Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
:~6643~3
1052-011-0
258/
TITLE OF THE INVENTION
THERAPEUTIC APPLICATION OF N-SUBSTITUTED
2-AMINOMETHYLENE-1,3-INDANEDIONES
BACKGROUND OF THE INVENTION
Field of the Invention:
The present invention relates to N-substituted
2-aminomethylene-1,3~indanediones and their therapeutic
application.
Discussion of the Background:
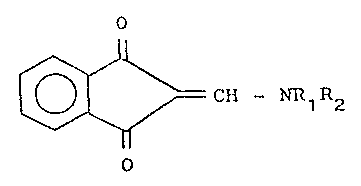
The present invention relates to prod~cts having
the structural formula
~ ~ CH - NR~R2 (I)
in which
Rl is a hydrogen atom or a Cl_6 group, such as,
for example, methyl or ethyl,
R2 is a straight or branched cyclopropylmethyl,
2-furyl methyl, benzyl, phenyl or Cl_4 alkyl group,
such as, for example, methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, tertiary butyl; the NRlR2 group can
~1~
~6643~3
--2--
also represent a nitrogenous heterocyclic compound
N(CH2)n, in w~ich n can be 4, 5 or 6.
The products of the above structural form~la have
been previously described in the l:iterature. Studies
reported by Gasjuna, L. et al. in Latv. P.S.R. Zinat.
Akad. Vest. Kim. Ser., 1980, 1:98-L01, can be cited by
way of example. ~owever, no therapeutic application
has been described for these derivatives.
There is a strongly felt need in the medical art
for compounds possessing good pharmacological
properties. For example, products having anti-
inflammatory, diuretic, bronchial dilating,
anticholinergic, antispasmodic and antidepressant
properties are much needed in medicine.
SUMMARY OF THE INVENTION
Accordingly, it is an object of this invention to
provide a novel pharmaceutical composition.
It is another object of this invention to provide
a novel pharmacological composition which possesses
anti-inflammatory propertiesO
It is another object of this invention to provide
a novel pharmacological composition which possesses
diuretic properties.
It is another object of this invention to provide
a pharmacological composition possessing bronchial
dilating properties.
~iG~3~
--3--
It is another object of this invention to provide
a novel pharmacological composition w~ich possesses
anticholinergic properties.
It is another object of -this invention to provide
a novel pharmacological composition which possesses
antispasmodic properties.
It is another object of this invention to provide
a novel pharmacological composition which possesses
antidepressan-t properties.
It is another object of this invention to provide
a novel pharmacological composition which possesses
anti-inflammatory, diuretic, bronchial dilating,
anticholinergic, antispasmodic or antidepressant
properties and which may be used in human or veterinary
therapy.
In is another object of this invention to provide
a novel anti-inflammatory treatment which comprises
administering an effective amount of the novel
pharmaceutical composition of this invention.
It is another object of this invention to provide
a novel diuretic treatment which comprises
administering an effective amount of the novel
pharmaceutical composition of this invention.
It is another object of this invention to provide
a novel bronchial dilatin~ treatment which comprises
the administration of an effective amount of the novel
pharmaceutical composition of this invention.
~LZ66~38
It is another object of this invention to provide
a novel anticholinergic treatment which comprises the
administration of an effective amount of the novel
pharmaceutical composition of this invention.
It is another object of this invention to provide
a novel antispasmodic treatmen-t which comprises the
administration of an eEfective amount: of the novel
pnarmaceutical composition o~ this invention.
It is another object of this invention to provide
a novel antidepressant treatment which comprises the
administration of an effec~ive amount of t'ne novel
pharmaceutical composition of the present invention.
Applicants have surprisingly discovered that each
and every one of the above objects of this invention
have been satisfied with a novel pharmacological
composition containlng (1) a pharmaceutically
acceptable excipient, and (2) an effective amount of at
least one product having the structural formula
O
~ ~ ~ =CH - NR1R2 (I)
in which
Rl is a hydrogen atom or a Cl_6 alkyl group, such
as methyl or ethyl;
~Z6~ 3l3
R2 is a straight or branched cyclopropylmethyl,
2-furyl. methyl, benzyl, phenyl or Cl_4 alkyl group such
as, for example, methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, and tertiary butyl, the NRlR2 group
can also be a nitrogenous heterocyclic compound 1,
N(CH2)n, wherein n may be 4, 5 or 6.
