Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-19-
WE CLAIM:
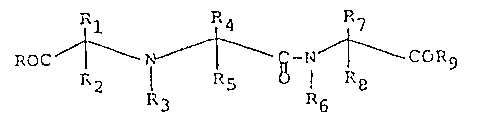
1. Process for the preparation of compounds of the formula:
<IMG> I
wherein
R and R9 are independently hydroxy, lower alkoxy,
lower alkenoxy, di(lower alkyl)amino-lower alkoxy, hydroxy-
lower alkoxy, acylamino-lower alkoxy, acyloxy-lower alkoxy,
aryloxy, aryloxyl-lower alkoxy, amino, lower alkylamino,
di-lower alkylamino, hydroxyamino, or aryl-lower alkylamino,
R1, R2, R3, R4, R5, R7, and R8 are independently H
alkyl, alkenyl or alkynyl containing up to 20 carbon atoms,
aryl or aryl-lower alkyl containing up to 12 carbon atoms,
heterocyclic or heterocyclic-lower alkyl containing up to
12 carbon atoms, or cycloalkyl or cycloalkyl alkyl containing
up to 20 carbon atoms.
R6 is heterocyclic or heterocyclic alkyl;
R2 and R3 taken together with the carbon and
nitrogen to which they are respectively attached and R3
and R5 taken together with the nitrogen and carbon to which
they are respectively attached form an N-heterocycle containing
from 3 to 5 carbon atoms or 2 to 4 carbon atoms and a sulfur
atom,
and wherein said alkyl, alkenyl, and alkynyl
groups can be substituted with hydroxy, lower alkoxy, thio,
lower alkylmercapto, amino, lower alklamino, di(lower alkyl)
amino, halogen, and nitro;
-20-
aim 1 - continued
comprising
A. reacting under amide-forming conditions an amino compound
of the formula
<IMG> II
wherein R6, R7, R8 and R9 are as defined above, with an acylating.
derivative of an acid of the formula
<IMG> III
wherein R, R1, R2, R3, R4 and R5 are as defined above;
B. reducing the corresponding imine; or
C. reacting a compound of the formula
<IMG> VI
wherein R, R1, R2, R3, R4, R5 and R6 are as above defined, with
a halo compound of the formula
<IMG>
wherein R7, R8 and R9 are as above defined,
-21-
and if desired, before forming a salt, reacting a
product obtained to form a product (wherein the R groups
are defined as above) wherein one or more R groups are
converted from one value of the R groups to another
value of the R groups, and converting a product obtained
if desired into a salt thereof.
2. Process as in Claim 1 wherein
R and R9 are independently hydroxy and lower
alkoxy,
R1 and R2 are independently hydrogen, lower alkyl,
aryl- lower alkyl having from 7 to 12 carbon atoms, or
heterocyclic- lower alkyl having from 6 to 12 carbon atoms,
R3, R4, R5, R7 and R8 are hydrogen or lower alkyl,
and
R6 is heterocyclic or heterocyclic alkyl;
3. Process as in Claim 2, wherein R6 is
pyrrole, pyrroline, pyrrolidine, pyridine, di-hydropyridine,
piperidine, morpholine, thiamorpholine, imidazole, imidazoline
imidazolidine, furan, furfuryl, thiophene, benzimidazole,
thiazole, thiazoline, thiazolidine, indole, quinoline, iso-
quinoline, tetrahydroquinoline, or tetrahydroisoquinoline.
4. Process as in Claim 3, wherein the
heterocyclic groups have 1 or more substituents selected
from the group consisting of lower alkyl, lower alkenyl,
lower alkynyl, hydroxy, lower alkoxy, amino, lower alkyl-
amino, di(lower alkyl)amino, thiol, lower alkylmercapto,
hydroxy-lower alkyl, amino-lower-alkyl, thio-lower alkyl,
nitro, halogen, trifluoromethyl, methylenedioxy, ureido
or guanidino.
-22-
5. Process as in claim 1, wherein R1 is lower alkyl
or phenyl-lower alkyl, R4 is lower alkyl, and R2, R3, R5, R7
and R8 are hydrogen.
6. Process as in claim 1, wherein R and R9 are
hydroxy.
7. Process as in claim 1, wherein R is ethoxy and
R9 is hydroxy.
8. Process as in claim 1, wherein R1 and R4 are
each methyl.
9. Process as in claim 1, wherein R1 is benzyl
or phenethyl and R4 is methyl.
10. Process as in claim 1, wherein R6 is indolyl-
ethyl.
11. Process as in claim 1, wherein R1 is benzyl.
12. Process as in claim 1, wherein R1 is phenethyl.
13. Process as in claim 1, wherein R is ethoxy,
R1 is phenethyl, R4 is methyl, R6 is 2-(3-indolylmethyl) and
R9 is hydroxy.
14. Process as in claim 1, wherein R2, R3, R5, R7
and R8 are each hydrogen.
