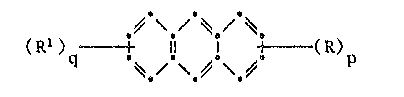Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ 3~l
-- 1 -
3-15061/-tMAC 186
Anthracene Darivatives
The pr~sent invention relates to new anthracene compound~,
especially functionally alkylated anthracene compounds us~ful as
ssnDltisors in the photo-crosslinking of resin system~.
Substitutld anthracene derivatives containing polar-group bearing
substituents are known in the art. Thus M.5. New~an and S. Otzuka
describe in J. Org. Chem., 23, 797 (1958) 1-snthryl derivatives
carrying ftmctionally substituted alkyl groups. These alkyl groups
may have a methyl group attached to the carbon atom that i6 bound to
the anthracena ring and the~s alkyls carry polar groups, such as
-COOH, -OH or -CN. The compounds are used a8 lnter~edlates for the
preparation of 1,2-b~nzanthracenes.
P. Arjunan et al. describe ~n J. Org. Chem., 46, 626 (1981) 2-and
i 9-anthrylalkane carboxylic acids and the corre~ponding esters. These
~ compounds are used as fluorescent probes in membranes.
:
According to the present invantlon, there are provided compounds
having the formula:
~t
(Rl )q~ + 5 ! +~ (R)p (I),
wherein p is 1 or 2 and q ls O or 1 provided that p~q is 1 or 2,
each R is independently a residue of for~ula:
`~
-- 2 -- .
-A-(Q)k (II),
wherein Q ls a re~idue -COZR2 wherein Z i3 0 or NR3, and R2 ia H,
straight or branched chain alkyl having from 1 to 18 carbon atoms, a
straight or branched chain alkenyl group having 3 to 18 carbon
atoms, a cycloalkyl group having from 3 to 12 carbon atom~ or an
aralkyl group having 7 to 9 carbon atom~: and when Z is -NR3, R3 i3
hydrogen or a straight or branched chain alkyl group having from 1
to 8 carbon atom~l or R2 and R3, together ~ith the ni.trogen atom to
which they are each bonded, may form a 5- or 6-membered heterocyclic
ring; or Q i~ a re~idue -OX wherein X i3 R3 or CoR4, wherein R3 ha~
itB previous significance, and R" is H, a 3tralght- or branched
chain alkyl group having from 1 to 18 carbon atoms, a C3-Cl~
~traight or branched chain alkenyl group or a C3-Cl 2 cycloalkyl
group; a residue CN; k i~ 1 or 2, A i9 a residus of formula
~ ~CnH2n+1-k) (III),
wherein n is an integer from 1-17, k is as defined above, Rs and R5
are ths sa~e o~ differ~nt and each i9 a straight- or branched chain
alkyl group having 1 to 5 csrbon ato~s and, when Q i~ COORZ eithes
Rs or R5 iB opt~onally substituted by a COOR2 group the R2 group~
being the same or different, prov~dQd that when Rs or R5 is sub-
stituted by a COOH group (i.e. RZ~H), then tha co~pound of formula I
doss not contain more than three COOH group~ or R5 or R6 may be
llnkod to the residue CnH2n+1_k to fo m 5 12 Y Y
residue sub~tituted by tha group -(CO2RZ)k wherein the R2 groups
independently have thelr previous significance and k has ~ts
previous significance; each Rl, independently,
i5 a Cl-C~ ~traight- or branched chain alkyl, a C7-Cg aralkyl
group, optionally ~ubstituted by one or two halogen atoms, an
alkyl-(~1-C4~-cycloalkyl-(Cs-C12) group; or R1 ls a re~ldue of
~ 31L
_ 3 -
formula ~I as hereinbeforQ d~fln~d, and when Rl 1~ a re~ldue of
formula II, then Rl nnA R may be the same or diEorent; and ~alt~ o~
carboxyllc acld6 of Eormula I with organic or lnorganic bases.
~hen the group Rl i~ a Cl-ca ~traight- or branched chain alkyl group
group it may be, for example~ a methyl, ethyl, n-propyl, i30propyl,
n-butyl, iso-butyl, t-hutyl, t-pentyl, or 1,1,3,3-tetramethylbutyl
r~?sidue.