The inventors have now discovered that the
products according to the formula (I) exhibit
pharmacological properties, viz~ of an anti-
inflammatory, diuretic, bronchial dilating,
anticholinergic, antispasmodic and-antidepressant
nature, which allows them to be used in human and
veterinary therapy.
DETAILED DESCRIPTIOR OF THE PREFERRED EMEODIMERT5
The present invention thus relates to a novel
pharmacological composition which comprises (1) a
pharmaceutically acceptable excipient, and (2) an
effective amount of at least one product having the
following structural formula
~ ~ =CH - NR1R2 (I)
~266~3~
--6--
where Rl is a hydrogen atom or a Cl_6 alXyl group, such
as methyl or ethyl; and R2 is a straight or branched
cyclopropylmethyl, ~-furyl methyl, benzyl, phenyl or a
Cl_4 alkyl group, such as, methyl, ethyl, propyl,
isopropyl, butyl, isobutyl, or tertiary butyl; and the
NRlR2 group can also be a nitrogenous heterocyclic
compound N(CH2)n, where n can be 4, 5 or 6.
In a preferred embodiment of this invention the
novel pharmacological composition is useful for the
treatment of edema or arterial hypertension.
In another preferred embodiment of this invention,
the novel pharmaceutical composition i5 useful for the
treatment of spasmodic condition.
In another preferred embodiment, the novel
pharmaceutical composition is useful as a psychotropic
drug in the treatment of depressions.
In anot'ner preferred embodiment, the novel
pharmaceutical composition is useful for the treatment
of asthmatic conditions.
The novel pharmaceutical composition of this
invention is characterized by the fact that it contains
an effective amount of at least one product of
formula I in combination with a pharmaceutical or
veterinary vehicle or appropriate excipient.
The products of the present invention are
generally prepared by a reaction which involves
--7--
2-formyl-1,3-indanedione and an amine of the formula
HNRlR2~ in which Rl represents a hydrogen a-tom or a
Cl_6 alkyl group, such as methyl or ethyl: R2
represents a straight or branched cyclopropylmethyl,
2-furyl methyl, benzyl, phenyl or a Cl_4 alkyl group,
such as methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, or tertiary butyl. The NRlR2 group can also
represent a nitrogeneous heterocyclic compound N(CH2)n,
w~ere n may be 4, 5 or 6.
A variation of the preparation of these compounds
consists of reacting 2-formyl-1,3-indanedione and the
hydrochloride of the HNR1~2 amine defined above in the
presence of a hydracid acceptor, such as sodium
carbonate.
Other featues of the invention will become
apparent in the course of the following descriptions of
exemplary embodiments w~ich are given for illustration
of the invention and are not intended to limiting
thereof.
Example I
2-Methylaminomethylene-1,3-indanedione. (Product
of formula (I) in which Rl = H and R2 = CH3; code name
COR3726.)
~6~3~3
--8--
Preparation
A solution of 35 ml of ethyl orthoformate and
70 ml acetic anhydride is added to 25 g
1,3-indanedione. The mixture is heated to 80C for one
hour. The solution is filtered and immediately cooled
in an ice bath. A quantity of 130 ml of previously
boiled water is added while shaking; the solution is
monitored to ensure that the temperature remains at
10C, left to crystallize in the cold for ~ive hours,
and then filtered. The yield is 67~. The precipitate
is poured into 400 ml of boiling ethyl alcohol. The
mixture is main-tained in reflux for fi~e minutes and
then filtered into a previously heated flask. The
solution contains 20 g 2-formyl-1,3-indanedione and
must be used rapidly.
A solution of 1.2 g 2-formyl-1,3-indanedione in
50 ml ethyl alcohol is added to a methylamine solution
in excess in 2 ml acetic acid and 50 ml ethyl
alcohol. The yellow precipitate is filtered and washed
in cold ethyl alcohol. The yield is 86%.