15. Process as in claim 1, wherein R is ethoxy,
R1 is phenethyl, R4 is methyl, R6 is 3-pyridylmethyl and R9
is OH.
16. Process as in claim 15 wherein R2, R3, R5, R7
and R8 are each hydrogen.
-23-
17. Compounds of the formula
<IMG>
wherein
R and R9 are independently hydroxy, lower alkoxy,
lower alkenoxy, di(lower alkyl)amino-lower alkoxy, hydroxy-
lower alkoxy, acylamino-lower alkoxy, acyloxy-lower alkoxy,
aryloxy, aryloxyl-lower alkoxy, amino, lower alkylamino,
di-lower alkylamino, hydroxyamino, or aryl-lower alkylamino,
R1, R2, R3, R4, R5, R7, and R8 are independently H
alkyl, alkenyl or alkynyl containing up to 20 carbon atoms,
aryl or aryl-lower alkyl containing up to 12 carbon atoms,
heterocyclic or heterocyclic-lower alkyl containing up to
12 carbon atoms, or cycloalkyl or cycloalkyl alkyl containing
up to 20 carbon atoms.
R6 is heterocyclic or heterocyclic alkyl,
R2 and R3 taken together with the carbon and
nitrogen to which they are respectively attached and R3
and R5 taken together with the nitrogen and carbon to which
they are respectively attached form an N-heterocycle containing
from 3 to 5 carbon atoms or 2 to 4 carbon atoms and a sulfur
atom,
and wherein said alkyl, alkenyl, and alkynyl
groups can be substituted with hydroxy, lower alkoxy, thio,
lower alkylmercapto, amino, lower alklamino, di(lower alkyl)
amino, halogen, and nitro;
and salts and pharmaceutically acceptable acid
and base salts thereof,
-24-
18. A compound of the formula
<IMG>
wherein:
R and R9 are independently hydroxy and lower
alkoxy,
R1 and R2 are independently hydrogen, lower alkyl,
aryl- lower alkyl having from 7 to 12 carbon atoms, or
heterocyclic- lower alkyl having from 6 to 12 carbon atoms,
R3, R4, R5, R7 and R8 are hydrogen or lower alkyl,
and
R6 is heterocyclic or heterocyclic alkyl; and salts
thereof with pharmaceutically-acceptable acids and bases.
19. Compound according to claim 18 wherein
R6 is pyrrole, pyrroline, pyrrolidine, pyridine, di-hydro-
pyridine, piperidine, morpholine, thiamorpholine, imidazole,
imidazoline, imidasolidine, furan, furfuryl, thiophene,
benzimidazole, thiazole, thiazoline, thiazolidine, indole,
quinoline, isoquinoline, tetrahydroquinoline, or tetra-
hydroisoquinoline and pharmaceutically acceptable acid
and base salts thereof.
-25-
20. Compound according to claim 19 wherein the
heterocyclic groups have 1 or more substituents selected
from the group consisting of lower alkyl, lower alkenyl,
lower alkynyl, hydroxy, lower alkoxy, amino, lower alkyl-
amino, di(lower alkyl)amino, thiol, lower alkylmercapto,
hydroxy-lower alkyl, amino-lower alkyl, thio-lower alkyl,
nitro, halogen, trifluoromethyl, methylenedioxy, ureido
or guanidino , and pharmaceutically acceptable acid and
base salts thereof.
-26-
21. A compound according to claim 17, wherein R1
is lower alkyl or phenyl-lower alkyl, R4 is lower alkyl, and
R2, R3, R5, R7 and R8 are hydrogen, and pharmaceutically
acceptable acid and base salts thereof.
22. A compound according to claim 17, wherein R
and R9 are hydroxy and pharmaceutically acceptable acid and
base salts thereof,
23. A compound according to claim 17, wherein R
is ethoxy and R9 is hydroxy, and pharmaceutically acceptable
acid and base salts thereof.
24. A compound according to claim 17, wherein R1
and R4 are each methyl, and pharmaceutically acceptable acid
and base salts thereof.
25. A compound according to claim 17, wherein R1
is benzyl or phenethyl and R4 is methyl, and pharmaceutically
acceptable acid and base salts thereof.
26. A compound according to claim 17, wherein R6
is indolylethyl, and pharmaceutically acceptable acid and base
salts thereof-
27. A compound according to claim 17, wherein R1
is benzyl, and pharmaceutically acceptable acid and base salts
thereof.
-27-
28. A compound according to claim 17, wherein R1
is phenethyl,and pharmaceutically acceptable acid and base
salts thereof.
29. A compound according to claim 17, wherein R
is ethoxy, R1 is phenethyl, R4 is methyl, R6 is 2-(3-indolyl-
methyl) and R9 is hydroxy, and pharmaceutically acceptable
acid and base salts thereof.
