Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~2637~9
This invention relates to a method and apparatus
for the treatment of contaminated water, in particular
water which is contaminated by oxidizable substances such
as ferrous iron, ferric iron, manganese and hydrogen
sulphide.
The use of aeration processes in the removal of
contaminants from water is known, with the intended result
being the oxidation of such contaminants. Traditional
methods involve the use of either an open system, in which
the water i5 sprayed into the air or allowed to flow or
trickle down over cascades or similar obstructions; or a
closed system, in which air is forced under pressure into
a closed empty tank through which the water is flowing.
Other existing methods in general use a static mixer in
order to achieve the oxidation, which has the disadvantage
of low efficiency, and of requiring air venting to release
excess air. E~uipment required for such methods is
relatively complex to manufacture, and tends not to remove
a satisfactory quantity of contaminants.
United States Patent No. 3,521,752 (William
Edward Lindman) describes a water purification system in
which a gaseous mixture containing sulfur dloxide and
oxygen i~ passed through the water which is then violently
agitated. The apparatus include~ means for adding scrap
lron to the water being treated.
United States Patent No. 3,649,53Z ~John Oliver
McLean) discloses a method of treating water in a one-
tank system, to remove iron and reduce acidity, by adding
air to the water to precipitate iron and raising the pH of
the water while filtering iron hydroxide therefrom by
passing air and water thus mixed through a mineral bed.
United States Patent No. 4,430,228 ~aurence 0.
Paterson) relates to the removal of iron compounds from
water by aerating the water in an in~ection-mixer,
maintaining the aerated water under pressure and then
passing the water through particulate material having a
surface charge capable of attracting and removing
dispersed iron hydrates from the water.
12~37~
United States Patent No. 4,534,867 (Edward D.
Kreusch et al) desçribes a similar system for removing
iron or other chemically reducing substances from potable
water, including a tank containing a bed of activated
5carbon. Untreated water is first aerated and then
permitted to flow through the bed to permit the activated
carbon to provide a catalytic action and oxidize the iron
in the water. The precipitated oxidized particles are
retained in the bed by a filtration effect thereof.
10United States Patent No. 4,695,3~8 (Terry E.
Ackman et al) discloses a water aeration and treatment
system in which a ~et pump is employed to effect aeration
of the water to be treated, using atmospheric air. The
water discharged from the jet pump then enters a static
15mixer to provide further aeration of the waste water.
Other methods of removal of contaminants require
the use of chemicals, which gives rise to the
disadvantages of cost and of environmental or other
problems. Most existing systems require regular time
20consuming maintenance for their operation.
An object of this invention therefore is to
provide a method of removal of contaminants from water
which has increased efficiency, little maintenance, avoids
chemical use, and is simple to construct and operate.
25Accordingly, one aspect of the invention
provides a method for the treatment of contaminated water,
which comprises:
(1) introducing a predetermined quantity of
oxygen containing gas, such as air, oxygen or a mixture
30thereof, into the water to be treated;
(2) maintaining the gas and water under
pre~sure and applying centrifugal force thereto causing
carbonation thereof facilitating the oxidation of
contaminants in the water and resulting in precipitation
35of the contaminants in particulate form;
(3) separating said oxidized particles from the
water by filtration; and
(4) recovering the treated water.
1:~6~7~;~
Another aspect of the invention provides an
apparatus ~or the treatment of contaminated water
comprisina a mixing chamber. a water input line
communicating with said chamber, pump means capable of
introducing oxygen-containing gas into said chamber,
pressure means for maintaining the contents of said
chamber under pressure. mixer means capable of application
of centrifugal force to a pressurlzed water and gas
mixture in said chamber so as to produce a carbonation to
effect oxidation of contaminants in the water, filtration
means capable of separating precipitated particulate
conta~inants from the water after the application of
centrifugal force thereto, and means for recover~ng the
treated water.
The chemlcal reaction which takes place during
the oxidation process may be illustrated by the following
equation:
2 Fe tHC08)2 + ~2 2 Fe (OH)3 particles + 4 ~2 ga8
This oxidation takes place rapidly at a pH of
about 6.5 and almost immediately in the higher pH range of
normally alkaline natural water. The end product is
ferric hydroxide which in particular precipltates easily,
settles in a retention chamber or can be filtered out by
normal sediment filtration. The carbon dioxide being a
26 gas, when released, escapes into the atmosphere. The
resulting pH of the water is, of course, higher, and thus
corrosion by carbonic acid is reduced.