Physiochemical Properties
Melting point: 254C (ethyl alcohol), sublimates.
Infrared spectrum: y(C0) bands at 1705 and
1650 cm~l.
NMR spectrum in DMS0 (d6): 9.5 ppm, singlet,
1 proton, NH; 8 ppm, singlet, 1 proton, CH; 7.73 ppm,
~Z~6~L3~3
multiplet, 4 protons, aromatic phthalyl hydrogen;
3.3 ppm, singlet, 3 protons, CH3.
Example II
2-Ethylaminomethylene-1,3-indanedione. Product of
formula (I) in which R1 = ~ and R2 = C2H5; code name
COR3755.
Preparation
A quantity of 4 ml of a 70~ aqueous ethylamine
solution is added to a solution of ~.4 g 2-formyl-1,3-
indanedione in 40 ml ethyl alcohol. The mixture is
heated slightly in a water bath for 15 minutes. After
cooling, the product formed precipitates. It is
recrystallized in ethyl alcohol. The yield is 86.7%.
Physiochemical Properties
Melting point: 170~C (ethyl alcohol).
NMR spectrum in CDC13: 9.33 ppm, singlet,
1 proton, ~H; 8 ppm, singlet, 1 proton, CH; 7.75 ppm,
multiplet, 4 protons, aromatic phthalyl hydride;
3.55 ppm, multiplet, 2 protons, CH2; 1.38 ppm, triplet,
3 protons, C~3-
~xample III
2-Anilinomethylene-1,3-indanedione. Product of
formula (I) in which Rl = H and R2 = C6~15; code name
COR3756.
~6643~ I
--1 o -
Preparation
Three grams of aniline is added to a solution of
2.4 g 2-formyl-1,3-indanedione in 40 ml ethyl
alcohol. The mixture is heated sl:ightly in a water
bath for 20 minutes. After cooling, the product formed
precipitates. It is recrystallized in ethyl alcohol.
The yield is 67.1%
Physiochemical Properties
Melting point: 197C (ethyl alcohol).
NMR spectrum in CDC13: 11 ppm, multiplet,
1 proton, NH, 8.38 ppm, singlet, 1 proton, H at 4 on
aniline; 8.13 ppm, singlet, 1 proton, CH; 7.77 ppm, I
multiplet, 4 protons, aromatic ,~hthalyl hydride;
7.38 ppm, multiplet, 4 protons, H at 2,3,5,6 on
anlllne .
Example IV
2-Isobutylaminomethylene-1,3-indanedione. Product
of formula (I) in which Rl = H and R2 = CH2CH(CH3)2,
code name COR3754.
Pre~aration
Three grams of isobutylamine is added to a
solution of 2 g 2-formyl-1,3-indanedione in 40 ml ethyl
alcohol. The The mixture is heated slightly in a water
bath for 10 minutes. The ethyl alcohol is removed by
evaporation and the desired product is recrystallized
in ethyl alcohol. The yield is 56~.
3~
Physiochemical Properties
Melting point: 149C (ethyl alcohol~.
NMR spectrum in CDC13 9.42 ppm, singlet,
1 proton, NH; 7~93 ppm, singlet, 1 proton, =CH;
7.77 ppm, multiplet, 4 protons, aromatic phthalyl
hydride; 3.3 ppm, duplet, 2 protons, CH2; 1.98 ppm,
multiplet, 1 proton, CH; 1.025 ppm, duplet, 6 protons,
c~3.
Example V
2-Isopropylaminomethylene-1,3-indanedione.
Product of formu~a (I~ in which Rl = ~ and R2 =
CH(CH3)2; code name COR3762.
Preparation
Two milliliters of isopropylamine is added to a
solution of 2.7 g 2-formyl-1,3-indanedione in 60 ml
ethyl alcohol. The mixture is heated in a water bath
for 30 minutes, The ethyl alcohol is removed by
evaporation and the desired product is recrystallized
in an isopropyl ether/ethyl alcohol (9/1) mixture and
then in ethyl alcohol. The yield is 60%.
Physiochemical Properties
Melting point: 122C (ethyl alcohol).