When the groups R2, R3 or R~ are Cl~Cla straight or branch~d chain
alkyl they may be the same or different snd may be e.g. methyl,
athyl t n-propyl, iso-propyl, n-butyl, ~ec-butyl, n pentyl, n-hexyl,
n-heptyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-dodecyl,
n-hexadecyl or n-octadecyl.
When the groups Rs and R6 are Cl-C5 straight- or branched chain
alkyl, they may be the same or different and may be e.g. methyl,
ethyl, n-propyl, isopropyl, n-butyl, sec.-butyl or n-pentyl.
When R2 or R4 is a C3-Clg straight or branched chain alkenyl group,
it may be for example, a prop-2-enyl, n but 2-enyl, 2-methyl-
prop-2-enyl, n-pent-2-enyl, D-hex-2-enyl, n~hexa-2,4-dienyl,
n-dec-10-0nyl, n-heptadec-~-enyl, or n-heptadec-8,11-dienyl group.
When the group R2 os Rt is a C3 C~2 cycloalkyl group, it ~ay be, for
exampla, a cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cyclooctyl or cyclododecyl group.
When the groups R2 and R3, together with the nitrogen atom to which
~hey are bonded form a 5- or 6-membered heterocycllc ring this ring
may be a pyrrolldin~, piperidin0 or morpholine ring.
When the group Rl is a C7-Cg aralkyl group, optionally substituted
by one or ~wo halogen atoms it may be, for example, a benzyl,
a-methyl-ben7yl, p-chloro-~-methyl-benzyl, cumyl, p-chlorocumyl or
2 ? 4-dichlorocumyl group.
When the group R2 iu a C7-Cg aralkyl group lt may be for example, a
benzyl, ~-methylben~yl, or cumyl group.
When the growp R1 i9 an alkyl (C1-C4)-cycloalkyl-~Cs-C12) group it
may be, for example, a 1-methyl-cyclopent-1-yl, l-methyl-cyclohex-
1-yl, l-methyl-cyclohept-l-yl, l-methyl-cyclooct-l-yl, l-ethyl-
cyclohex-l-yl or 1-n-butyl-cy&lohex-1-yl group.
Examples o~ ~alts where Q i9 an acidic group, e.g. COOH, include
salts with al~all and alkaline esrth metals and amines.
Examples of aminss which caD form ~alts when Q is an acidic group
include aliphatic- cycloallphatic-, aromatic-, araliphatic- and
heterocycllc amines.
Exampleg of aliphatic amlnes are 1-20C aliphatic amine3 such as
ethylamlne, triethylam~ne, decyl~mine, dodecylamine and o~ta-
decylamine; examples of cycloaliphatic smineg are 5-8C cycloali-
phatic amines ~uch as cyclohexylamine; examples of aromatic aminea
are aniline and toluidine; an example of an araliphatic amine i9
benzylamlne; and heterocyclic amines lnclude morphol~ne and
pyridine.
In one preferr~d embodiment p~q ~ 2, more prefarably the gro~ps R
and R1 are each a re6idue of formuls II, it i8 egpecially preferrPd
that the residues of formula II are the same and are preferably
bonded in the 2,6- or 2,7-positions of the anthracene molecule.
Othar preferred compoundg of the invention are tho~e having the
formula IV
RI
Rt !~ i i ( IV),
~./ \,~ \0~
-- 5 --
wherein R i8 a resldue of formula II as hereinbefore defined and R~
iB a residue of formula II, as hereinbefore defined, or is a Cl-C8
straight- or branched chain alkyl re~idue;
as well as ~alts thereof.
Yet other preferred compound~ are those wherein p-l and qeO~
Still other preferred compounds of formula I are those in which ~ is
1.
Partlcularly preferred compounds of formula I are those in which
i8 1 and Q i8 a residue CO2R2 whereln R2 has its previous signi-
ficance.
