Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
3~
METHANOL VAPORIZATION FOR METAL PROCESSING FURNACES AND THE LIKE
This invent;on relates generally to metal processing furnaces
requiring a reducing atmosphere, for example continuous brazing
furnaces, annealing, sintering, neutral hardening and carburizing
furnaces. Specifically, this invention relates to a method and
apparatus for providing a reducing atmosphere within the furnace in
an efficient and cost-2ffectiYe manner.
BACKGROUND OF THIS INVENTION
Open ended brazing furnaces and the like are ~ell known.
Typically, a part may have a dwell time of up to 1-1/2 hours in such
a furnace in order ~or the particular operation to be accomplished.
In a brazing furnace, for example, parts are "brazed" together using
copper or other brazing alloy between the parts. The furnace is
typically at a temperature above the melting point of the brazing
material.
In order to allow the brazing material to bond to the steel,
iron or alloy parts, a reducing atmosphere is required which will
reduce (remove) the oxides from the parts. By using a reducing
atmosphere, no flux is required since the brazing material will run
over and bond with the part when it has been cleaned due to the
reducing atmosphere. The most common gaseous substance used for
reducing is hydrogen (H2), and the most typical conventional mekhod
currently used to provide the hydrogen ~s to spray atom k ed methanQl
into the furnace cracking the methanol into two molecules of hydrogen
and one of carbon monoxide. However~ this injection of atom k ed
droplets of liquid methanol into the furnace, normally called
sparaging, gives rise to a number of problems. In the first place,
the atomization jets create undesirable turbulence at the furnace
atmosphere, which leads to a high atmosphere consumption. This
turbulence can also upset furnace performance. Although the methanol
is in association with nitrogen, these ~wo components do not dwell
in a heated surrounding, and the nitrogen simply acts as an atomizing
medium for the methanol in the atomization jets of the sparger. This
means that the methanol is not vaporized while in contact with the
nitrogen prior to the atomization jets, and that all vaporization of
the methanol in the conventional process takes place within the
furnace and draws active heat away from the furnace interior.
~ f ~
Another problem with sparging relates to the fact th~t the
conveying belt through the furnace is of a high alloy material in
order to withstand the furnace heat. Droplets of liquid methanol
must not be allowed to hit the belt, because at the high temperature
of the belt the methanol would crack directly on the belt and would
result in carbon being transferred into the belt, thus weaken;ng the
belt~
Another disadvantage of the sparging method is that all of the
energy for vaporizing the methanol (which must take place before it
cracks) must come from the furnace, thus risking a decrease in
furnace productivity.
An alternative method which is also presently known is t~
utilize a separate device to vaporize methanol~ The current cost of
such devices is quite high, and of course extra electrical or other
high priced energy must be provided in order to accomplish the
vaporization.
Of course, pure hydrogen could also be provided to the
furnace, but at the present time such a proposal is uneconomical
compared to methanol.
GENERAL DESCRIPTION OF THIS INVENTION
.
In view of the foregoing problems with conventional ~ethods of
proYiding a reducing atmosphere in a furnace of the kind under
discussion, a method has been developed of vaporizing methanol
without requiring additlonal electr~cal or other energy for khe
vaporization, the method being one which simply utilizes waste heat
already provided by the furnace itself.
It is not enough simply to pass the methanol through a conduit
which receives the waste heat from the furnace. A sustained
temperature above 60~C is re~uired for 100% methanol.
The essence of the invention relies on a recognition that
Dalton's law o~ partial pressure permits, in effect, a lowering of
the vaporization temperature of methanol when it comprises only a
fraction of a mixture in which the other component is gaseous. Thus,
for a mixture in which methanol is present at between 10~ and 20~, a
temperature between 15C and 30C will suffice to rapidly vaporize
all of the methanol. When the mixture consists of 60~ methanol,
temperatures in the region of 50C are required. Specifically,
nitrogen utilized as the gaseous component of the mix~ure and liquid
,;
.
3~
- 3
methanol is passed along with the nitrogen through a conduit,
preferably copper heated in heat-transfer contact with a wall of the
furnace through which waste heat passes.
For example, in a continuous brazing furnace operation a gas
flow of 2,000 cubic feet per hour at atmospheric pressure is
required. If the mixture consists of 20~ methanol approx;mately
5,500 BTU/hour of heat is needed for vaporization. If this heat is
obtained from the waste heat of the furnace itself instead of from
anelectrically heated sparger, considerable savings are achieved.
It is therefore an object of one aspect of this invention to
provide a method of providing methanol within a metal processing
furnace or the like which methanol comprises the steps of:
a) passing a mixture of liquid methanol and gaseous nitrogen
through a conduit which is in heat transfer relationship with
the waste heat from said furnace whereby the waste heat
vaporizes the methanol into the nitrogen, and
b) injecting the nitrogen and methanol gas into the furnace.
It is an object of another aspect of this invention to provide
a furnace for metallic objects in a reducing atmosphere comprising:
20 a) an open ended furnace body having walls through which waste
heat passes from a heat source within said body,
b) a conveyor belt passing through said furnace,
c) a conduit connected at i~s ups~ream end ~o a source of li~uid
methanol and gaseous nitrogen and to at least one lnjection
inlet in the Furnace wall at its downstream end,
d) a portion of sa~d conduit posi~ioned in clost proximity ~o the
wast heat from said furnace wall whereby the liquid methanol
is vaporized into the nitrogen prior to injection into the
furnace.
