates the desired metal
sulfide from the sulfide ore is suitable. Preferably
between about 0.005 and 0.25 lb of disulfide carbamothio-
ates or disulfide carbamodithioates per ton of ore, mostpreferably between about 0.015 and 0.08 lb per ton of ore
is used.
Mixtures of O,O'-dithiodialkylene-bis(mono- or
dihydrocarbyl carbamothioates~ and S,S'-dithiodialkylene-
-bis(mono- or dihydrocarbyl carbamodithioates) are usually
used in froth flotation of sulfide ores, because the
process described hereinbefore prepares mixtures of the
compounds. Each of the species can be used alone for
froth flotation of sulfide ores. O,O'-dithiodialkylene-
-bis(mono- or dihydrocarbyl carbamothioates) and S,S'-
-dithiodialkylene-bis(mono- or dihydrocarbyl carbamothio.-
ates are the preferred species as they are generally better
collectors, with the O,O'-dithiodialkylene-bis(mono- or
dihydrocarbyl carbamothioates) being most preferred.
The froth flotation processes in which the
disulfides of this invention are used, are those which are
well known in the art. In most of these processes, use of
'
31,361-F -18-
,, .
.
. .,
.
.
- 1 9 ~
frothing agents is re~uired. It is contemplated that -the
disulfide carbamothioa-tes and disulfide carbamodithioates
of this invention will be used along wi-th fro-thers.
Further, the collectors of this invention can be used in
mixtures with other known collectors.
Numerous collectors are known in flotation
practice or have been proposed in the technical and patent
literature; Generic examples include xan-thates, thio-- -
carbamates, dithiophosphates, thiocarbanilide, xanthogen
formates, alkylamines, quaternary ammonium compounds,
sulfonates and the like. Any collector which is known in
the art as suitable for the beneficiation by flotation of
sulfide mineral ores can be used in this invention.
Further blends of known collectors can also be used in
this invention.
Suitable frothers include some compounds which
are also useful as collectors such as fatty acids, soaps,
and alkyl aryl sulfonates, but the best frothers are those
which have a minimum of collecting properties. They are
polar-nonpolar molecules of the type C5~11OH, amyl alcohol
or CloH17OH, the active constituent of the well-known
frother pine oil. The aliphatic alcohols used as frothers
preferably have chain lengths of 5 to 8 carbon atoms,
provided there is sufficient branching in the chain.
Alcohols in the 10 to 12 carbon atom range are good
frothers. Other examples include polyalkylene glycols,
polyoxyalkylene paraffins and cresylic acids. ~lends of
frothers may also be used. All frothers which are
suitable for beneficiation of sulfide mineral ores by
3Q froth flotation can be used in this invention.
31,361-F -19-
-
-20-
The disulfide carbamothioate and disulfide
carbamodithioate collectors of this invention demonstrate
good recoveries and rates of recovery.
~cific Embodiments
The following examples are included for
illustration and do not limit the scope of the invention
or claims. Unless otherwise indicated, all parts,
fractions and percen-tages are by weight.
In the following examples, the performance of
the frothing processes described is shown by giving the
rate constant of flotation and the amount of recovery at
infinite time. These numbers are calculated by using the
formula
-kt
y = R~ [1- kt ]
. . ,
wherein: y is the amount of mineral recovered at time t,
k is the rate constant for the rate of recovery and R~ is
the calculated amount of the mineral which would be
recovered at infinite time. The amount recovered at
various times is determined experimentally and the series
of values are substituted into the equation to obtain the
R and k. The above formula is explained in Klimpel,
~n
"Selection of Chemical Reagents for Flotation", Ch. 45,
pp. 907-934, Mineral Pr_cessin~ Plant Desiqn, 2d Ed., Eds.
Mular and Bhappu, published by Society of Mining Engineers,
N.Y. ~1980).
, .