In the above chemical equation, it can be seen
only one atom of oxygen is needed to oxidize two atoms of
ferrous iron, with a valence of 2, to ferric iron, with a
valence of 3. The atomic weight of oxygen is 16, that of
iron 55.8, thus, 16 ppm of oxygen will oxidize 2 x 55.8 =
111.6 ppm of iron, or 1 ppm of ferrous iron will require
only 1/7 ppm of oxygen to oxidize it. Water in contact
with air will dissolve 12.5 ppm of oxygen at 5C, 10.2 ppm
at 15C, and 8.4 ppm at 25C. The lower the temperature,
the higher the solubility. Water saturated with even 8.4
ppm of oxygen can oxidize about 8.4 x 7 = 58.8 ppm of
~.2~i376~
ferrous iron found in any but the most exceptional
normally alkaline waters.
Thus, the invention utilize~ an in-line air
inducer using the water line pressure to draw room air
into the water stream containing the iron, manganese, and
H2S, thus achieving an air-water mix and oxidation. The
oxidation is then further expanded, enhanced and
accelerated by means of a centrifugal in-line mixer
resulting in a final air-water mix solution.
Because of this, there is no excess of air in
the system, and the oxidation is lOOX complete. There are
many ways of introducing air into the water stream
including through venturi or air compression. ~he pre~ent
8y8tem does not rely on the manner of introducing the air,
but on how to attain total mixing of the air with every
portion of water to achieve the complete oxidation
process.
For example, the use of the system of the
invention enables recovery of treated water with
oxidizable contaminants removed to a level of less than
0.1 mg/l of iron, less than 0.05 mg/l of manganese, and
hydrogen sulfide at a non-detectable level, commencing
from contaminated water, for example containing dissolved
iron in an amount of about 40.0 mg~l, dissolved manganese
in an amount of about 8.00 mg/l and a hydrogen sulfide
content of about 3.0 mg/l.
~mbodiments of the invention will now be
described, by way of example, with reference to the
accompanying drawings, in which:
Figure 1 show~ a schematlc partly cros~
sectional view of an apparatus embodyin~ the invention;
and
Figure 2 shows a partly cross sect ional view of
an embodiment of mixer.
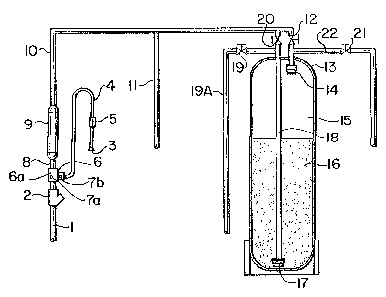
Referring now to Figure 1, the apparatus
comprises an input supply conduit 1 leading to a strainer
2, which prevents particles larger than about 1 mm from
pa~sing. The strainer 2 leads, in turn, to an air
1263~9
injector 6 attached by suitable means to the conduit 1.
The in~ector 6 can be any suitable pump means, but
preferably includes a venturi 6A, and has connected
thereto ~n air input conduit 4 leading from a ~creen 3.
An air flow monitor 5 i5 attached to the conduit 4. At
the air injector 6, ad~ustment ~crews ~A and ~B provide
adJustment means for the air and water flow~. From the
in~ector 6, a conduit 8 leads to a centrifugal mixer 9,
which is described in greater detail below.
From the mixer 9, conduit 10 leads to a
filtration unit 13, the entry thereto being regulated by
valves 12 and 20. The conduit 10 terminate~ in~ide the
filtration unit 13 ~n an upper screen 14, located in a
free zone 15 within the filtration unit 13. In the lower
portion of the unit 13, filter medium 16 i8 d~posed. The
medium 16 can be any suitable catalytic filter, but is
preferably BI~M , which is manufactured by Clack
Corporation of Windsor, Wi~consin, USA, or a catalytic
sand. Other suitable filter material~ include, silica
sand, aridsorb, activated carbon, greensand and Filter-
Ag . From a point near the bot~om of ~he unit 13, a
conduit 18, havin~ an entry screen 1~, leads upwards
through the unit 13 to an exit point at-~he top thereof,
and thence through valve 19 and conduit l9A to a water
outlet ~not shown).