Non limiting examples of compound~ of formula I include:
2-(3-Methoxycarbonyl-2-methyl-prop-2-yl)-anthracene
2-(5-Carboxy-2-methyl-pent-2-yl)-anthracane and its sodium salt
2-(5-Methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-(5-Ethoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-(5-n-Butoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-(5-n-Hexyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-[5-(2-Ethylhexyloxycarbonyl)-2-methyl-pent-2-yl]snthrscene
2-(5-n-Decyloxycarbonyl-2-methyl-pent-2-yl)anthracene
2-(5-n-Octadecyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-(7-Methoxycarbonyl-2,6-dimethyl-hept-2-yl)-anthracene
2-(5-N-n-Butylcarbamoyl-2-methyl-pent-2-yl)-anthracene
2-(7-Cyano-2,6-dimethyl-hept-2-yl)-anthracene
2-(6-Hydroxy-2-methyl-hex-2-yl)-anthracene
2-(6-Acetoxy-2-methyl-hex-2-yl)-anthracene
2-(8-Hydroxy-2,6-dimethyl-oct-2-yl)-anthracene
2-(6-Hydroxy-2,6-dimethyl-hept-2-yl~-anthracene
2-(8-Acetoxy-2,6,dimethyl-oct-2-yl)-anthracene
2-(8-Acryloyloxy-2,6-dimethyl-oct-2-yl)-anthracene
2-(8-Methoxy-2,6-dimethyl-hept-2-yl)-anthracene
Ci8 and trans-2-(4-methoxycarbonyl-1-methyl-cyclohex-1-yl)-
anthracene
73~
- 6 ~
2-t-Butyl-6-(3-methoxycarbonyl-2-methyl-prop-2 yl) anthraceno
2-t-Butyl-7-(3-methoxycarbonyl-2-methyl-prop-2 yl)-inthracene
2-(S-Car~oxy 2-methyl-pent-2-yl)-6-methyl-anthrscene and its ~odlum
Ralt
2-(5-Carboxy-2 Methyl-pent-2-yl)-7-methyl-anthracene and it~ sodlum
~alt
2-~5-Methoxycarbonyl-2-methyl-pent-2-yl)-6-methyl-anthracene
2-(5-Methoxyca~bonyl-2-methyl-pent-2-yl)-7-methyl-anthracene
2-15-Etho~ycarbonyl 2-methyl-pent~2-yl)-6-methyl-anthracene
2-(5-Ethoxycarbonyl-2-methyl-pent-2 yl)-7-methyl-anthracene
2-~S-n-Butyloxycarbonyl-2-methyl-pent-2-yl)-6-methyl-anthracene
2-~5-n-Butyloxycarbonyl-2 methyl-pent-2-yl)-7-methyl~anthracene
2-(5 n-Hexyloxycarbonyl-2-methyl-pent-2-yl)-6-methyl-anthracens
2-~5-n-Hexyloxycarbonyl-2-methyl-pent-2-yl)-7-methyl-anthracene
2-(5-n-Decyloxycarbonyl-2-methyl-pent-2-yl)-6-methyl-anthracene
2-(S-n-Decyloxycarbonyl-2-methyl-pent-2-yl)-7-methyl-anthrAcene
2-Ethyl-6-(5-methoxycarbonyl-2-methyl-pent-2-yl~-anthracene
2-Ethyl-7-~5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-(S-carboxy-2-methyl-pent-2-yl)-anthracene and its sodium
Ealt
2-t-Butyl-7-(5-carboxy-2-methyl-pent-2-yl)anthracene and its sodium
æalt
2-t-Butyl-6-(S-methoxycarbonyl-2-methyl-pent-2-yl)-anthrscene
2-t-Butyl-7-(5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-~utyl-6-[5-ethoxycsrbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-7-(S-ethoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-~5-n-propyloxycarbonyl-2-methyl-pent-2-yl)-anthraGene
2-t-Butyl-7-(5-n-propyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-(5-n-isopropyloxycarbonyl-2-~ethyl-pent-2-yl)-anthracene