Example 1 - Preparation of 0,0'-dithio(l,l'-dimethyl)di-
ethylene-bis(ethyl carbamothioate)
A 250 ml 3-necked flask is equipped with a
stirrer, thermometer, condenser (vented through a drying
.,~ .
31,361-F -20-
,
. .
: `
,
.; ,
-21-
tube), and an addi-tional funnel. To the flask is added
13.42 parts of 5-methyl-1,3-oxathiolane-2-thione and
42 parts of THF. The solution is cooled at 0C wikh an
ice-water bath. To the mixture is added, dropwise,
5.90 parts propylamine o~er approximatel~ 15 minutes. The
temperature ls maintained below 20C. After the exotherm
is complete, the reaction solution is allowed to stand at
0C-20C for 30 minutes. The solution is cooled to 0C
and 11.3 parts of 30 percent H202 in H2O is added, drop-
wise, maintaining the temperature below 20C. After theexotherm is complete, the solution is allowed to warm to
room temperature and remain there for 1 hour or longer.
The tetrahydrofuran and water are removed in a ro-tovap at
up -to 80C in an aspirator vacuum. The crude product of
O,O'-dithio(l,l'-dimethyl)diethylene-bis(ethyl carbamo-
thioate), 18.36 parts, is thus obtained.
Examples 2-12 - Experimental Procedure for Flotation
of Copper Sulfide Ores
Several of the O,O'- or S,S'-dithiodialkylene-
-bis(mono- or dihydrocarbyl carbamothioates) and S,S'-
dithiodialkylene-bis(mono- or dihydrocarbyl carbamo-
dithioates~ of this invention and prior art collectors are
used for the flotation of copper sulfide. The procedure
for such flotation is described hereinafter. The results
are compiled in Table I.
Pro~edure:
The flotation cell used is a 6.5 x 6.5 x 8-inch
plexiglass container which holds approximately 2.8 liters
of deionized water, ore, collector and frother. A rotating
paddle is provided for skimming the frother from the top
of the cell. An air inlet is placed in the bottom of the
cell.
31,361-F -21-
i
-22-
A copper sulfide ore from the Inspiration
Consolidated Copper Company is preground to -10 mesh.
Immediately before floatlng the ore is yround in a rod mill
for an additional period of time to obtain the desired mesh
size. The process for -this gri~ding is as follo~7s. Eigh-t
rods of one inch each are put in a rod mill along ~ith
lO00 g of ore, 0.6 g of lime (to bring the pH to 10.6),
600 g of deionized water, 0.05 lb of collec-tor per ton of
ore (0.025-g), and the mixture ls ground at 60 rpm for
about 25 minutes, until approximately 80 percent of the
particles had a size of less than 200 mesh.
Thereafter, the slurry is transferxed to the
float cell as described hereinbefore. The frother,
Dowfroth~ 1012 (a polypropylene glycol ether available
from The Dow Chemical ~ompany, Midland, Michigan) is added
to the cell, 0.0~ lb per ton of ore (0.04 g). Deionized
water is added to bring the water up to the desired level
in the float cell. The mixture in the float cell is
stirred at 900 rpm for 2 minutes to condikion the ore.
After 2 minutes of stirring, the air flow of 9 liters/-
minute is started, wi.th continued stirring, and a paddle
rotation of 10 rpm is started. Further water is added to
maintain the water level. The froth from the cell is
skimmed by the paddle into a collection tray. The froth
25 skimmed off is collected at intervals of 0.5, 1.5, 3.0,
5.0 and 8.0 minutes. Each sample is dried overnight in a
forced air oven at 95C.
The samples are weighed and analyzed for copper
` content by plasma emission spectroscopy.
`:
The recovery and rate are calculated from the
copper content and time of each sample using the equation
` described hereinbefore.
~ .
31,361-F -22-
.
.