GENERAL DESCRIPTION OF THE DRAWINGS
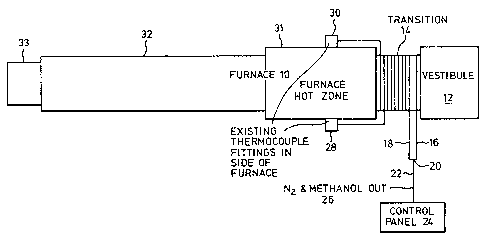
The single figure is a schematic representation of a furnace
with the methanol vaporization system of this invention installed.
DETAILE~ DESCRlPTION OF THE DRAWINGS
In the figure, a furnace 10 is shown in plan view. The
furnace includes a vestibule 12 for entry of parts intented to pass
through the furnace 10. A transition portion 14 is a typical part of
such furnaces, and may have a round or rectangular cross-section.
The transition portion 14 incorporates a wall or walls through which
waste heat from the furnace 10 passes.
. .,
~7~3!7;~
- 4
Wrapped around the transition portion 14 are two distinct
coils 16 and 18 of copper pipe. The length of the coils is not
critical, since it depends on the pipe diameter and the flow of
methanol. As an example, however, the coi1s may each be about lO0
feet of 3/8" or l/2" tubing. A combined length of at least 150 feet
and a diameter of 1/2" usually performs satisfactorily. The copper
pipe thus constitutes a conduit in heat-trans~er relationship with
the walls of the transition portion 14 through which waste heak
passes, and therefore picks up waste heat from this region. The
upstream ends of the two coils 16 and 18 come together at a "T" 20
and connect with a single conduit 22 from a control panel 24, the
control panel hav1ng the capability of metering nitrogen and methanol
into the upstream end 26 of the conduit 22. At the downstream end of
coil 16 is an access fitting 28 which allows the mixture of nitrogen
and vaporized methanol to enter the furnace at one side. A similar
fitting 30 is provided for the coil 18 at the other side of the
furnace. Downstream of hot zone 31 is an elongated cool down zone
32. A portion of conveyor belt 33 is shown at the discharge end of
the furnace for removing the metal objects from the furnace.
Another means of heat transfer involves using the waste heat
of the cooling water from the furnace cooling pipes. In normal
brazing furnace operations the cooling water would be at
approximately 60~C. Since the heat transfer relat~onsh1p between the
methanol/nitrogen coil and the cooling water pipes is more efflcicnt
than between the coll and the furnace wall, a short coil can be used
between "T" 20 and access fitting 28.
It is quite satisfactory to operate the system at atmosphere,
or at a pressure only sllghtly above atmosphere in order to cause the
mixture to travel through the coils. However, the high pressures
conventionally used for nitrogen sparging are not required~
A built-in safety feature is incorporated into the system in
addition to the fact that the system is operated at substantially
atmospheric pressure. Specifically, an energized-open solenoid is
used for the methanol and the nitrogen entry into the conduit 22, so
that upon power failure or induced power failure by safe~y interlocks
the solenoid will close and the methanol and nitrogen feed will
cease. At the same time, there is provided a parallel feed of
nitrogen alone into the pipe, with a solenoi~ that is
energized-closed. This ensures that, upon power failure. The
furnace will be purged with n~trogen, and that no further methanol
will enter it.
. .
. ~ .,: .
It will thus be appreciated ~hat I have provided a me~hod and
apparatus by which a furnace can be furnished with a reducing
atmosphere, without requiring the considerable expense involved in
purchasing a separate methanol vaporizor unit, and without drawing
away active heat from the interior of the furnace. The method of
apparatus I have proposed utilize only waste heat, and furthermore
avoid the problems created by the presence of liquid methanol
droplets within the furnace. As indicated earlier, these droplets
and the associated high velocity nitrogen can disrupt the furnace
atmosphere9 lead to higher a~mosphere consu~ption, and cause changes
in flow rate that can upset furnace performance. Also, the high
alloy material of the conveying belt ~ould be destroyed if droplets
were to contact it. A further problem with sparging is that it can
create cold spots in the furnace.
It will be appreciated that the method of this invention could
also employ other inert or near-inert gases in place of nitrogen, for
example argon or helium. However, at the present time nitrogen
appears to be the least expensive of the suitable gases available.
For a carburizing furnace, it is appropriate to use a mixture
of gases containing approximately 60~ methanol, thus requiring a
somewhat higher temperature of vaporization. The me~hanol gives CO
and H2 upon cracking within the furnace
If it is des~red to slow the velocity of the yaseous mixture
out of the colls 16 and 18 where they enter at the access fixtures 28
and 30, the copper piping can be expanded to a larger diameter, ~or
example from 1/2" to 1".
While one embod~ment of this invention has been illustrated in
the accompanying drawings, and described hereinabove, it will be
evident to those skilled in the art that changes and modifications
, 30 may be made therein, without departing from the essence of this
invention as set forth in the appended claims.