Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~6~
TITLE OF THE INVENTION
TREATMENT OF SOLIDS CONTAINING CALCIUM
SALT OF SULFURIC OXYACID AND METHOD OF
PURIFYING EXHAUST GAS UTILIZING SAME
FIELD OF THE INVENTION
The present invention relates to a dry method of
trea-ting solids containing the calcium salt of a sulfuric
oxyacid such as gypsum, and to a process utili~ing -the
method for purifying exhaust gases from boilers or furnaces
such as incinerators by efficiently removing harmful acid
subs-tances from the yas.
BACKGROUND OF THE INVENTION
A dry method is already proposed oi purifying
the exhaust gas from a boiler or waste incinerator or like
furnace by dispersing particles of a Ca-type absorbent
(quick lime, slaked lime, limestone, dolomite or the l:ike)
directly into the furnace or flue to cause the absorbent
parti.cles to absorb harm-ful ac.id substarlces, such as sulfur
oxicles (SOx), Erom the cJaS fo.r removal. With this method,
the absorbent. partlcles a:re part.:Ly convertecl to cJypsum
(CaSO~I) by the rcact:ion w:ith thc suleur oxides and collected
by a clust collecto.r aloncJ with other solid particles as
soot and clust. q'he soot and dust obtained, although
containi.nc3 cJypsum, further contain fly ash and unreacted
.
~l _
"
~ .
.
~6~
absorbent components and therefore are not usable for
preparing gypsum board or cement for which gypsum is
generally used. Fur-ther if the furnace is large-sized,
the soot and dust, which are produced in large quantities,
can not be discarded directly, since various problems will
then result. Further if the gypsum obtained has a relatively
high purity and is useful, problems will be encountered in
treating the gypsum in the event of excess supply.
Aeeordingly, methods have been proposed whieh
inelude one eomprising ealeining gypsum in a kiln in the
presenee of an insufficien-t amount of air to obtain quick
lime and a yas eontaining SO2 and reeovering sulfurie aeid
from the SO2, and a me-thod eomprising eonverting gypsum to
ealeium sulfide in a redueing atmosphere in a kiln with
a limited supply of air, preparing an aqueous slurry ~rom
the resulting ealeium sulEide aEter pulverization and passlng
earbon dioxide through the slurry to eolleet hydrogen sulEide.
~ owever, the eonventional methocls not only
require a larcJe-slzed apparatus hut also :invo;Lve uneven
heat:ing by ~he kiln to render the reswlting c~uick lime or
ea:lcium su:lE:lde less reactive ow:ing to aceelerated crystal-
:li.zation ancl ea-lse trouble to the subsecluent treatment.
Moreover, the rnethocls are unsatisfaetory Erom the viewpoint
oE savings in energy.
~5 SUMMARY OF THE INVENTION
,
~ .
An object of the present invention is to overcome
the foregoing problems heretofore encount~red.
According to a first aspect of the invention, thare
is provided a dry method of treating solids containing the
calcium salt of a sulphuric oxyacid comprising feeding to a
reduction reactor a reducing gas stream containing hydrogen
and~or carbon monoxide and dry particles containing the calcium
salt of sulphuric oxyacid and ha~ing a mean particle size of up
to 10 microns, reducing the calcium salt to calcium sulphide at
700 to 900~C in the reduction reactor, feeding the gas stream
entraining the produced calcium sulphide obtained ~rom the
reduction reactor to a carbonation reactor, cooling the gas
stream entraining the calcium sulphide to 300 to 500C in the
carbonation reactor to react in a dry state the calcium
sulphide with carbon dioxide and water vapour and produce
calcium carbonate and hydrogen sulphide, yuiding the resulting
gas stream ~rom the carbonation reactor into a dust collecting
unit to collect solid particles, separating the hydrogen
sulphide ~rom the gas stream ~lowing out from the dust
collecting unit, then ~eeding back to the reduction reactor the
remaining gas containing hydrogen, carbon monoxide and carbon
dioxide as a carrier yas ~or Eeedi.ng the dry particle~ into the
reductlon reactor.