2-t-Butyl-7-(5-n-isopropyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-(5-n-butyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-7-(5-n-butyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-S-(S-n-hexyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-7-(5-n-hexyloxycarbonyl-2-methyl-pent-2-yl~-anthracene
2-t-Butyl-6-L5-~2-ethylhexyloxycarbonyl)-2-methyl-pent-2-yl]-
anthracene
~2~
2-t-Butyl-7-[5~(2-ethylhexyloxyclrbony~ 2-methyl-pent-2-yl~-
anthr~cene
2-t Butyl-6-(n-decyloxycarbonyl 2-methyl-pent-2~yl)-anthracene
2-t-Butyl-7-(n-decyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-(cylcohexyloxycarbonyl-2-methyl-pent-2-yl~-anthracene
2-t-Butyl-6-(S-csrbamoyl-2-methyl-pent-2-yl)-anthracene
2-t-Butyl-6-(5-N-methylcarbamoyl-2-methyl-pent-2-yl)-anthracene
2-(1,1,3,3-tetramethylbutyl)-6-(S-methoxycarbonyl-2-methyl-pent-
2-yl)-anthracene
2-(l,1,3,3-tetramethylbutyl)-7-(5-methoxycarbonyl-2-methyl-pent-
2-yl)-anthracene
2-Cumyl-6-(5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-Cumyl-7-(5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
2-(l-methyl-cyclohex 1 yl)-6-(5-methoxycarbonyl-2-methyl-pent-2-yl)-
anthracene
2-(1 methyl-cyclohex-l-yl)-7-(S-methoxycarbonyl-2-methyl-pent-2-yl)-
anthracene
2-tert-Butyl-6-(7-methoxycarbonyl-2,6-dimethyl-hept 2-yl)-anthracene
2-tert-Butyl-7-(7-methoxycarbonyl-2,6-dimethyl-hept-2-yl)-anthracene
2-tert-Butyl 6-(7-cysno-2,6-di~ethyl-hept-2-yl)-anthracene
2-tert-Butyl-7-(7-cyano-2,6-dimethyl-hept-2-yl)-anthracene
2-tert-Butyl-6-(8 hydroxy-2,6-dimethyl-oct-2-yl)-anthracene
2-tert-Butyl-7-(8-hydroxy-2,6-d1methyl-oct-2-yl)-anthracene
2-tert-Butyl-6-(8-acetoxy-2,6-dimethyl-oct-2-yl)-~nthracene
2-tert-Butyl-7-(8-acetoxy-2,6-dim0thyl-oct-2-yl)-anthracene
ci~ und trans 2-t-B~tyl-6-(4-methoxycarbonyl-1-methyl-cyclohex
1-yl)-anthracene
cis und trans 2-t-Butyl-7-(4-methoxycarbonyl-1-methyl-cyclohex-
l-yl)-~nthracene
2,6-Bis-(3-methoxycarbonyl-2-methyl-prop-2-yl~-anthracene
2,7-Bis-[3-methoxycarbonyl-2-methyl-prop-2-yl?-anthracene
2,6-Bis-(5-carboxy-2-methyl-pent-2-yl)-anthracene and its 30dium
salt
2,7-Bis-(~i-carboxy-2-methyl-pent-2-yl)-anthracene and its sodium
salt
2,6-Bis-(S-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene
8 -
2,7-Bi~-(5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracqne
2,6-Bi~-(5-ethoxycarbonyl-2-m~thyl-pqnl:-2~yl)-anthraccne
2,7-Bis-(5-ethoxycarbonyl-2-methyl-pent 2-y].)-anthracene
2,6-Bis-(S-n-propyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,7-Bls-(5-n-propyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,6-Bis-(5-n-isopropyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,7-Bis-(5-n-lsopropyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,6-Bi~-(5-n-butyloxycarbonyl-2-methyl-pent-2-yl)-anthracena
2,7-Bis-(5-n-butyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,6-Bis-(5-n-hexyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,7-Bis-(5-n-hexyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,6~Bis-~5-(2-ethylhsxyloxycarbonyl?-2-methyl-pent-2-yl]anthracene
2,7-Bis-[5-(2-ethylhQxyloxycarbonyl)-2-methyl-pent-2-yl]anthracene