23 '~J'~ 3~
The procedure for the analysis by plasma emission
spectroscopy is as follows. Into a 100-cc flask is placed
0.2 to 0.25 g of ore sample (approximately 2.0 y i it is
a tailings sample, the ore lef-t in the cell after flo-
tation). -To this is added 3.5 ml of concen-trated hydro-
chloric acid and 5.0 ml of concentrated nitric acid. The
- mixture is heated to boiling and boiled for 25 minutes,
and then allowed to cool. To this is added 25 ml of
~ dei-onized water. The mixture is heated to boiling then
allowed to cool. The mixture is filled to the volumetric
line. A plasma emission spectrometer (Spectrospan IV) is
used to determine the copper level in the solutions
prepared. The copper emission line at 2135.98 nm is found
to give a linear response with copper concentration. The
instrument is standardized by the use of copper solution
standards. When the sample solution is aspirated into the
plasma, the concentration in ppm of Cu is shown by the
- instrument by digital display. This ppm of Cu is converted
into percent Cu in the original sample by the following
e~uation:
% Cu in original sample = (gPrPamsCUOf(lsOamp)eluOsed) x 100%
The results are compiled in Table I.
, .
:,~
!.
:,'
:~ 1
, . ~
,2,.~
~`i
~ ~ 31,361-F -23-
~''
`: '
'.'..~
,,.,~,
.
,~:
~: .
- 24 - ~ 6~6g3-3795
TABLE :[
Copper R Ganque
Exam-
ple Collector _ _ K8 minl- R K
2 Blank2 0.16 2.7 O.l6 0.03 4,5
3 Z-]l 0.55 4.3 0.54 0.03 3.7
4 Sodium Aerofloat 0.55 4.6 0.54 0.03 4.1
AFT 2083 0.55 2.7 0.56 0.03 0.8
~-200 0.65 7.7 0.63 0.14 4.2
7 (cH3cH~NHcocHcH2s~2 0.62 4.5 0.62 0.07 3.7
S CH3
8 (CH3CH2NHC0CHcH2s~2 0.71 6.5 0.70 0.08 3.7
CH2CH3
g (cH3cH2cH2NHcolcHcH2s~2 0.67 7.1 0.66 0.15 4.6
CH3
(CH3CH2NHCSCHCH2S~2 0.55 4.8 0.52 0.04 4.8
0 CH3
11 (cH3cH2NHcsclHcH2s~2 0.65 4.40.65 0.04 3.6
CH2CH3
12 (CH3CH2NHCSCIHCH2S~2 0.54 3.80.53 0.54 4.1
CH2CH3
R 8 min is the actual recovery after 8 minutes.
2Blank means no collector has been added.
3AFT 208~ Trade-mark of American Cyanamid is a mixture of Na
diethyl dithiophosphate and Na di-sec-butyl dithiophosphate.
.
3 ~
-25-
Examples 2 6 are not embodiments of -this
invention.
Examples 13-22 - Experimental Procedure for Flotation
of Copper Sulfide Ores
Several of the disulfide carbamothioates of this
invention and prior art collectors are used for the flo-
tation of copper sulfide. The procedure for such flotation
is described hereinafter. The results are compiled in
Table II.
Procedure:
The flotation cell used is a container which
holds approximately 1.7 liters of deionized water, ore,
collector and frother. A rotating double-paddle is
provided for skimming t~e frother from the top of the
cell into a collecting tray. An air inlet is placed in
the bottom of the cell.
` Kennecott ore containing copper sulfide from the
Arthur Mill in Utah is preground to -10 mesh. Immediately
before floating, the ore is ground in a rod mill for an
additional period of time to obtain the desired mesh size.
', The process for this grinding is as follows. Eight rods
of one inch each are put in a rod mill along with 500 g of
~t ore, 1 g of NaCO3 and 333 g of deionized water. Lime is
added to adjust the pH to between 10.0 and 10.2, the
collector is added and the mixture is ground at 60 rpm for
about 5 minutes, until approximately 52 percent of -the
s~ particles had a size of less than 200 mesh.