The embodiment shown i8 also provided with
reverse flow means, by which the filtration unit can be
periodically cleansed and restratified. For this purpose,
a water ~upply conduit 11 leads into the conduit 10 and
thence to valve 20 which can function as an input means to
the conduit 18 within the filtration unit 13. Conduit 22
can be used as an exit mean~ controlled by valve 21 to any
suitable waste disposal means (not shown), such as a
drain.
*Trademark
f ~
~.263769
Referring now to Fi~ure 2, a centrifugal mixer 9
is shown which compri~es a cylindrical main tube 23 having
an input end 28 and an output end 27. A spiral element 26
ls contained within the main tube 23 along substantially
the entire len~th thereof. The main tube 23 ~s encased in
a cylindrical sleeve 24, with endcaps 25. The spiral
element 26 preferably has an angle of deflection within
the range of 2~ to 89 degrees, in order to achieve the
best results from the centrifugal force produced by the
element 26 on the water and air passing through the mixer
9. The function of the apparatus and its method of
operation will now be further de~cribed.
Water which is contaminated by contaminants,
such a~ ferrous iron, ferric iron, manganese and hydrogen
sulphide, i8 pumped into conduit 1 and through the
strainer 2 which separates out any relatively large solid
particles having a particle size of about 1 mm or larger.
Room air entering through the screen 3 and monitored by
the monitor 5 pa~ses through air conduit 4 into the alr
injeotor 6, whlch can be any suitable pump means, but is
preferably an air pump, a ~et pump or venturi ~eans. In
the embodiment shown, the water flow i~ regulated by an
adjustment screw 7A which can provide or prevent a bypass
channel around a venturi 6A. The flow of water regulates
the ~uction created by the venturi 6A, cau~ing air to
enter from conduit 4. The desired air flo~ can be
achieved by regulating the ad~ustment crew ~B.
The air and water then pass throu~h conduit 8
into the mixer 9, entering by input conduit 28 and fl~wing
around and through the spiral ele~ent 26 within the main
tube 23. The effect of the ~piral element 26 i8 to exert
a centrifugal force to the water and air which a~sists air
to dissolve in the water ~uch a~ to cause a carbonation
effect (i.e. a disperson of the oxygen-containing gas into
the water). As the mixer i8 pressurized, the carbonation
effect is maintained until the water i8 no lon~er under
pressure. The flow rate and the system pressure must be
lZ~376~
6a
sufficient to cause pressure loss over the spiral element
26.
The effects of the carbonation are of ~reat
si~nificance to the invention. Firstly, as the air is not
released from the mlxture until the pressure is released,
there is no necessity for air venting for any excess alr.
,.. ~
1;~637.~
Secondly, the fine division of the air and its even
distribution throughout the water maximize the contact of
contaminants with the air so as to achieve a high
efficiency of the oxidation process.
The result of the oxidation is to convert the
contaminants to precipitated particulate matter, which can
then be filtered out of the water. The water leaves the
mixer unit 9 by conduit 10 and passes to the filtration
unit 13, entering through opened valve 12. At this point
in the process, valves 20 and 21 are closed. The water
and particulate matter pass through upper screen 14 and
enter free zone 15 containing the air-water mix, in which
any remaining unoxidized contaminants can become oxidized.
The water then passes down through the filter
medium 16 to the lower portion of the filtration unit 13.
Such medium 16 operates to separate the particulate matter
and any unoxidized solid contaminants from the water. The
filtered water can then pass through screen 1~ and rise up
through conduit 18 and out of the filtration unit 13,
passing through opened valve lg to leave the system for
further use.
Periodically, the filter medium 16 may be
cleansed and restratified. For this purpose, a reverse
flow means can be provided, as shown in Figure 1.
To operate the reverse flow, the valves 12 and
10 are closed, and the valves 20 and 21 are opened. Water
is then pumped from a supply (not shown) through conduit
11 and thence into the filtration unit 13 through valve 20
and conduit 18 to the lower portion of the unit 13. The
water then rises through the medium 16, cleansing it and
restratifying it, before being directed out of the unit 13
by means of conduit 22 through valve 21 to a waste
disposal means (not shown), such as a drain. This reverse
flow process can be provided as an automatic process.
It should further be noted that the method and
apparatus are suitable for use in respect of any
contaminants which can be axidized by air, in addition to
more common contaminants specifically mentioned herein.