2,6-Bi~-(5-n-decyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,7-B1~-(5-n-decyloxycarbonyl-2-methyl-pent-2-yl)-anthracene
2,6-Bis-(7-methoxycarbonyl-2,6-dimethyl-hept-2-yl~-anthracene
2,7-Bis-(7-methoxycarbGnyl-2,6-dimethyl-hept-2-yl)-anthracene
2,6-Bi~-(5-N,N-dimethylcarbamoyl-2-~ethyl-pent-2-yl)-anthracene
2,6-Bls-(5-N-n-butylcarbamoyl-2-methyl-pent-2-yl)-anthracene
2,7-Bis-(5-N-n-butylcarbamoyl-2-mathyl-pent-2-yl)-anthracene
2,7-Bis-(5-N-allylcarbamoyl-2-methyl-pent-2-yl)-anthracene
2,7-B~s-(5-N-cyclohexylcarbamoyl-2-methyl-pent-2-yl~-anthracene
2,7-Bis-(5-N-ben~ylcarbamoyl-2-methyl-pent-2-yl)-anthracene
2,7-Bis-(5-morpholinocarbonyl-2-methyl-pent-2-yl~-anthracene
2,6-Bis ~-cyano-2,6-dimethyl-hept-2-yl)-anthracane
2,7-Bi3-(7-cyano-2,6-dimethyl-hept-2-yl)-anthracene
296-Bis-(8-m~thoxy-2,6-dimethyl-hept-2-yl~-anthracene
2,7-Bis-(8-methoxy-2,6-dimethyl-hept-2-yl)-anthracene
2,6-Bls-(8-hydroxy-2,6-dimethyl-hept-2-yl) anthracene
2,7-Bis-(8-hydroxy-2,6-diemthyl-hept-2-yl?-anthracene
2,6~Bis-(8-acetoxy~2,6-dimethyl-hept-2-yl?-anthracene
2,7~Bis-(8-acetoxy-2,6-dimethyl-hept-2-yl)-anthracene
2,6-Bi~-(8-methoxy-2,6-dlmethyl-hept-2-yl)-anthracene
2,7-Bis-(8-methoxy-2,6-dimethyl-hept-2-yl)-anthracene
2,6-Bi~-(8-acryloyloxy-2,6-dimethyl-hept-2-yl?-anthracene
2,6-Bis-(8-cyclohexanoyloxy-2,6~dimethyl-hept-2-yl)-anthracene
2,6 Bi~-(4~methoxycarbonyl-l-methyl-cyclohex-1-yl)-anthrncene
2,7-B1~-(4--methoxyc~rbonyl-1-methyl-cyclohe~ yl)-anthr~cene
The compound~ of formula I may be prepared by methods well known to
thoae ~killed in the art, for instance they may be made by func-
tional slkylatlon of anthracene, 2-methyl anthracene, 2-t-butyl
anthracene, 2-(3-methoxycarbonyl-2-methyl-prop-2-yl)-anth~acene,
2-(5-methoxycarbonyl-2-methyl~pent-2-yl)-anthracene and the like.
Alternatively, compound~ of formula I may be prepared by first
acylatlng anthracene, 2-methyl anthracene, 2-t-butyl anthracene,
2-(5 methoxycarbonyl-2-methyl-pent-2-yl?-anthracene, 2-(3-carboxy-
propanoyl)-anthrscene and the like, with appropriate acylating
agentg BUCh ag 8uccinic anhydride, glutaric anhydride, methyl
3-chloroformyl-propanoate, ethyl-S-chloroformyl-pentQnoate, methyl
9-chloroformyl-nonanoate and the like; followad by conversion of the
acyl carbonyl into a hydrocarbyl residue, by known methods. Examples
of such conversions to a hydrocarbyl residue are the converaion of
the carbonyl group to a ~C(CH3)2 residue by geminal alkylation.
Gemlnal alkylation of the carbonyl group may be carried out for
in~tance by the method describ0d by Weidmann and Seebach 9
Angew.Chem.Int.Ed., Engl. 22 (l983?, 31 45.
~hen the compounds of formula I contain two residues R or a residue
R and a residue R~, then the functional acylation andJor acylations
and conversion may be carried out in stepwise manner. When the two
residues R or two resldues R and Rl are ldentical it 1~ convenient
to introduca them simultaneously.