Thereafter, the slurry is transferred to the
~t~ float cell as described hereinbefore. The frother, methyl
;~ 30 isobutyl carbinol (50 ~l) is added to the cell. Deionized
~ water is added to bring the water up to the desired level
;~
`~ 31,361-F -25-
. .
::j
,.,
. j .
,.. .
~,, 7~ ~;3
-26-
in the float cell. The mixture in the float cell is
stirred at 1050 rpm for 2 minutes to condition the ore.
After 2 minutes of s-tirring, the air flo~l o~ l9 ft3/hour
is started, with continued stirring, and a paddle
rotation of 12 rpm is starte~. Further water is added
to maintain the water level. The froth from the cell is
skimmed by the paddle into a collection tray. The froth
skimmed off is collected at intervals of 0.5, l.0, 2.0,
4.0 and 8-.0 minute~s. Each sample- is dried overnight in
a forced air oven at about 100C.
The samples are weighed and analyzed for copper
content by plasma emission spectroscopy.
The procedure for the analysis by plasma
emission spectroscopy is as follows. Into a lO0-ml flask
is placed 0.2 to 0.25 g of ore sample (approximately 2.0 g
if it is a tailings sample, the ore left in the cell after
flotation). To this is added 3.5 ml of concentrated hydro-
chloric acid and 5 0 ml of concentrated nitric acid. The
mixture is heated to boiling and boiled for 25 minutes,
and then allowed to cool. To this is added 25 ml of
deionized water. The mixture is heated to boiling then
allowed to cool. The mixture is filled to the volumetric
line. A plasma emission spectrometer (Spectrospan IV) is
used to determine the copper level in the solutions
prepared. The copper emission line at 2135.98 nm is found
to give a linear response with copper concentration. The
instrument is standardized by the use of copper solution
standards. When the sample solution is aspirated into the
plasma, the concentration in ppm of Cu is shown by the
instrument by digital display. This ppm of Cu is
converted into percent Cu in the original sample by the
following equation:
31,361-F -26-
:
. . .
-27-
% Cu in original sample = ((PrPmsCOU)(1amp~ used) x 100% .
The percent recovery and rate are calculated by
subs-tituting the weight of the copper and the time each
sample was taken into the e~uation described hereinbefore.
Table II demonstrates that the compounds of this
invention demonstrate activity comparable to -the activity
of collectors presently being used commercially. Further,
the preferred collectors of this invention can give
recoveries around 90 percent with rates of 9.0 or better.
,
31,361-F -27-
'' ':
- ~ .
,, .
~ . .
-28-
TABLE II
Copper
Exam- R 8
ple Collector CC1 R K min~ R K
13 sodium isopro- 0.02 0.90 12.4 0.90 0.093 15.2
pyl xanthate
14 potassium amyl 0.02 0.91 11.5 0.91 0.106 18.8
xanthate
15A-211 0.02 0.89 7.8 0.89 0.052 8.3
16Z-200 0.02 0.91 7.3 0.91 0.091 6.8
10 17Z-200 0.01 0.92 6.8 - 0.128 10.7
S
183.(RlNHCOCHCH2St2 0.05 0.90 9.8 0.90 0.096 11.2
R
193 (RlNHCOCHCH2S~2 0.025 0.89 10.7 0.90 0.089 12.6
R
S
203 (R NHcocHcH2st2 0.025 0.89 11.0 0.89 0.092 14.1
R
S
213 ~RlNHCOCHCH2S~2 0.015 0.88 9.0 0.88 0.091 12.1
R2
O
22 (RlNHCSCHCH2S~2 0.015 0.69 2.9 0.69 0.066 16.0
R
1Collector Concentration.
2R 8 min is the actual recovery after 8 minutes.
3R1 and R2 are CH3CH2 above.
.,; .
`
~ 31,361-F -28-
~.:
.
: