Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
'; ' , ~,~t~ 7~ ,
PHENYLE~ MONO - AND DIESTER
PERACID PRECURSORS
Field of the Invention
.
This relates to novel peracid precursors and the in situ
generation of peracid in aqueous solution by combining a source of
hydrogen peroxide, and the novel peracid precursor, exemplary of
which are phenylene mono - and diesters, in water, said
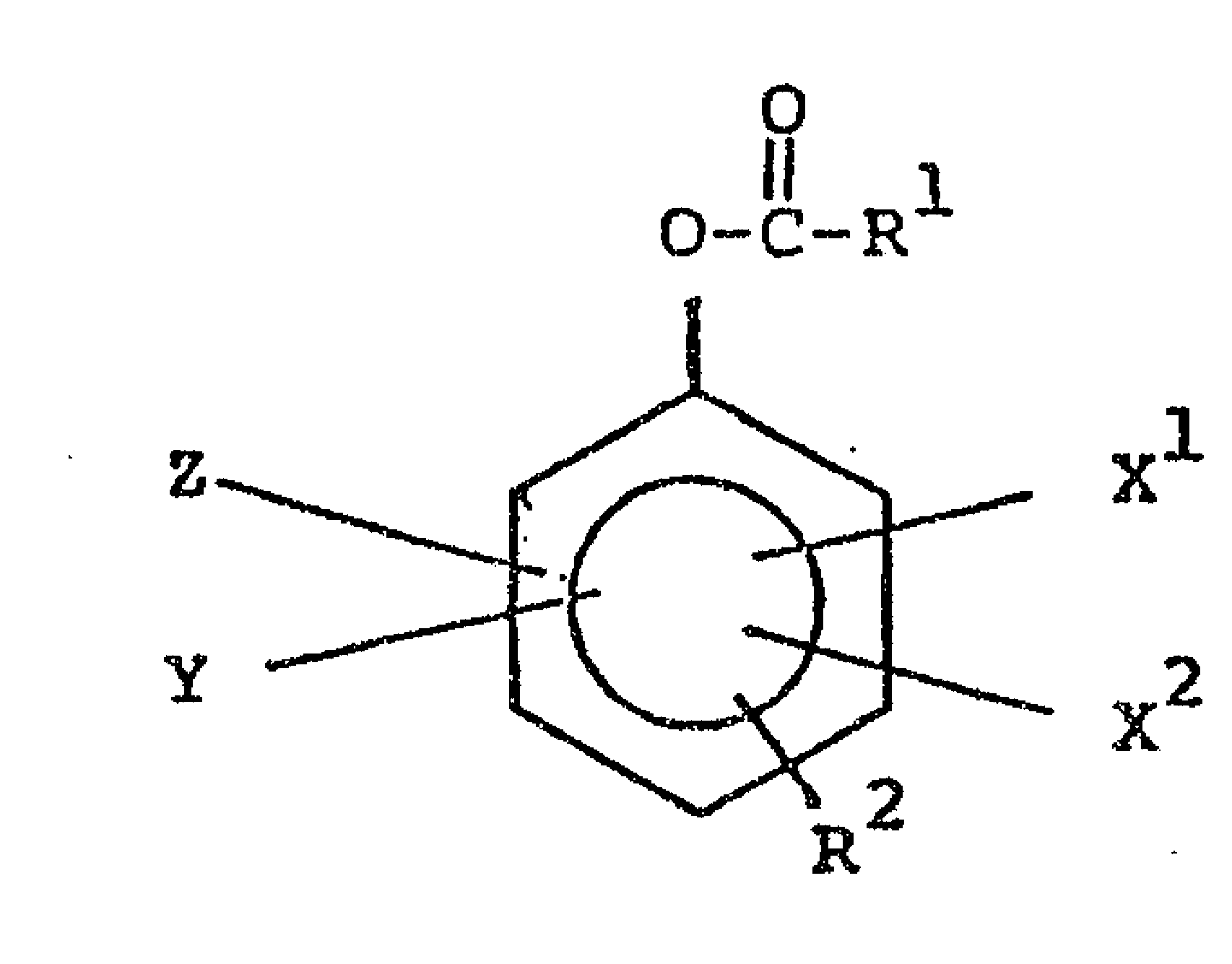
precursors being of the general structure:
O-C-R
~~,,~Xl
wherein Rl, R2, Xl, X2, Y and z are defined within the
specification.
Background of the Invention
. _
Peroxygen bleaching compounds, such as hydrogen peroxide,
sodium percarbonate, so~ium perborate monohydrate or tetrahydrate
are useful for bleaching fabrics, textiles and other materials.
Unfortunately, these sorts of pecoxygen bleaches appear less
effective when bleaching temperatures o less than 70 C are
utilized. Thus, the low wash temperatures found in American
washing machines make the use of these bleaches less effective
than in European-type washing machines, which typically use water
temperatures above 70C. Therefore, attempts have been made to
use activators in combination with these peroxygen bleaches. It
may be more accurate to call these activators peracid precursors,
--1--
' ~7~7~7
since it is generally accepted that when a molecule of a compound
such as sodium acetyloxy benzene sul~onate (rNABS~) is combined
with a source of hydrogen peroxide, such as sodium perborate
monohydrate, in aqueous solution (as indicated in GB 864,798), the
result is production of peracetic acid, O
CH3C~O-
However, nothing within the prior art shows, discloses, orsuggests that di-substituted benzenes, more specifically,
phenylene diesters, may be appropriate for use as peracid
precursors.
,
Summary of the Invention
The invention provides a compound of the general structure
o
O -C~R
" Z ~ Xl
R2
wherein Rl is alkyl of l to 20 carbon atoms; R is
OH, -O-R , or -O-C-R ; and X , X , Y and Z are
individually selected from H, SO3, CO2, NO2,.
NR4-,- halogen,-R and mi-xtures~ hereo~-
Oherein ~3 of -o-R3 is alkyl of l to 20 carbon atoms;
R4 of -o-C-R4 is~alkyl---of l-to 20 carbon atoms; R5 of
NR5 is selected from H, alkyl of 1 to 24 carbon atoms and
0 mixtures thereof; and R6 is alkyl of l to Z0 carbon atoms;
wherein when R2 is OH, Rl has m8re than about 3
carbon atoms.; and wherein when RZ is -o-c-R4 t and Rl and
R comprise individually alkyls of less than 3 carbon atoms,
R ~ R .
~2--
7~
The invention also provides a 501id or liquid bleaching
composition comprising:
(a) A hydrogen peroxide source; and
(b) A bleach effective amount of a precursor of the general
structure:
O-C-R
y ~x2
wherein Rl is alkyl of 1 to 20 carbon atoms; R2 is
OH -o-R3 or O C R4 and Xl x2 Y d
individually selected from H, S03, CQ2, N02,
0 NR4 , halogen, R6 and mixtures thereof; ~o
wherein R3 of -o~R3 is alkyl of 1 to 20 carbon atoms;
R4 of ~o-C-R4 is alkyl o~ 1 to 20 carbon-atoms; R of
NR4~ is selected from H, alkyl of 1 to 24--carbon atoms--and
mixtures thereof; and R6 is alkyl of 1 to 20 carbon atoms.
Preferred embodiments include phenylene ~onoesters wherein
R is OH and Rl is straight chain alkyl of 1 toOll carbon
atoms; and phenylene diesters wherein R2 is ~o-C-R4, bot~ R2
and R4 straight chain comprislng alkyls of L to 11 carbon atoms.
Selected adjuncts can be added to these bleaching
compositions, such as surfactants, stabilizers, buffers and
builders. The invention also includes a method for synthesizing
the above noted precursor compounds and a method of bleaching.
~7~7~L~
Detailed DescriptiOn o:E the Invention
The invention generally relates to novel peracid precursors
~ypical precursors are esters, imide or enol ester compounds which
are combined with a source of peroxygenr such as hydrogen
peroxide, sodium percarbonate or sodium perborate. These
particular types of precursors are commonly used in Europe where
washing temperatures are generally higher than is prevalent in the
United States. Washing temperatures of up to 100C are common
in Europe.
However, there remains a need to provide peracid precursors
which are effective to promote good bleaching in wash temperatures
below 70 C, more preferably-below 60 C, and most--preferably
below 50C.
The preferred peracid precursors of this invention have the
general structure:
O-C-R
~R2
wherein ORl is alkyl of 1 to 20 carbon atoms; R2 is
OH, -O-R , or -O-C-R ; and X , X , Y and Z are
individually selected from H, SO3, CO2, NO2,
0 NR45+, halogen, ~6 and mixtures thereof;
wherein R3 of -o-R3 is alkyl of l to 20 carbon atoms;
R of -O-C-R is alkyl of 1 to 20 carbon atoms; R of
NR4 is selected from H, alkyl of 1 to 24 carbon atoms and
7~7
mixtures thereof; and R6 is alkyl of 1 to 20 carbon atorns;
wherein when R is OH, R has more than about 3
carbon atoms; and wherein when R2 is -o-C-R4, and Rl and
R comprise individually alkyls of less than 3 carbon atoms,
Rll R4
The embodiments of this general structure include:
O -C-Rl
~,~ X
wherein Rl, Xl, X2, Y and Z are defined as above;
O--C--E'~l
(II) ~ X
wherein Rl, R3, Xl, X2, Y and Z are defined as above;
and O
I i 1
(III) ~ ~ x2
~1 4
O-C-R
wherein Rl, R4, Xl, X2, Y and Z are defined as above.
The substituents ~1, R4 and R6, all being alkyls o~ 1 to
20 carbon atoms, may additionally be either straight chain,
branched chain, have some unsaturation ~for example, if Rl, R4
or R is derived from natural oils or fatty acids, e.g , oleic
acid), and may be substituted at various positions on the carbon
chain. substituents of R , R and R may include halogen
(Cl , Br , T ), NO2, NR4 ~R5 defined as in the
foregoing, and representing, e.g., NH4 and other quaternary
ammonium compounds), SO4, CO2, and OH.
With respect to the ring substituents X , X2, Y and Z,
which are selected from H, S03, CO2, NO2, NR4 ,
halogen, R6 and mixtures thereof (wherein R5 of NR4+ is
selected from H, alkyl of 1-24 carbon atoms, and mixtures thereof;
and R6 is alkyl of l to 20 carbon atoms~, any combination of
these substituents may be present in the precursors of this
invention. When the substituents are charged moieties, e.g.
SO3, appropriate counterpart ions (counterions) may be
present. With respect to SO3, CO2, Cl , Br , and
F , appropriate counterions may be
chosen from H , alkali metal salts (Na , Li , K ),
although alkaline earth salts (calcium, magnesium, barium) or even
- 20 ammonium salts may be possible. With respect to a quaternary
ammonium substituent, i.e., NR4 , appropriate counterions can
include halides, (CI , Br , I ), methosulfates~ sulfates and
nitrates. These aforementioned counterions may also be present
with respect to the substituted Rl, R4 and R6 groups, as
appropriate.
When compounds of (I), i.e., phenylene monoesters, are
considered, it is preferred that R comprise alkyl of l to 20,
more preferably l to 15, and ~nost preferably l to ll carbon
atoms. Particularly preferred are phenylene monoesters of about
6-ll carbon atoms in length, which appear to provide surface
active peracids when combined with a hydrogen peroxide source in
-6-
~X~717
-
aqueous solution. As exemplified below, in EXPERIMENTAL, E~ample
II, these particular compounds were found to be excellent in
perhydrolysis, giving good yields of the desired peracid, with
surprisingly low levels of diacyl peroxide, which, as described in
Chung it al, U.S. 4,412,934, may be problematic.
Compounds of ~II), i.e., phenylene esters with an ether
substituent, -o-R5, wherein R5 is alkyl of 1 to 20, more
preferably 1 to 10, and most preferably 1 to 6, carbon atoms, may
be very reactive compounds. Especi~lly preferred may be when R
= CH3. As with the substituents R , R and R , R may
be straight chain, branched, unsaturated or substituted.
With compounds of (III), i.e., phenylene diesters, wherein
R is -O-C-R , R and R are preferably 1 to 20, more
preferably 1 to 15, and most preferably 1 to 11 carbon atoms.
These particular compounds have the advantages of containing two
potential sites for perhydrolysis and thus appear to greatly
increase peracid yields over prior art precursors when the same
amount of precursors, based on molar equivalents, is used~
Additionally, unexpected salutary benefits appear when R and
R are unequal, i;e., the compound is a mixed diester. In
particular when R or R is less than 5 carbons, and the other
is greater, it is believed that both hydrophobic and hydrophilic
peracids are generated. Therefore, if used in aqueous media with
a source of hydrogen peroxide ~e.g., sodium perborate
monohydrate), for example, as an all fabric bleach, two different
oxidizing species appear to be present which can attach to
different types of soils, i.e., hydrophilic soils such as tea and
wine, and oily soils, such as sebum.
The phenylene diesters of ~III) include ortho, meta and
Dara substituted phenylene diesters, such as diacetate,
dihexanoate, dioctanoate and mixed (i.e., wherein Rl T R4)
ester derivatiVes of resorcinol, hydroquinone and catechol, which
S are exemplified below: OH
-
Hydroquinone (1,4-benzenediol; 1,4-dihydroxybenzene;
p-dihydroxybenzene) is a white crystalline compound which can be
obtained by dry distillation of quinic acid or by reduction of
1~ quinone. OH
1 OH
Resorcinol (1,3 benzenediol; 1,3-dihydroxybenzene;
m-dihydroxybenzene) is a crystalline compound with a faint
aromatic odor, and a sweet/bitter taste. It may be produced by
the alkali fusion of galbanum and asafetida resins.
~ OH
Catechol (1,2-benzenediol; 1,2-dihydroxybenzene;
o~dihydroxybenzene) is a crystalline compound with a phenolic odor
and a sweet and bitter taste. It may be obtained by dried
distillation of catechin which is found in the aqueous extract of
catechu, which is an extract of an East Asian acacia plant.
7~
A11 three oE these dihydroxybenzene startiny materials are
commercially available.
The dihydroxybenzenes are weak acids with two dissociation
constants. They are generally classified as antioxidant agents
and are useful analytical reagents. Their structures, uses and
chemistries are more thoroughly explored in Kirk-Othmer,
Encyclopedia of Chemical Technology, 3rd Ed., vol 13, pages 39-69
( 19~
.,
The diesterified derivatives of these dihydroxybenzene
compounds are generally produced by reacting them with an
appropriate acid anhydride in the presence of a strong acid. The
general procedures for making these precursors are set forth below
in EXPERIMENTAL. Additionally, the preferred phenylene monoesters
are depicted below in EXPERIME~TAL.
15It is believed that in situ peracid generation occurs when
these novel precursors are combined with a source of hydrogen
peroxide in aqueous solution as follows: O- !
+ OOH ~ Rl OOH +
~ O-C-R O-C-R
Step II ~ ~ + 0O~_ > R4Coo~ +
20wherein the phenylene diester precursors revert back to the
appropriate dihydroxybenzene compound.
_9_
~'7~7~L7
While the foregoing is believed to occur, in fact, the
mechanism behind peracid generation may occur simultaneously, or
in rapid sequence, or a combination of these reactions.
~ hatever the mechanism, it was surprisingly discovered that
when the novel precursors were combined with hydrogen peroxide in
aqueous solution, high yields of peracid were produced, even at low
temperatures such as those found in U.S. wash water temperatures.
It was even move surprising to see these high yie~ds given that the
byproducts of reaction, dihydroxybenzenes, are noted antioxidants
which one would expect to consume the peracids thus produced.
Applicants have found these particular substituted phenylene
diesters to be particularly effective in low temperature bleaching
applications. It was surprising that, given the large number of
carbons on the disclosed compositions, the reactivities thereof
lS were suitable for low temperature bleaching applications. Large
alkyl groups ar~'hydrophobic, hence solubility or dispersibility
in cold water was assumed to be problema-tic. While enhanced
bleaching activity occurs when vari.ous solubili-ing ~roups are
added to these composl.tions, sufficient pero~yacid generation for
20 bleach applications has béen observed even in their''abs'ence.
Addtionally, applicants observed that with increasing
chain lengths of the phenylene diester'precursors, decreasing
bleaching performance may be observed due to decreasing solubility
or dispersibility. Therefore, solubility~dispersibility and hence
performance can be improved by the addition of solubilizing groups
such as SO3, CO2, NR43 . Placement of these
solubilizing groups may have different effects on the precursor
compositions. For example, if the solubil-izing groups are placed
--10--
07~7
on the aromatic rinq or at or near the end of the alkyl groups of
the esters, increased solubility may be observed. Placing the
solubilizing groups next to the carbonyl carbon on the ester group
or electron withdrawing substituents on the aromatic leaving group
may increase the perhydrolysis rate. These theories are by way of
explanation and not intended to thereby restrict the invention
herein.
Addition of the above described substituent groups can be
accomplished by ways known to thosë skilled in the art. For
example, halogen groups may be added by typical halogenation
reactions, in which a typical source of halogen is combined with
the selected dihydroxybenzene starting material in the presence of
a Lewis Acid. Nitration, on the other hand, occurs when the
dihydroxybenzene is reacted with nitric acid in the presence of
sulfuric acid. Sulfonation occurs when the dihydroxybenzene is
reacted with concentrated sulfuric acid. on the other hand,
amination will generally be produced by reacting a source of amino
with the dihydroxybenzene in the presence of liquid ammonia.
Further, as with typical benzene-derived compounds, acylation and
2~ al~.ylation can occur via Friedel-Crafts reactions.
Especially preferred are solubilizing groups, such as
sulfonate (-S03) or carboxylate (~C02) groups. These
- appear to impart good solubilit~/dispersibility properties to the
peracid precursors of this invention. Additionally, it is
preferred that a counterpart ion (counterion) to the sulfonate or
carbonate group be chosen from H~ or an alkali metal ion
selected from sodium, potassium or lithium, although alkaline
earth counterions and even ammonium counterions may be appropriate.
The precursors can be incorporated into a liquid or solid
matrix for use in liquid or solid detergent bleaches by dissolving
L'7
into an appropriate solvent or surfactant or b~ dispersing onto a
substrate material. Examples of appropriate solvents include
acetone, non-nucleophilic alcohols, ethers or hydrocarbons. Other
more water-dispersible or -miscible solvents may be considered.
As an example of affixation to a substrate material, the
precursors of the present invention could be incorporated onto a
non-particulate substrate such as disclosed in published European
Patent Application EP 98 129
In a further embodiment of the phenylene diesters of this
invention, it has ~en found that precursors containing mixed
chain lengths, i.e., a shorter carbon chain length of at least one
ester functionality, and a longer carbon length at the second
ester functionality, provides extremely proficient bleaching For
example, it is believed that when one of the ester functionalities
has an alkyl straight chain length of less than 5, e.g., wherein
R or R is CH3, and the other alkyl group's chain length is
greater than 5 carbon atoms, peroxyacids which are, respectively,
hydrophilic and hydrophobic are generated. The believed advantage
thereof is that particulate soils, e.g~, clay soil/ and
hydrophilic stains, e.g., tea and wine, can be attacked with a
hydrophilic peroxyacid bleach while oily soils, e.g., sebum, can
be attacked with a hydrophobic peroxyacid bleach Different
pre-formed hydrophobic and hydrophilic peroxyacid bleaches were
combined in published European Patent Application EP 68 547
Pre-formed
peracids appear, however, to have storage stability problems and
may lose significant amounts of active oxygen (A.O) upon prolonged
storage. EP 98 129, mentioned above, discloses in one embodiment,
separate peracid precursors which are impregnated on a fabric
substrate. Problematic to this approach are the added
manufacturing steps to producing different peracid precursors and
-12-
7~'~
using slurrying, emulslfyiny or other techniques to bind the
different precursors to the substrate. A particularly preferred
combination of the present invention is when one ester is an
acetate te.g., Rl is CH3) and the other is an hexanoate,
heptanoate, octanoate or nonanoate (e.g, R is -(CH2)4CH3
to -(CH2)7CH3). In a preferred embodiment, the total number
of backbone carbons of Rl plus R4 should be in the range of
2-20, more preferably 5-20, most preferably 7-14.
Additionally, it was surprisingly found that while the
positioning of the ester groups with respect to each other on the
phenyl ring is significant, it is not critical. This was
surprising since some references had suggested that activators
which comprise a substituted phenyl ring must have the active
substituent in para configuration with respect to other
substituents, likely, it is assumed, to avoid steric hindrance.
~ nder wash conditiorls and at temperatures below 70C, it has
been surprisingly found that any dihydroxybenzene, whether
catechol, hydroquinone or resorcinol, can be used as perhydrolysis
leaving groups, and that the resul-ting antioxidant does not
appreciably or rapidly consume the oxidant formed, i.e., the
peroxyacid(s). Resorcinol and catechol may be the preferred
leaving groups because, of the byproducts of perhydrolysis of
ortho, meta and para phenylene diesters, hydroquinone may be the
most readily oxidizable.
In the disclosure of Chung, et. alO, U.S. 4,412,934, it is
contended that the molar ratio of hydrogen peroxide to bleach
activator must exceed 1.5 or else a competing reaction is favored
wherein peracid generated reacts with the bleach activator itself
to form diacyl peroxide. In contrast to the Chung, et. al. bleach
activator, the present invention has ~een surprisingly discovered t~".
~ 7~
form low levels of diacyl peroxi~e. This is further depicted
below in EXPERIME~TALi Examples II and IV. Although it is not
definitely understood why this phenomenon occurs, it appears that
the phenylene diester precursors may have different surface active
properties. And, because of two reactive sites, which provides
two equivalents of peracid per equivalent of precursor, lower
concentrations of precursor are needed. There also is no need for
a hydrogen peroxide/precursor ratio of greater than 1.5, as
rnandated in the Chung, et. at. disclosure. ~ased on two reactive
sites, i.e., the ester equivalents of the phenylene diester
precursors, a ratio of l:l hydrogen peroxide: ester is possible,
although ratios greater than this are also within the invention.
It is preferred that the molar ratio of hydrogen peroxide: ester
be from about 1:20 to 20:1, more preferably about 1:10 to 10:1,
most preferably about 1:1 to 5:1.
While it has been disclosed by applicants that substituting
solubilizing groups on the phenyl ring will improve the solubility
and enhance the reactivity of these precursors, an alternate mode
and preferred embodiment is to combine the precursors with a
20 -surfactant. Particularly effective surfactants appear to be
nonionic surfactants. Preferred surfactants of use include linear
ethoxylated alcohols, such as those sold by shell Chemical Company
under the brand name Neodol. Other suitable nonionic surfactants
can include other linear ethoxylated alcohols with an average
length of 6 to 16 carbon atoms and averaging about ~ to 20 moles
of ethylene oxide per ~ole of alcohol; linear and branched,
primary and secondary ethoxylated, propoxylated alcohols with an
average length of about 6 to 16 carbon atoms and averaginy 0-10
moles of ethylene oxide and about l-to 10 moles of propylene oxide
per mole of alcohol; linear and branched alkylphenoxy (polyethoxy)
alcohols, otherwise known as ethoxylated alkylphenols, with an
average chain length of 8 to 16 carbon atorns and averaging 1.5 to
~7~7~.J7
30 moles of ethylene oxide per mole of aleohol; arld mixtu~es
thereof.
Further suitable nonionic surfactants may inelude
polyoxyethylene earboxylie aeid esters, fatty aeid glyeerol
esters~ fatty aeid and ethoxylated fatty aeid alkanolamides,
certain bloek copolymers of propylene oxid~e and ethylene oxide,
and block polymers of propylene oxide and ethylene oxide with
propoxylated ethylene diamine. Also ineluded are such semi-polar
nonionic surfactants like amine oxides, phosphine oxides,
sulfoxides, and their ethoxylated derivatives.
Anionic surfactants may also be suitable. Examples of such
anionie surfactants may inelude the ammonium, substituted ammonium
~e.g., mono-di-, and triethanolammonium), alkal; metal and
alkaline earth metal salts of C6-C20 fatty acids and rosin
acids, linear and branehed alkyl benzene sulfonates, alkyl
sulfates, alkyl ether sulfates, alkane sulfonates, ole~in
sul~onates, hydroxyalkane sulfonates, fatty aeid monoglyceride
sulfates, alkyl glyeeryl ether sul~ates, aeyl sarcosinates and
acyl N-methyltaurides.
Suitable eationic surfactants may include the quaternary
ammonium eompounds in whieh typically one o~ the groups linked to
the nitrogen atom is a C12-C18 alkyl group and the other three
groups are short chained alkyl groups which may bear inert
substituents such as phenyl groups.
Further, suitable amphoterie and zwitterionie sur~aetants
which contain an anionic water-solubilizing group, a cationic
group and a hydrophobic organie group may inelude amino earboxylic
acids and their salts, amino diearboxylic acids and their salts,
--15--
~7~37~7
alkylbetaineS, alkyl aminopropylbetaines, sulfobetaines, alkyl
imidazolinium derivatives, certain quaternary ammonium compounds,
certain quaternary phosphonium compounds and certain tertiary
sulfonium compounds. other examples of potentially suitable
5 zwitterionic surfactants can be found described in Jones, U.S.
4,005,029, at columns 11-15.
Further examples of anionic, nonionic, cationic and amphoteric
surfactants which may be suitable for use in this invention are
10 depicted in Kirk-Othmer, Encyclopedia of Chemical Technology,
Third Edition, Volume 22, pages 347w387, and McCutcheon's
Detergents and EmulsifierS, North American Edition, 1983.
As mentioned hereinabove, other common detergent adjuncts may
15 be added if a bleach or detergent bleach product is desired. If,
for example, a dry bleach cornposition is desi.red, the ollowing
ranges (weight %) appear practicable:
0.5-50.0% Hydrogen Peroxide Source
0.05~25.0% Precursor
1.0-50.0~ Surfactant
1.0-50.0% Buffer
5.0-99.9% Filler, stabilizers, dyes,
Fragrances, brighteners, etc.
The hydrogen peroxide source may be selected from the alkali
25 metal salts of percarbonate, perborate, persilicate and hydrogen
peroxide adducts and hydrogen peroxide. Most preferred are sodium
percarbonate, sodium perborate mono- and tetrahydrate, and
hydrogen peroxide. other peroxygen sources may be possible, such
as monopersulfates and monoperphosphates. In liquid applications,
30 liquid hydrogen peroxide solutions are preferred, but the
precursor may need to be kept separate therefrom prior to
combination in aqueous solution to prevent premature decomposition.
--16-
7~
The buffer may be selected Erom sodium carbonate, sodium
bicarbonate, sodium borate, sodium silicate, phosphoric acid
salts, and other alkali metal/alkaline earth metal salts known to
those skilled in the art. organic buffers, such as succinates,
5 maleates and acetates may also be suitable for use. It appears
preferable to have sufficient buffer to attain an alkaline pH,
i.e., above at least about 7Ø
The filler material, which, in a detergent bleach application,
may actually constitute the major constituent, by weight, of the
10 detergent bleach, is usually sodium sulfate. SodiUm chloride is
another potential filler. Dyes include anthraquinone and similar
blue dyes. Pigments, such as ultramarine blue (UMB), may also be
used, and can have a-bluing effect by depositing on fabrics washed
with a detergent bleach containing ~MB. Monastral colorants are
15 also possible for inclusion. Brighteners, such as stilbene,
styrene and styrylnapthalene brighteners (fluorescent-whit-ening
agents), may be included. Fragrances used for esthetic purposes
are commercially available from Norda, Irlternational FlaVors and
Fragrances and GiVaudon. Stabilizers include hydrated salts, such
20 as magnesium sulfate, and boric acid.
!
In one of the preferred embodiments in which a monoester
compound such as in (I) above is the precursor, a preferred bleach
composition has the following ingredients:
15.5% Sodium Perborate Tetrahydrate
11.93 Resorcinol Monooctanoate
7.0% Nonionic surfactant
15.0% Sodium Carbonate
50.6~ Sodium Sulfate
100 . 0%--
The above composition is ormulated to deliver, desirably, 14
30 parts per million total available oxygen (ppm A.O.), at a pEI of
about 10.5
~.~ 7~
In another one of the preferred embodiments, in which a mixed
diester compound as in (III) above is the precursor, a preferred
bleach composition has the following ingredients:
15.5% Sodium Perborate Tetrahydrate
7.0% Resorcinol octanoate Acetate
7.0% Nonionic Surfactant
15.0~ Sodium Carbonate
55.5% Sodium Sulfate
100 . 0%
The above composition is formulated to deliver, desirably,
about 14 ppm A.O. at a pH of about 10.5. Other peroxygen sources,
such as sodium perborate monohydrate or sodium percarbonate are
suitable. If a more detergent-type product is desired~ the amount
of filler can be increased and the precursor halved or further
decreased.
The novel precursocs of this invention are synthesized by the
methods which are disclosed below. Additionally, perforrnance
results are showri below in the EXPERIMENTAL section.
~18-
EXPERI MRNTAL
I synthesis of 1 Octanoyloxy-3-Hydroxy Benzene
O
-~-C7E~15
/\
~ 1 OH
Adapting the method of synthesis disclosed .in D. Johnston,
5 ~Preparation of Hydroquinone Monoacetate, n Chemistry & Industry
24:1000 ~lg82),it i~ .
expected that resorcinol may be combined with about an equimolar
amount of dioctanoic acid anhydride, and ethyl acetate solvent, a
non-nucleophilic solvent, in the presence of 4-dimethylaminopyri-
10 dine, a catalyst, and a base, such as triethylarnine, at roomtemperature, to produce the desired 1 octanoyloxy-3-hydroxy
benzene (resorcinol monooctanoate).
Therefore, the followinq procedure was performed:
~esorcinol (2.75 g, 0.025 mole), 4~dimethylaminopyridine (0.3
15 g, 0.0025 mole), triethylamine (2~5 g, 0.025 mole) were dissolved
in 50 ml of ethyl acetate in a 100 ml round bottom flask equipped
with a magnetic stir bar. Dioctanoic acid anhydride (6.76 g,
0.025 mole) was added dropwise, via an addition funnel, to the
stirred solution over a 100 minute time period~
The resulting solution was stirred ~or an additional 30
minutes, at which time the solvent was removed via rotary vacuum
evaporation The remaining oil was dissolved in 200 ml of ethyl
ether and extracted with a 200 ml portion of 3g HCl to remove the
4-dimethylaminopyridine catalyst, and four 100 ml portions of 5
25 NaHCO3 were used to remove the octanoic acid byproductO
--19--
After drying the organic phase with ~0 grams of Na2S0~,
the ether was removed by rotary vacuum evaporation and the
remaining oil was redissolved in 15 ml of chloroform. The sample
was then chromatographed in a column on 200 grams of silica gel G
5 with chloroformJpetroleum ether (1:2 vol/vol ratio) and pure
resorcinol monooctanoate (2.36 g) was collected. Yields of the
desired monoester were typically about 40%~wt.).
Surprisingly, unlike in the synthesis described in Johnston's
report, the~ high yields of desired monoester, resorcinol
10 monooctanoate, were not achieved. However, beneficially,
-symmetrical diesters, resorcinol dioctanoate, were co-produced in
a slightly greater portion (about 50~(wt.)) and available for use
in the present invention.
In the foregoing synthesis, and in those depicted in III and
15 IV, it is believed that any of the dihydroxybenzenes are suitable
for use as starting materials. If non nucleophilic solvents are
required, as in base catalysis, acetone tdimethyl ketone), ethyl
or methyl acetate, tetrachloromethane, dichloromethane, ethylene
chloride, chloroform, and others appear appropriate to the
20 synthesis. The catalyst, 4-dimethylaminopyridine, appears to
promote transesterification by acting to for~ a reactive
intermediate. other suitable catalysts may include pyridine-and
other tertiary aliphatic and aromatic amines. The base, which may
act to tie up any carboxylic acid moieties formed in the reaction,
25 may include triethylamine, tetramethyl piperidine, NaHCO3,
Na2C03, and suitable tertiary amines. In the selection of
suitable bases, care must be taken to insure solubility of the
ingredients in the reaction. Similarly, if acid catalysis is the
chosen route of synthesis, concentrated sulfuric acid,
30 hydrochloric acid, and methanesulfonic acid are among the
catalysts of choice known to those skilled in the art.
-20-
~'7~71>7
II. Comparison of Diacyl Peroxide Formatlon
In order to ascertain the amounts of diacyl peroxide formed
when less than a 1.5: 1 H2O2: precursor ratio are used,
applicants compared the levels of diacyl peroxide formed when two
peracid precursors were separately combined with H2O2, namely,
resorcinol monooctanoate (representing a mono ester functionality
of one of the embodiments of the present invention,
7 15 .
~0~
and sodium octanoyloxy benzene sulfonate (NABSl,
7 15
10 [~ '
a+SO
which is one of the activators shown in U.S. 4,412,~34.
The two precursors were subjected to the following conditions:
H2O2: 1.25 X 10 M
(a) precursor~ 1.25 X 10 3~ predissolved in surfactant)
buffer: 0.02M NaHCO3/NaOH
pH: 10.5
temperature: 25C
~7~
(b) all conditions in (a), but H~02 at
2.5 X 10 3M. ~J. :~
The results were:
H202/~ster equiv. Diacyl Peroxide Content(M)
Monooctanoate NABS
(a) 1:1 0.3X10-4 Z.OX10-4
(b) 2:1 - b.3X10-4 1 OX10-4
The results show that at lower than 1.5:1 H202:precursor
ratios, the inventive precursors will maintain low amounts of
10 diacyl peroxide. The activators of U.S. 4,412,934, on the other
hand, will form significantly higher levels of diacyl peroxide.
Comparing the results, it should be noted that the activators of
U.S. 4,412,934 produce several times more diacyl peroxide as the
precursors of the present invention.
III. Synthesis of 1,3 Dihexanoyloxybenzene
O-~-C5~
o-c-cs~ll
In a reaction vessel, resorcinol is placed with an equimolar
amount of hexanoic acid anhydride (from Aldrich Chemicals~.
Concentrated sulfuric acid (98~) is added to the solution and
20 heated at 100C for 3 hours. A crude reaction product was
obtained from this acid catalysis containing the 1,3
dihexanoyloxybenzene (resorcinol dihexanoate) and hexanoic acid.
-22~
~ ~ 7 ~7 ~
The reaction mixture is diluted with diethyl ether and the
hexanoic acid removed by extraction with 5~ NaHCO3. The ether
phase is dried under Na2SO4 and rotary evaporated to remove
the solvent. For hydroquinone dihexanoate, the resulting solid is
5 recrystallized with EtOH/H2O to give a pure solid (m.pt.
56-57C). For resorcinol dihexanoate, the liquid is distilled
and the product fraction collected at 175-180/0.5~m Hg. Isolated
yields are generally 903 for either synthesis.