Mono-substituted anthracene~ not bearlng a functional group useful
a~ intermediates are known or ~ay be prepared by method~ known in
the art, e.g. the proce~s de~cribed in US Patent Specification
4 255 343~ Friedel Crafts abnd Related Reactions Vol. III by
G. Olah, and Houben Weyl, Band VII/Teil 2a page 76 et seq.
~L2~
~- L0 -
Functional fllkylation of aptlonally sub~tltllted anthrscene has the
advantage that lt i8 a slngle stage proeess for producin~ ompoullds
of formulA I which ls conventiently effected at a ta~peratllre
ranging from 20 to 170~C, convenlently 20 to 120C. Functional
acylation may llkewise ba effscted at similar elevated temperatures.
The reaction is ePfected in the presence of a cataly3t e.g. a
Bron~ted acid, an active earth, a metal salt or a metal ~alt in
combination wLth a Bronsted acid. Bronsted acids used may be organic
or inerganic ~tcid~, or partial ~alts thereof, and may be an lnor-
ganic mineral acid e.g. hydrochloric-, sulphuric-, perchloric-or
orthophosphoric acid; an alkyl-, haloalkyl-, aryl- or alkaryl-
substituted inorganic ac~d e.g. methane, dlfluoromethane, tri-
fluoromethane or ethane sulphonic aclds5 benzene sulphonic acidl
p-toluena sulphonic acid or methane phosphic acid; or an organic
acid e.g. dichloroacetic acld, trichloracetic acid or trifluoro-
acetic acid. Active earths used may be those commercially-available
under the trade mark Fulmont~ 237 and Fulcat~ 22; while aluminium
chloride and zinc chloride and stannic chloride are suitable metal
salts.
Dapending on whether one or two functional alkyl or functional acyl
groups are being introduced up to one or at lea~t two moles of
functional alkylating or functional acylating agent are used per
mole of anthracene. When one alkyl-, acyl-, or functional alkyl
group is being introduced into a substituted anthracene then up to
one mole of ~1kylating agent, acylating agent, or functional
alkylating agent is used.
The reaction may be carried out with or without a solvent, of which
benzene, chlorobenzene, toluene, xylene, nitromethane and acetic
acid are suitabla.
Function11 al~ylating agents whlch may be used contain a reacti~e
centre, a.g. an olefinic- or hydroxy group.
Examples of olefinic functlonal alkylating agent~ are:
5-Methylhex-5-enoic acid
Methyl-5-methyl~hex-4-enoate
Methyl-5-methylhex-S-enoate
Ethyl-5-methylhex-5-enoate
n-Propyl-5-methylhex-5-en~ate
iso-Propyl-5-methylhex-5-enoatQ
n-Butyl-5-methylhex-5-enoate
iso-Butyl-S-methylhex 5-enoate
sec-Butyl-5-methylhex-5-enoste
n-Pentyl-5-methylhex-S-enoate
i~o-Pentyl-5-methylhex-5-enoate
~ec-Pentyl-S-methylhex-5-enoate
n-Hexyl-5-methylhex-5-enoate
Cyclohexyl-5-methylhax-5-enoate
2-Ethylhexyl-5-methylhex-5-enoate
n-Octyl-5-methylhex-5-enoate
Methyl 5,7,7-trimethyl-oct-4-enoate
1,7-Di-methoxycarbonyl-4-methyl-hept-3-ene
4-Carbomethoxy-l-methylcyclohex-l-ene
4-Acetyl-l-methylcyclo-hex 1-ene
Methyl-citronellate
Citronellol
Citronellyl-acetate
Citronellyl-methyl-ether
Citronellyl-n-butyl-ether
Cltronellyl-nitrila
Ethyl-2-ethoxycarbonyl-5-methyl-hex-4-enoate
Examples of alkylating agents are isobutylene, diisobutylene,
t-butanol, t-butylacetate, 1,1,3,3-tetramethylbutan-1-ol,
l-methyl-cyclohex-l-ene, ~-methylstyrene, cumyl alcohol and cumyl
acetate.