NMR spectrum in CDC13: 9.3 ppm, singlet,
1 proton, ~H; 8.05 ppm, singlet, 1 proton, =CH;
7.83 ppm, multiplet, 4 protons, aromatic ,bhthalyl
~2~ 3~
-12-
hydride; 3.77 ppm, multiplet, 1 proton, CH; 1.41 ppm,
doublet, 6 protons, CH3.
Example VI
2-Cyclopropylmethylaminomethylene-1,3-
indanedione. Product of formula (I) in which Rl = H
and R2 = CH2CH-CH2; code name COR3757.
Preparation CH
Three grams of cyclopropylmethylamine
hydrochloride and 2.9 g sodium carbonate are added to a
solution of 2.4 g 2-formyl-1,3-indanedione in 40 ml
ethyl alcohol. The mixture is heated in a water bath
for 30 minutes and then filtered in the presence o~
heat. After cooling, the product formed
precipitates. It is recrystallizea in ethyl alcohol.
The yield is 43%.
Physiochemical Properties
Melting point: 157C (ethyl alcohol).
NMR spectrum in CDC13: 9.47 ppm, singlet,
1 proton, ~H; 8 ppm, singlet, 1 proton, -CH; 7.78 ppm,
multiplet, 4 protons, aromatic phthalyl hydride;
3.37 ppm, doublet, 2 protons, CH2; 1.1 ppm, multiplet,
1 proton, CH; 1.7 ppm, multiplet, 2 protons, cyclical
CH2; 0.38 ppm, multiple, 2 protons, cyclical CH2.
;438
-13-
Example VII
2-(2-Furyl methylaminomethylene)-1,3-indanedione.
Product of ~ormula (I) in which Ul = H and R2 = ~2
code nama COR3758.
Preparation
A quantity of 2.7 g 2-furyl methylamine is added
to a solution of 2.4 g 2-formyl-1,3-indanedione in
40 ml ethyl alcohol. The mixture is heated in a water
bath for 20 minutes. It is subsequently filtered aEter
cooling. The precipitate is recrystallized in
dioxane. The yield is 40.4%.
Physiochemical Properties
Melting point: 216C (dioxane).
NMR spectrum in CDC13: 9.38 ppm, singlet,
1 proton, ~H; 7.97 ppm, singlet, 1 proton, CH;
7.77 ppm, multiplet, 4 protons, aromatic phthalyl
hydride; 7.5 ppm, multiplet, 1 proton, H at 5 on the
furyl nucleus; 6.45 ppm, multiplet, 2 protons, H a~. 3
and 4 on the furyl nucleus; 4.67 ppm, singlet,
1 proton, CH2; 4.53 ppm, singlet, 1 proton, C~?-
Example VIII
2-Piperidinomethylene-1,3-indanedione. Product of
formula (I) in which NRlR2 = ~(CH2)5; code name
COR3759.
3L;Z6~38
-14-
Preparation
A quantity of 2.5 g plperidine i3 added to a
solution of 2.4 g 2-Eormyl-1,3-indanedione in 40 ml
ethyl alcohol. The mixture is heated in a water bath
for 20 minutes. It is subsequently filtered after
cooling. The precipitate is recry~tallized in ethyl
alcohol. The yield is 44~.
Physiochemical Properties
Melting point: 167~C (ethyl alcohol).
NMR spectrum in CDC13: 7.77 ppm, multiplet, 4
protons, aromatic phthalyl hydride; 7.6 ppm, singlet, 1
proton, CH; 4.53 ppm, multiplet, 2 protons, CH2 at 2 or
6 on the piperidine nucleus; 3.67 ppm, multiplet, 2
protons, CH2 at 2 or 6 on the piperidine nucleus; 1.83
ppm, multiplet, 6 protons, CH2 at 3, 4 and S on the
piperidine nucleus.
Example IX
2-Dimethylaminomethylene-1,3-indanedione. Pro~uct
of formula (I) in which Rl - R2 = CH3; code name
COR3761.