30. A compound according to claim 17, wherein R2,
R3, R5, R7 and R8 are each hydrogen, and pharmaceutically
acceptable acid and base salts thereof.
31. A compound according to claim 17, wherein R
is ethoxy, R1 is phenethyl, R4 is methyl, R6 is 3-pyridylmethyl
and R9 is OH, and pharmaceutically acceptable acid and base
salts thereof.
32. A compound according to claim 17, wherein R2,
R3, R5, R7 and R9 are each hydrogen, and pharmaceutically
acceptable acid and base salts thereof.
33. Process according to claim 1, wherein
R2, R3, R5, R7 and R8 are each hydrogen,
R1 is methyl, benzyl or phenethyl,
R4 is methyl,
R6 is indolylethyl, 2-(3-indolylmethyl) or 3-pyridyl-
methyl, R9 is hydroxy, and
R is hydroxy or ethoxy.
-28-
34. A compound according to claim 17, wherein
R2, R3, R5, R7 and R8 are each hydrogen,
R1 is methyl, benzyl or phenethyl,
R4 is methyl,
R6 is indolylethyl, 2(3-indolylmethyl) or 3-pyridyl-
methyl, R9 is hydroxy, and
R is hydroxy or ethoxy,
and pharmaceutically acceptable acid and base salts.
-29-
35. Process according to claim 1 wherein R2, R3,
R4, R7 and R8 are hydrogen, R9 is hydroxy, R is C2H5O, R1 is
<IMG>, R5 is -CH3, and R6 is
<IMG> or <IMG>,
36. Compound according to claim 17 wherein R2, R3,
R4, R7 and R8 are hydrogen, R9 is hydroxy, R is C2H5O, R1 is
<IMG>, R5 is -CH3, and R6 is
<IMG> or <IMG>
and pharmaceutically acceptable acid and base salts thereof.
37. Process according to claim 1 wherein R2, R3,
R4, R7, and R8 are hydrogen, R9 is hydroxy, R is C2H5O, R1 is
<IMG> C2H4, R5 is-(CH2)4NH2 and R6 is <IMG>,
38. Compound according to claim 17 wherein R2, R3,
R4, R7, and R8 are hydrogen, R9 is hydroxy, R is C2H5O, R1 is
<IMG> C2H4, R5 is-(CH2)4NH2 and R6 is <IMG>.
and pharmaceutically acceptable acid and base salts thereof.
39. Process according to claim 1 wherein R2, R3,
R4, R7 and R8 are hydrogen, R9 is hydroxy, R5 is CH3, R1 is
<IMG>, R is C2H5O or HO, and R6 is <IMG>.
40. Compound according to claim 17 wherein R2, R3,
R4, R7 and R8 are hydrogen, R9 is hydroxy, R5 is CH3, R1 is
<IMG>, is C2H5O or HO, and R6 is <IMG>.
and pharmaceutically acceptable acid and base salts thereof.
41. A pharmaceutical composition comprising a compound of
claim 17, 18, or 19, together with a pharmaceutically acceptable
carrier therefor.
42. A pharmaceutical composition comprising a compound of
claim 20, 21 or 22, together with a pharmaceutically acceptable
carrier therefor.
43. A pharmaceutical composition comprising a compound of
claim 23, 24 or 25, together with a pharmaceutically acceptable
carrier therefor.
44. A pharmaceutical composition comprising a compound of
claim 26, 27 or 28, together with a pharmaceutically acceptable
carrier therefor.
45. A pharmaceutical composition comprising a compound of
claim 29, 30 or 31, together with a pharmaceutically acceptable
carrier therefor.
46. A pharmaceutical composition comprising a compound of
claim 32, 34 or 36, together with a pharmaceutically acceptable
carrier therefor.
47. A pharmaceutical composition comprising a compound of
claim 38 or 40 together with a pharmaceutically acceptable
carrier therefor.
31
48. A compound of the formula:
<IMG>
wherein
Rg is hydroxy, R2, R3, R4, R7, and R8 are hydrogen,
R6 is pyrrolidine, R is ethoxy, and R1 is phenethyl, and salts
thereof with pharmaceutically acceptable acids and bases.
49. Use of the compound of Claim 17, 18 or 19, as a
hypertensive and angiotensin inhibitory agent.
50. Use of the compound of Claim 20, 21 or 22, as a
hypertensive and angiotensin inhibitory agent.
51. Use of the compound of Claim 23, 24 or 25, as a
hypertensive and angiotensin inhibitory agent.
52. Use of the compound of Claim 26, 27 or 28, as a
hypertensive and angiotensin inhibitory agent.
53. Use of the compound of Claim 29, 30 or 31, as a
hypertensive and angiotensin inhibitory agent.
54. Use of the compound of Claim 32, 34 or 36, as a
hypertensive and angiotensin inhibitory agent.
55. Use of the compound of Claim 38, 40 or 48, as a
hypertensive and angiotensin inhibitory agent.
32