73
1;~
-- 12 --
Any functlonal clerivative of a compound of formula I may be con-
verted to a different unctlonal derlvative. For example when (~
the acid group -C0211 it may be esterlf:Led with an alcohol R20H to
give the corre~pondlng ester -C()2R2, or when Q ls the ester group
-CO2R2 it may be transesterlfied to give a different R2 group, or
alternatively the ester group -CO2R2 may be converted to an amide
-CoNR2~.3 by treatment with NHR2 R3, wherein R2 and R3 have their
previous significance or may be converted to an alcohol by reduct:Lon
of by reaction wlth a Grignard reagent. When the group Q i8 sn
alcohol group, it may be esterified with an acylating agent and
converted into the group oco~4 in which R~ has its previous signi-
ficance.
The compound~3 of formula I are useful as sen~itisers for photo-
curable polymer systems of use for protective coatings or photo
image production.
The following Examples further illustrate the present invention.
Parts are parts by weight.
Exsmple 1
To a stirred solution of 15.0 parts of aluminium chloride in
50 parts of nitromethane at room temperature are added 8.9 parts of
anthracene followed by 14.2 parts of methyl 5-methyl-hex-5-enoate
dropwise over 15 mlnutes. On completing the addition stirrlng at
room temperature is continued a further 24 hours before the reaction
mixture is poured into lSO parts of 2N hydrochloric acid. The
organic phase is ether extracted washed wlth water and evaporated.
The resldual oll is then refluxed for 3 hours with 200 parts of
methanol which had previously been saturated with hydrogen chloride
gas. After ~tripplng down the methanol solution on a rota-evaporator
the residual oil is diluted with ether and the ether solution washed
with sodium bicarbonate solution, then water, and evaporated.
Distlllation of the residue gives a fractlon bo 7 250-300C con-
taining 94 % of 2,6 and 2,7-bis-(5-methoxy-carbonyl-2-methyl-pent-
lZ7;~73~
- 13 -
2-yl~-anthrac~ne. Cry~talll~atlon o~ thl~ mer mLxturo from
methanol ~ives, a~ a Elrat crop of crystAIs, the 2,6-iDomcr mp
111-3C with the following percentage compo~ltion by wei~ht.
Carbon Hydrogen
Found 77.82 8.65
Calculated for C30H3gOI~ 77.89 8.28
The mother llqllorD from the abov~ crystallisatlon are concentrated
and then ylPld, a3 a second crop of crystals, the 2,7-l~omer m.p
94-96C having
Carbon ~ydrogen
Found (C30H3~04) 77 94 8.38
Example 2
8.~ Parts of anthracene, 14.2 parts of methyl 5-methyl-he~-5-enoate,
and 26.1 parts of ~tannlc chloride ln 50 parts of p-xylene are
heated on steam-bath for 24 hour~. The reaction mixture is then
poured into 250 part~ of 2N hydrochlorlc acld and the organic phase
extracted with ether. The ether solution after washing with in turn
water, sodium bicarbonate solutlon and water is evaporated. The
re~idue is treated with 100 parts of ~ethanol and thers is then
filte~ed off 3.7 part~ of anthracene. The methanol solution is
evaporated under reduced pre~sure to leave a residue whlch distilled
to give a fraction bo 7 220C. Crystallisation of this fraction f~om
40-60C petroleum-ether gives 2-(5-methoxycarbonyl-2-methyl-pent-
2-yl~-anthracene with m.p 82-4C and the followlng percentage
co~position by weight.