Pre~aration
Five milliliters of a 25~ dimethylamine solution
in ethyl alcohol is added to a solution of 2.7 g
2-formyl-1,3-indanedione in 60 ml ethyl aLcohol. The
mixture is heated at approxi~ately 40C for four
669L38
hours. The solvent i8 removed by evaporation. The
precipitate i9 isolated, then recrystallized in a
mixture o isopropyl ~ther/ethyl alcohol (8/2). The
yiela is 58%.
PhysicochemicaL Properties
Melting point: 138C (ethyl alcohol).
MNR spectrum in CDC13: 7.8 ppm, multiplet, 4
protons, aromatic p~thalyl hydride; 7.6 ppm, singlet, 1
proton, =CH; 3.93 ppm, singlet, 3 proton, CH3;
3.42 ppm, singlet, 3 protons, CH3.
Example X
l-Perhydroazepinyl-2-methylene-1,3-indanedione.
Product of formula (I) in w~ich NR1R2 = N(CH2)6; code
name COR3790.
Preparation
Three milliliters hexamethylenimine is added to a
solution of 1.6 g 2-ormyl-1,3-indanedione in 40 ml
ethyl aIcohol. The mixture is 'neated in a water bath
form 60 minutes. The ethyl alcohol is partially
evaporated. The residue i8 refrigerated for 12
hours. The precipitate is filtered and then purified
by passing over a silica column using ethyl ether as
the eluent. The yield is 43%.
38
-16-
Physicochemical Properties
Melting point: 125C (ethyl alcohol).
IR spectrum: ~O bands at 1700 and 1650 cm-l
NMR spectrum in CDC13: 7.78 pprn, multiplet, 4
protons, aromatic phthalyl ~ydride; 7.63 ppm, singlet,
1 proton, CH; 4.50 ppm, multiplet, 2 ~rotons, CH2 at 2
or 7 on the perhydroazepinyl nucleus; 3.67 ppm,
multiple-t, 2 protons, CH2 at 3, 4, S and 6 on the
perhydroazepinyl nucleus.
The results of the toxicological and
pharmacological studies performed on the products
according to the present invention are reported below.
Toxicity
CoR3726, COR3754, COR3755, COR3756, CoR3757~
COR3758, COR3759, COR3751, and coR379o caused no deaths
in mice in an oral dose of 300 mg/kg or an
intraperitoneal dose of 200 mg/kg. When administered
to mice orally, dissolved in 20% TWEEN*, COR3761
exhibited an LD50 of 520 (457-592~ mg/kg.
Pharmacolo~y
Diuretic activity was determined in several
experimental models. In rats subjected to excess water
intake, CoR376 and COR3761, administered in an oral
dose of 20 mg/kg, increased urinary sodium excretion by
* Trademark
,
,
~6~
-17-
a factor of 4.4 and 3.5, respectively. Spironolactone
adminis-tered under -the same conditions and in the same
dose increased sodium excretion by 3.6. ~hen given
orally to rats subjected to excess sodium chloride
intake, COR3761 produced the following increments in
volumetric urinary excretion measured at the sixth
hour: 10~ in a dose oE 10 mg/kg, 42!.5% in a dose o~
20 mg/kg and 68.4% in a dose o~ 40 mg/kg. COR3761 was
administered to rats subjected to excess water intake
and volumetric urinary excretion and sodium and
potassium elimination were measured over a period of
six ~ours. These parameters were, respectively, -13%,
+73~, +77% af-ter administration of 10 mg/kg; +9%,
+241%, ~114~ after 20 mg/kg and -4%, +532~, +138% after
40 mg/kg. The sodium/potassium ratio which was 0.4 in
the control animals was 0.47 at 10 mg/kg, 0.62 at 20
mg/kg and 1.48 at 40 mg/kg. In the course of another
experiment, volumetric urniary excretion and sodium and
potassium elimination were, respectively: +1%, +100%,
+54% at 20 mg/kg; +10%, +313~, +69~ at 40 mg/kg and
+16%, ~356~ and +89% at 60 mg/kg. The sodium/potassium
ratio went from 0.43 in the control animals to 0.53 at
20 mg per kg, 1.02 at 40 mg/kg and 1.12 at j60 mg/kg.