The above method is not limited only to the treatment
o~ gyp~um resultincJ ~rom the purif.ication o~ exhaust gases but
is use~ul also eor solids containiny the calcium salt of any
sul:Euric oxyacid capable of.giving calcium sulfide upon
reduction.
--3--
~1
t~
.
. .
Examples of calcium salts of sulfuric oxyacids are,
in addition to gypsum (calcium sul~ate), calcium sulfite
(CaSo3), calcium thiosulfate (CaS203) and calcium polythionate
( CaSX6 ) -
According to a second aspect of the invention, there
is provided a dry method of purifying an exhaust gas containing
sulphur oxides by spraying dry particles of a Ca-type absorbent
into the exhaust gas to cause the absorbent to absorb the
sulphur oxides and thereafter collecting by a dust collecting
unit the absorbent particles along with other particles
contained in the exhaust gas, the method being characterised by
feeding to a reduction reactor the used absorbent particles
collected by the dust collecting unit and having a mean
particles size of up to 10 microns, and reducing gas stream
containing hydrogen and/or carbon monoxide, reducing at 700 to
900C in the reduction reactor calcium sulphate contained in
the used absorbent particles to calcium sulphide, feeding the
gas stream entraining the produced calcium sulphide and
obtained .~rom the reduction reactor to a carbonation reactor,
cooling the gas stream entraining the calcium sulphide 300~ to
500~ in the carbonation reactor to react in a dry state the
aalcium sulphide with carbon dioxide and water vapour and
produce calcium caxbonate and hydrogen sulphide, guiding the
re~ulting gas ~tream Prom the carbonation reactor into a second
duct collecting unit to collect solid particles, and spraying
the solid parti.cles again into the exhaust yas as regenerated
absorbent particles, the hydrogen sulphide being separated from
the gas stream flowing out from the second dust collecting
-4-
(~,
I
~2~Ç~9~3
unit, the remaining gas containing hydrogen, carbon monoxide
and carbon dioxide then being fed back to the reduction reactor
as a carrier gas for feeding the used absorbent particles into
the reduction reactor.
The Ca-type absorbents to be used in the present
method include limestone (CaC03), slaked lime [Ca(OH)2~,
quick lime (CaO), dolomite (CaC03.MgC03~, slaked dolomite
[Ca(OH)2.Mg(OH)2 or Ca(OH)2.MgO], calcined dolomite (CaO.MgO
or CaC03.MgO) and any material containing CaO or capable of
~orming CaO at a high temperature. Accordingly, the
particulate Ca-type absorbent originally contains CaO or
produces CaO through the following reactions at a high
temperature.
Ca(OH)2 ~ CaO + H20 (1)
CaC03 ~ CaO ~ C02 (2)
CaO absorbs sulfur oxides (SOx) from the exhaust gas upon
reacting therewith as represented by the ~ollowing equations.
CaO ~ 52 ~ ~2 ' CaSO~ (3)
CaO -~ S03 -~ CaSO~ (4)
The CaSO~ shells formed on the surfaces of absorberlt
particles by the reaction~ (3) and (~) have a compact texture
and lnhibit harmPul acid substances such as Sx from di~using
into the particles, consQquently reducing the reactivity o~ the
absorbent. However, the CaSO~ shells are converted to calcium
carbonate (CaC03) by the treatment according to the first
aspect described, whereby the particulate absorbent is
regenerated. The regenerated absorbent, if sprayed into khe
exhaust gas again, greatly reduces the amount of fresh
absorbent to be supplied.
3~0 Of the Ca-type absorbents mentioned above,
1~
--5--
';
9~3
dolomlte, slaked dolomite and calci~ed dolomite have a
large void volume, permit harmful acid substances to
readily diffuse into the particles thereof and are therefore
advan-tageous. Since the extraneous components such as MgO
do not participate directly in the regeneration reaetions,
-the change of such components will no-t be described for the
sake of simplified descrip-tion.
Exhaust gases, especially those from coal boilers
and waste incinerators generally contain fly ash of large
partiele sizes in addition to harmful acid substances. In
sueh a case, it is desirable to elassify the par-tieles
eolleeted by the first dust eollecting uni-t into a eoarse
partiele portion primarily eontaining fly ash and a fine
partiele portion primarily eontaining absorbent partieles
not larger than a predetermined partiele size and thereafter
regenerate the Eine partiele portion only. In the ease of
Euel oil boi.:Lers, the amount of fly ash is lesser than that
of absorbent partiel~s, so khat the elasslEieation step is
not always r~c~uirecl.
Va~lous Eeatures and aclvantacJes of the pre.sent
inventlorl w~ L be read:ily unclerstood Erom the embodlrnents
to be cleser.ibed below with referenee to the aeeompanyincJ
drawlncJs.
~RI~EF DE.SCRIPT~ON OF T~IE DR~W:tNGS
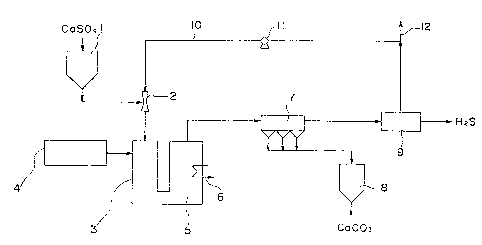
F:L~. 1 is a flow ehart showing a system for
--6--
~2~
treating sollds containing the calcium salt of sulfuric
oxyacid according to the invention; and
Fig. 2 is a flow chart showing a system for
purifying exhaust gases according to the inven-tion.
EMBODIMENTS
Indicated at 1 in Fig. 1 is a hopper containing
fine particles of used ea-type absorbent (par-tly eontaining
gypsum) or solids containing the caleium salt of ~ sulfurie
oxyaeid, sueh as high-purity gypsum. The fine particulate
material is sprayed from the hopper 1 lnto a reduetion
reaetor 3 as entrained in a carrier gas by an ejector 2
which is an example of a dispersing nozæle. Connected to
the reduetion reaetor 3 is a redueing gas generator ~, in
whieh a hydrocarbon, sueh as asphal-t, is partially burned
with oxygen and water vapor to produee a reducing gas having
a high temperature and eontaining earbon monoxide and
hydrogen. Within the reduetion reaetor 3, therefore, -the
caso4 componerlt Oe the p~rtieulate material ls redueed to
ealeium sulficle (CaS) aeeording to the following equation.
CaSO4 ~ ~2 ~ CaS ~ ~12
CaSO~ CO ~ CaS ~ ~CO2 (6)
Providecl clownstream Oe the reaetor 3 is a
earbonat:ion reaetor 5 equipped wlth a eooler 6 (whieh may
be a heat exchanger or a deviee adapted to evaporate wa-ter
on spraying). The gas s-trearrl from the reduetion reaetor 3
9~
is cooled in the carbonation reactor 5, whereby the CaS
is carbonated and further gives hydrogen sulfide according
to -the following equation.
CaS + H2O + CO2 ~ CaCO3 + H2S (7)
The gas stream flowing out from the reaetor 5 and
containing fine earbonated particles and H2S is then
subjeeted to solid-gas separation by a dust eollecting uni-t
7, and a fine solid partieulate portion eonsisting primarily
of fine carbonated particles is collected by a ealeium
carbonate hopper 8.
The gas stream from -the dust eolleeting unit 7 is
sent to a hydrogen sulfide eolleetor 9, by whieh the H2S is
eolleetecl. The eolleeted H2S may be eonver-ted to elemental
sulfur by the Claus proeess or -to sulfurie aeid by a we-t
lS proeess, or rnay be used as a material for ehemieal synthesis.
I'he yas stream separatecl:Erom the ~12S eontains
hydrogerl, earbon monoxide and earbon dioxide as unreacted
eomponents, so that l.t is a~vantacJeous to :reeyele the gas
stream throucJh a feedbaek l.ine 10 equipped with a eornpressor
l:L as a ea.rr:ier gas for Eeed:incJ :eine partieles frorn the
hopper 1 to the recluet:Lon reaetor 3. The earr:ier cJas~ when
eontaining :lmpur:it.Les (malnLy n:itrogen) at an exeess.ively
h:igh eoneent.ratl.on, recluires use of reaetors 3, 5 of inereased
eapaeity ancl adversely affee~ the Eorrnation of ealeiurn
earbonate. Aeeo:r~lingly the earrier gas is partially purged
~8--
sg
suitably via a purge line 12.
The smaller the fine particles passing through
the reactors 3, 5, -the better is the result, because the
reduction o~ the particle size assures smooth diffusion of
heat and the reactants into -the particles, consequently
shortening the reaction time required and permitting use of
reactors 3, 5 of reduced size. Further because of an improved
heat transfer efficiency then achieved, the reduction
reactor 3 assures uniform heating to inhibit crystallization
of CaS, while smooth carbonation takes place in -the
reac-tor 5, yiving highly reactive calcium carbonate. In
view of the power consumption needed for pulveriza-tion, the
fine particulate material to be used for practicing the
present invention is up to 10 microns, preferably 1 to 3
microns, in rnean particle size.
The ejector 2 serving as a disperslng nozzle and
cooperating with the carr.ier gas pressure-fed through the
.~eedback line 10 acts to un:i:Eormly fl:i.sperse the pre-~ulverlzed
particu:Late material from the hopper 1 into the .reducing gas
with:Ln the reaetor 3, whereby thc~ CaSO~ componen~ can be
reclueecl w:lth a high reactiv:Lty. In p:Lace Oe the Eine
pa:rk:ieulate ma-terial wh1ch has been f.inely cl:Lv:ided, soli~s
contain:ing the ealcium salt Oe sul:Euric oxyacid may he
pul.ver:Lzed on site by a jet mill or the like before being
fecl to the dispersing nozzle 2.
~6~i9
~ sually, the CaSO4 component contains crystal
water and loses the water at a high temperature of lO0 to 200
C. If this dehyd.ration reaction occurs at the por-tion of the
dispersing nozzle 2 which is exposed to a high temperature,
it is likely that the nozzle 2 will become plugqed up or
particles will agglomerate (to result in a reduced reactivity),
leading to poor economy. Accordingly, it is desirable to
calcine the particulate material to render the CaSO4 anhydrous
before the ma-terial reaches the nozzle 2.
For the regeneration of the CaSO~ component to
CaCO3, i-t is advantageous tha-t the reduclng gas concentration
wi-thin the reactor 3, and the water vapor concentration and
the carbon dioxide concentration within the reactor 5 be
maintained at a high level. For this purpose, concentrated
oxygen, especially concentrated oxygen having a concentration
of at least 95~, :ls more preferable than air which contains
large amounts of nitrogen and other impurities, as the oxygen
source to be used ~or part:ial:ly bu.rnlng a hyd.rocarbon within
the reducirlc3 gas generator ~.
The reduction reactiorlC of Equat:ions (5) and (6)
take p~.ace at a tcmpe:raturc Oe not lower than 700 C. While
these react:ions progress:iv~ly p:roceed :E:rom the surface Oe
the part:Lcles inwarcl, the reaction velocity is dependent on
the rate of diEfusion Oe H2 or CO from the surface of the
2S particle into the interior, so that the reac-tion is
--10--
~2~
accelera-ted with decreasing particle size. Because the rate
of diffusion is the predominant factor, the rise of the
reaction temperature to more than 900C is not very
effective for the reduction of CaSO~ to CaS. Further
temperatures over llaO C.promote crystallization of the CaS
produced, inhibiting the diffusion of H2 or CO into the
particles and adversely affecting the subsequent carbonation,
hece objectionable. Accordignly, i-t is advantageous that
the ternperature within the reduction reactor 3 be adjusted
to 700 to 900 C.
The carbonation reaction of Equation (7) i.s an
equilibrium reaction, and it is required to maintain the
tempera-ture at a level of up to 500 C for -the completion
of the reaction, while it is known -that this carbonation
reaction proceeds at a low velocity. It is therefore
necessary to maintain the reacti.on temperature at the highest
possib].e level within the range of up to 500 C for the
promot:lon oE the reaction. ~lthough it was not elear whethe.r
the carbonat:ion reaet:ion can be carr:iecl out wlthin a
pract:LcalL,y usefu:L periocl Oe reclct:ion (partlc:Le retention)
time, we have Eouncl that the clesi.recl.reactiv.i-ty can be
achieved w:Lth a react.Lon time Oe about 3 to 20 seconds by
us:i.n~ particJ.es oE reducecl mean particle size (especially
1 to 3 mlcrons) at a reaction temperature Oe 300 -to 500 C.
Ne~t, experiments will be described which were
conducted to substantiate the advantages of the foregoing
method of treatment.
EXPERIMENT I
Hollow cylindrical refractory bricks, 20 cm in
inside diameter, were stt~ked up to a height of 12 m to build
an experimental reaction column corresponding to the reactors
3 and 5 of Fig. 1 combined toge-ther. A combustion chamber
(corresponding to the reducing gas generator ~) made of
refractory bricks was provided on the top of the column. A
dispersin nozzle (corresponding to the ejector 2) was
installed at the column top under the chamber. A water spray
nozzle (corresponding to -the cooler 6) was attached to the
column at a positlon 1.5 m below -the nozzle. Tempera-ture
measuring thermocouples were inserted into the reaction
column at a position 0.5 m below the nozzle and at a position
6 m below the water spray nozzle. The portion of -the
reacti.on co~lumn above the water spray nozzle was externally
coverecl with an electrlc heater, which was protected with
heat insualt:Lnc3 brLcks and iron pipe from outside. The
resuLt:Lng as.sernbly wa9 Eurther eoverecl with a heat lnsula-tor.
The other portion Oe the column WC15 protected only w:ith an
iron p:i.pe. The water spray nozz:Le usecl was one called
a supersonic nozz:Le wh:ich is capable of producing atomized
water (fine water droplets). The outlet of the reaction
column was provicled with a Jetclone Collec-tor (high-
-12-
performance cyclone, product of Nippon Pneumatic MFG. Co.,
Ltd.) for trapping dust.
Town gas was burned in the combustion chamber to
heat the reaction column to a specified temperature. An
experiment was then conducted for 2 hours under -the following
conditions. Firs-t, the town gas was replaced by carbon
monoxide and hdyrogen. Carbon monoxide was fed to the
chamber at a flow rate of 14 Nm3/h (N representing stanclard
state), hydrogen at 6 Nm3/h, and air at 34.8 Nm /h for
partial cornbustion (to maintain a prede-termined reaction
temperature). On the o-ther hand, anhydrous gypsum (purity
99~) finely divided to a mean par-ticle size of 3 microns
and serving as a solid containiny -the calcium salt of a
sulfuric oxyacid was fed at a ra-te of 13.6 kg/h to -the
dispersing nozzle at the column top, as entrained in a carrier
gas composed of 50~ of carbon monoxide and 50~ of hydrogen
and suppl:Led at a rate o~ 24.2 Nm3/h, whereby the finely
d~vi.ded qypsum was di~persed into the hot combustion gas
from t.he cornbust:ion chatrlber~ the carr:i.er qas belng accelerated
to a Elow rate Oe 200 m/sec by khe nozzle. Atomized water
was suppll~d at a rate of ~1.2 kq/h from the water spray
nozzle. At th:is tlme, the reductlon reactor portion above
the water nozzLe hacl a temperature of 820 C, whlle the
carbonat:Lon reactor por-tion above the nozzle had a temperature
o~ 3~0 C.
-13-
..,
6~9~
Dust and particles were collected through the
cyclone at a rate of 9.5 ~g/h. When analyzed, the dust and
particles were found to contain 92.4~ of CaCO3, 3.5~ of CaS,
2.7~ of CaSO4 and 1.4% of others. On the other hand, a gas
stream having a temperature of 340 C and containing 2.4%
of H2S was collected from an upper portion of the cyclone.
EXPERIMENT Il
The procedure of EXPERIMENT I was repeated wi-th
the exception of the following.
*Solid containing calcium salt of sulfuric oxyacid:
Used Ca-type absorbent (anhydrous gypsum conten-t 43.7~)
*Mean particle size: 2 microns
*Rate of supply of particles: 20 kg/h
*Flow rate of carrier gas: 25 Nm /h
*Speed of carrier gas from dispersing nozzle: 300 m/sec
*Rate of supply Oe atomlzecl water: 20.0 kg/h
*Temperature of reduction reaction: 810 C
*Temperature of carbonation reaction: 335 C
Consecluently, dust ancl partlc:Les were collected
Erom the cyLone at a rate oE 21..l kg/h. When analyzec:l, the
clust and partLc.les were Eound to contaln 67.7'k Oe CaCO3,
0.7~ of CaS, 0.4~ Oe CaSO4 and 31.2~ Oe others. On the
other hancl, t~ gas ~tream containln~ 1.5~ of H2S ancl having a
l:emperature Oe 335 C was collected erom the ~pper portion
oE the cyclone.
-14-
~2~ 3
Eig. 2 shows an exhaust gas purifying system
including the treatment circuit of Fig. 1. The system is
an exma~le as used for a the~moelectr1c power plant of 1000
MW class specifically designed for burning coal. With
reference to the drawing, indicated at 13 is a coal burning
boiler, in which coal is burned with air sup?lied by a
blower 14 through an air heater 15 and which produces a
combustion exhaust gas con-taining Ely ash and harmful acid
substances. Fine particles of a Ca-type absorbent, for
exampl.e, finely divided limestone (CaCO3) having a mean
particle size of 1 -to 3 microns is sprayed in-to the top of
-the boiler 13 via a feed line 16. Consequently, the
particulate absorbent is retained at a -temperature of 900
to 1100 C for about 2 to abou-t 3 seconds, whereby CaCO3 is
thermally decoraposed instantaneously -to give highly reac-ti.ve
CaO according to Equation (2). The CaO .Eurther undergoes
the reactlons of Equations (3) and (~), absorbing sulfur
oxides and :forrning calcium sul:eate (CaSO~L) on the surface
oE the absorb~nt part;icle~. The reactivity oE the CaO is
about ~0 to about 50~, ancl unr0acted CaO remains as it is
in th~ :interlo:r oE th~ pa:rticles. The heat evolved .erorn
the r~actiorls is uki:Llzed by the bo:Lle.r 13.
The exhaust gas Erom the boiler 13 i5 passed
through a denitrating unit L7, which removes nitrogen oxi.des
Erom the gas. Via the air heater 15, the gas then.enters a
~6~9~
a dust collecting unit 18, in which the used particulate
absorbent and soot and dust containing rly ash are removed
from the gas stream. The exhaust gas thus purified is
-then released to the atmosphere through a chimney 19.
According to the invention, desulfurization is conducted
at a high temperature of 900 to 1100 C, so that the exhaust
gas has a reduced acid dew-point temperature. Consequently,
the exhaust gas as released from the chimney 19 to the
atmosphere has a temperature of about 100 C as reduced from
the conventional ternpera-ture level of 140 to 150 C. This
means that an increased amount of heat can be utilized by
the boiler 13. Before reaching the dust collectlng unit 18,
the particulate absorbent absorbs hydrogen ~luoride (HF) and
hydogen chloride (EICl) from the exhaust gas, in addition to
the sulfur oxlcles, but since the CaF2 and CaC12 consequen-tly
formed do no-t partlcipate in th~ subsequent treatment Oe
the absorbent, these halides will not be cleserlbed.
rrhe soot, dust and particles colleeted by the
unit 18 are led through a hopper 20 to an air elassi~ier
21, by which they are separated :into a coarse particle
portion primaril~ contai.nincJ ely ash of 12 to 15 microns
i.n rnean particle size, ancI a ~.ine particle portion c~Iiefly
contain:Lng the usecl absorbent. The coarse particle portion
separated o~f is s-tored in a container 22. The Eine particle
portion is led through a hopper 1 into a reyenerating
-16-
9~
treatment circuit.
The regeneration circuit shown in Fig. 2, like
the one shown in Fig. 1, includes an ejector 2, reduction
reactor 3, reducing gas generator 4, carbonation reac~or 5
having a cooler in the ~orm o~ a heat exchanger, dust
collecting unit 7, calcium carbonate hopper 8, hydrogen
sulfide collector 9, feedback line 10 having a compressor ll,
and purge line 12. The dust collecting unit 7 comprises
cyclones 23 and a bag filter 2~ in cornbination. The hydrogen
sulfide collector 9 comprises a cooler 25 having a cooling
water circulation pump 26, a hydrogen sulfide absorption
column 27 and a stripper 28. The stripper 28 is connected to
a hydrogen sulfide container 29.
The CaSO4 component contained in the used absorben-t
~ed to the reduction reactor 3 through the e]ector 2 is
reduced to CaS by the reactions of Equations (5) and (6) in
the hot reducing gas Erom the generator ~. The CaS further
- reacts with carbon dioxide and water vapor in the carbonation
reactor 5 accordiny to Equakion (7), forming CaCO3.
Consequentl~, the absorbent is regenerated, and H2S is
produced. ~uring -the carbonation reaction, the unreacted
component ~CaO) in~ide the ~bsorbent particles also undergoes
the following reaction and is carbonated.
CaO ~ CO2 ~ CaCO3 (~)
The reduction reac-tion and the carbonation reaction
-17-
~' ~
~2~9~
described above are each exothermic, and the heat of reaction
is recovered by the heat exchanger 6.
The gas stream flowing out from the carbonation
reac-tor S and containing the regenerated absorbent, ~2S and
other unreacted gas components is subjected to solid-gas
separation by the dust collecting unit 7. The particulate
absorber separa-ted off is fed back to the boiler 13 via the
calcium carbonate hopper 8, rotary valve 30, ejector 31 and
feed line 16. Since some of the absorbent particles are
los-t when classified by the air classifier 21, the feed line
is replenished with a fresh absorber (which need not always
contain CaCO3 but may contain CaO or Ca(OH)2) from an
absorbent feeder 32 via a hopper 33, rotary valve 3~ and
ejector 35 to compensate for the loss. Indicated at 36 is
a feeding compressor connected to the feed line 16.
The gas stream flowing out from the dus-t collecting
unit 7 is cooled to about ~0 C by the cooler 25 and then
subjected to an amine washing step by the absorption column
27 and the stripper 28, whereby H2S only is separated off
for collect,ion. The collected H2S is stored in the container
29 and is t.reated, Eor example, by the Claus process at a
~uitable t.Lme. ~ndicated at 37 and 38 are elemental sulfur
and water vapor resulting Erom the Claus process.
The gas stream flowing out from the hydrogen
suli~e collector 9 is returned to the redu~tion reactor 3
-18-
by the compressor 11 via the feedback line 10. The gas stream
is partly released to the outside via the purge line 12.
If it is attempted to reduce the amount of absorbent
particles to be lost at the air classifier 21, the particulate
S material to be sent to the absorbent regenera~ion circuit
downstream thereof will contain an increased amount of
impurities (fly ash, etc.), giving an increased load to -the
regeneration circuit. Accordingly -the ratio of separation
to be conclucted by the classifier 21 needs to be set in view
of the balance between the amount of loss of absorbent and
the load on the regeneration circuit.
The exhaust gas purifying system shown in Fig. 2
has the following advantages.
(a) Since used Ca-type absorbent is recycled for reuse
upon regeneration, the exhaust gas can be puriEied
eeficiently w,ith use of a smaller amount oE absorbent
without entalling the troublesome problem oE by-proclucts.
(b) RecyclincJ oE th~ absorbent wh:ich has been pulver:izecl
once serves to reduc~ the powcr eor pulver:iY.ation.
(c) The process erom the desuleur:i~ation with the
absorbent through the re~erl~ration Oe the abso~bent
(the ~eri0s of steps up to the dust collec-ting unit 7) :is
practlced cont:inuously in a clry state and is there~ore low
in hea-t loss. ~urther the heat developed from the
desulfurization and regeneration i.s effectively used by
-19-
9~
the boiler 13 and heat exchanger 6 to achieve an improved
energy efficiency. The present method is free of the
problem of effluent treatment which is encountered with
the wet or semi-wet process and which could cause
secondary pollution.
(d) The boiler 13 and dust collecting unit 18, which
serve also as desulfurizing units, render the system
compact.
(e) Because the desulfurization with the absorbent
is conducted at a high temperature of 900 to 1100 C,
SO3 which is more harmful than SO2 and diffucult to remove
at lower temperatures (SO3 suspends in the exhause gas
in the form of stable minute crystals at low temperatures)
can be removed effectively.
-20-
r~
.. ,~ .