IV. Synthesis of 1 octanoyloxy-3~acetoxy benzene
O-II-C H
10 ~ \ o-~o-c~3
An acetoxylated resorcinol is obtained through commercial
sources (from American Hoechst). It is placed in a reacti.on
vessel with an equimolar amount of dioctanoic acid anhydride (from
Aldrich Chemicals), in the presence of rnethanesulEonic acid to
lS promote acid catalysis, and reacted at room temperature (21C)
for one hour. A 95% yield of the 1 octanoyloxy 3-acetoxy benzene
(resorcinol acetate octanoate) and octanoic acid as a by-product
results.
The purpose of the next experiment was to see if a greater
20 than 1.5 molar ratio of H2O2: precursor as contended by U.S.
4,412,934 was actually necessary for the precursors of~this
invention to give good yields of desired peracids.
23-
~27(g7~7'
V. Yield of 1 Octanoyloxy-3-Acetoxy Benzene
-
a. The compound synthesi~ed in IV (resorcinol acetate
octanoate) was combined in aqueous solution with sufficient
hydrogen peroxide to yield a hydrogen peroxide: precursor ratio
(based on ester equivalents) of about 1.4:1. The reaction
conditions were pH 10.5 (based on 0.02M NaHCO33, temperature
25 C, and lg/l liter of a nonionic surfactant, Neodol 25-12
(which is a linear ethoxylated alcohol with predominant chain
length of 12-15 carbon atoms, averaging about 12 moles of ethylene
oxide per mole of alcohol). The concentration of II (resorcinol
acetate octanoate) was 4.375 X 10 M, H2O2 was about 1.225 X
M, to result in an H2O2: precursor (based on ester
equivalents) ratio of about 1.4:1. Yields of about 7s% peracid
were obtained Low levels of diacyl peroxide were detected
consistent with the high peeacid yield.
b. Repeating the above experiment, with the compound of IV
~resorcinol acetate octanoate) at 4.375 X 10 M, but with 1.75 X
10 3M H2O2, to result in-a ratio of H2O2: precursor of
about 2:1, the~ resulting yield was about 78%. Thé reason for the
absence of substantial diacyl peroxide formation in a competing
side reaction as posited by U.S. 4,412,934 are presently unknown.
It is speculated that there is a lack of interaction between the
recently formed peracid and that portion of unreacted-precursor.
This theory is-for purposes of explanation- and not meant to
restrict the scope of the invention. It is also believed that any
acetyl octanoyl diacyl peroxide formed may be rapidly
re-perhydrolyzed, i.e., converted back into peracid, without the
need for a large excess of hydrogen peroxide. Further experiments
appear to bear out the low diacyl peroxide formation in the
inventive compositions.
-24-
Perforrnance tests for the inventive precursors have also been
conducted. The pcecursors have been found to exhibit siynificant
improvements in bleaching performance over a commercial dry
perborate bleach:
VO % Stain Removal of crystal Violet stained Cotton Swatches
o~ L, 3 Dihexanoyloxy Benzene
Composition % Stain Removal
H2O2+ resorcinol dihexanoatel/
Neodol 25-12 90.45~+1.26
10 H22 + Neodol 25-12 76.77+1.24
H22 + resorcinol dihexanoatel 69.85+2.84
Neodol 25-12 80.45+1.05
Commercial Blea-ch ~sodium,
perborate, brighteners, builders) 73.45+2.39
H2O2 - 2.50 X 10 M
Resorcinol dihexanoate = 6O25 X 10 M
pH 10.5, 0.02M carbonate buffer, 38C
10 minutes wash time
Average of 5 swatches in 200 ml wash water
1 1,3 Dihexanoyloxy Benzene
~25
VI. ~ Stain ~emoval of Crystal Violet/Cotton swatches
of 1 Octanoyloxy-3-Acetoxy Benzenel
Composition % Stain Re~oval
Buffer only 29.7 5.7
H22 + Neodol 25-12 65.8+1.4
C2/C~ 1(7 ppm A.O. theoretical) 76.5_1.5
+ H2O2+ Neodol 15-12
C2/C8 1(10 ppm A.O. theoretical) 79.0+1.1
~H2O2+ Neodol 25-12
C2/Cg 1(14 ppm A.O. theoretical) 82.0+0.4
+H2O2+ Neodol 25-12 -
Peracetic acid (7 ppm A.O.) 50.4_3.0
Peroctanoic acid (7 ppm A.O.) 83.8_1.9
H2O2= 1.75x10 M
pH 10.5 0.02M carbonate buffer 22 C
10 minutes wash time
Average of 5 swatches in 200 ml wash water
1 ~esorcinol Acetate octanoate
. .
-~6
~'~'70~
The foregoing description and embodiments of the invention
have been presented for purposes of illustration and not intended
to restrict the scope of the invention. other non-limiting
embodiments of the invention are possible~ For example, standard
5 bleaching and detergent adjuncts may be added to the compositions
disclosed. Exemplary of such adjuncts are builders (sodium
carbonate, sodium tripolyphosphate, etc.), fille~s (e.g., sodium
sulfate), brighteners, enzymes ~e.g., alkaline proteases),
defoaming agents, and the like known to those skilled in the art.
10 Additionally, further esterification of the phenylene diesters may
be possible, for example, resulting in tri- and quaternary-,
substituted phenylene precursors. The claims hereto further
llustrate the invention.