Spasmolytic activity was determined in vitro by
measurement of t~e e:Efect of the test product on ileum
contractions in guinea pigs induced by transmural
~4~ 3~
-la-
electric transmission in an oxygenated Krebs solution
at 32aC. When used in a concentration of 0.5 mcg/ml,
COR3754 and COR3759 in~ibited these contractions by
50%. Domperidone ex~ibits ~he same activity in a
concentration of 1 mcg/ml~
Anti-inflammatory activity was determined in rats
by measurement of in~ibition oE the edema induc~d by
intraplantar injection oE 0.1 ml of a 1~ carragheenin
suspension. The test products were administered orally
one hour before the carragheenin injection and the
determination was made three hours after injection.
Doses of 100 mg/kg COR3755 and COR3761 produced 3G% and
49~ in~ibition, respectively. Aspirin administered
under the same conditions in a dose oE 150 mg/kg
produced 40% inhibition o the ede~a.
Bronchodilating activity was determined in vitro
by measuremsnt of the flow of Tyrode's solution through
an isolated and perfused guinea pig lung which had been
contracted by addition of 0.05 mcg/ml methacholine to
the perfusion liquid. CoR3755, CoR3757, COR3759,
COR3761, COR3762 and COR3790, which were used,
respectively, in concentrations of 50, 100, 10, 10, 25
and 100 mcg/ml, increased the flow by 50~.
Amino~hylline produces t'nis same efEect in a
concentration of 100 mcg/ml.
3~3
--19--
Anticholinergic activity was evaluated by
measuremPnt of the cont~actions inauced by 0.1 mcg/ml
acetylcholine ln isolated segments of guinea pig
ileum. When used in concentrations of 100, 50, 10, 50,
10 and 25 mcg/ml, respectively, CoR3755~ COR3757,
COR3761, COR3762 and C0~3790 produced an 80~ inhibition
of these contractions.
Central anticholinergic activity was evaluated by
measurement of inhibition, under the test product, of
muscular tremors induced in mice by ~ubcutaneous
injection of 0.5 mg/kg oxotremorine. The tremors are
rated according to an index ranging from O to 6, one
hour after intraperitoneal injection of the test
product. An index of O indicates absence of tremor
while 6 represents maximum tremor. COR3762
administered in a dose of 100 mg/kg resulted in an
index of 3; imipramine, given under the same conditions
in a dose of 25 mg/kg produced the same activity.
~ ntidepressant activity was determined in the test
involving potentiation of yohimbine toxicity and in the
test involving in~ibition of reserpine-induced ptosis.
One hour before oral administration of the test
product, a non-lethal dose of yohimbine, i.e.,
15 mg/kg-doses of COR3761 and COR3762 produced 80% and
100% mortality, respectively.
-20-
Mice were given an oral dose oE the test product
ninety minute~s beEore intraperitoneal injection of
5 mg/kg dissolved reserpine. Ptosis was evaluated
according to an index oE 0 to 6, ~here 0 signifies
ptosis and ~ indicates absence of pto~is. Under the
experimental conditions, COR3761 and COR3762 in doses
of 200 and 50 mg/kg, respectively, produces respective
ratings of 5 and 4.
In view oE their p~armacological activity, which
is associated with low toxicity, the products of the
present invention can be used in human or ve-terinary
therapy.
~ len combined with -the conventional vehicles, they
can e.g., be utilized in -the treatment of cardiac,
renal or hepatic edema and arterial hypertension, in
the -treatment of spasmodic symptoms, especially of the
digestive and respiratory systems, in the treatment oE
asthma conditions or for depressive states.
When combined with the conventional vehicles,
depending on the indication, they can be administered
e.g., by the oral route in the form of dragees, tables,
syrups, vials; rectally in the form of suppositories:
by -the intramuscular route or the intravenous route.
According to the indication and the subject, dodes
administered range ~rom 1-100 mg/day given in one to
six administrations orally, from 1-100 mg/day given in
~166~3~3
-21-
one or two admini~trations rectally and from 0.5-50 mg
given by parenteral injection.
Obviously, numerous modificatiolls and variation~
of the present invention are possible in light to the
above teaching.s. It is therefore to be understood that
within the scope of the appended cla:ims, the inv~ntion
may be practiced otherwise than as specifically
described herein.