Carbon Hydrogen
Found 82.59 7.79
Calculated for C22H2 42 82.46 7.5S
~L;2~
- 14 -
E _
26.1 Par~ of anhydroufl ~tannic chlorlde aro dL~olved ln 100 pArt~
of p-xylene, and to this ~olution i9 added 1.5 part~ t~lEluoro-
methane culphonic acid, 14.2 part~ of methyl 5-methyl-hex-5-enoate,
and 17.8 parta of anthracene. This mlxture is then heated on a ~team
bath for 8 hour~ and then poured into 500 parts of water. The
organic pha~e is taken up in ether, the ether solution wafihed wlth
water snd evaporated. To the residue 300 psrt~ of methanol are added
and brought to reflux before flltering to remove 6.7 psrt~ of
in~oluble snthracene. The methanol filtrate, to which is added
1.0 part of p-toluene sulphonic acid~ i8 refluxed for 4 hour3 to
e~terify any free acid produced in work up. After removlng the
~ethanol under reduced pressure, the residue is taken up in ether,
and the ether solution washed with sodium bicarbonate solution, then
water, and evaporated. Distillation of the residue gives as a first
~raction bo 5 210-40C 2-~5-methoxycarbonyl-2-methyl-pent-2-yl)-
anthracene m.p 82-4C identical with that obtainad in Example 2.
Continuation of the destillation give~ a second fraction bo 5
240-90C which is a mixture of 2,6 and 2,7-bis-(5-methoxycarbonyl-
2-methyl-pent-2-yl)-anthracene. Fractlonal crystall~Qation of thi~
mixture from methanol give~ the 2,6 ~nd 2,7-isomers with m.p 111-3C
and 94-6C respectively which are identical with tho3e obtained in
Example 1. The th~ee esters described in thi~ Example, on alkal~ne
hydrolysis, give ~helr re~pecitve acid as ~ollwos.
Acid m.p Carbon Hydrogen
2-(5-Carboxy-2-methyl- 149-51C Fo~nd 81.95 7.29
pent~2-yl?-anthracene Calc.C2lH2202 82.32 7.24
2,6-bi~-(5-Carboxy-2-methyl- 263-5~C Found 77.52 7.66
pent-2-yl)~anthracene Calc.C28H3404 77.39 7.89
2,7-bi~(S-Carboxy-2-methyl- 1$9-61C Found 77.40 8.12
pent-2-yl-)anthracene Calc.C28~13404 77.39 7.89
,. . .
- 15 -
Exampl~ 4
8.9 Parts Oe anthlacene, 18.4 parts o~ methyl cltrollellAte,
13.0 parts of stannic chloride, and 1.5 part~ of trifluoromethane
sulphonic acid1 in 50 parts of p-xylene are heated on a ~team-bath
for 24 hours. The work-up of Example 1 is followed and gives, on
distillation, a fraction bo 3 220-80C containlng 84 % of 2-(7-
methoxycarbonyl-2,6-dimethyl-hept-2~yl)-anthracene. The ester i8
obt~ined a~ a white cry~talline solid m.p 41-3C from 40-60
petroleum-ether, following column chromatography on a silica column
prspared in 40-60 petroleum-ether eluted with the same.
Carbon Hydrogen
Found 82.75 8.17
Calculated for C2sH~ 02 82.83 8.34
Alkaline hydrolysis of this ester gives 2-(7-carboxy-2l6-dimethyl-
hept-2-yl)-anthracene m.p 127-9C after crystalllsation from acetic
acid and containing a llttle ~ater.
Carbon Hydrogen
Found 82.70 7.76
Calculated for C24H2~Oz 82.72 8.10
Continuation of the above di~tillation gives a further fraction bo 6
290-310C which i~ a mixture of 2,6 and 2,7-bis-(7-methoxycarbonyl-
2,6-dimethyl-hept-2-yl~-anthracene.
Carbon Hydrogen
Fo~nd 79.38 9.19
Calculated for C36HsoO4 79.08 9~22
Example 5
Example 3 i5 rep~ated except that 30.8 parts of 4-methoxycarbonyl-
1-methyl-cyclohex-1-ene is used instead of the methyl 5-methyl-S-
hax-S-enoate and the reaction period iB 24 hours. Distillation gives
a fraction bo 5 200-60C of which 91 % is cis and trans -2-(4-
methoxycasbonyl-l-methyl-cyclohsx-l-yl~-anthracene. Trsatment of
this di~tillate with a mixture of ~ther and 40-60C petroleum-ether
~Llæ67~
givo~ a ~olld containing almo~t equal proportion~ of the ~i~3 and
trans i~omer~ and the~ are then separaterl by fractlonal cry~tallL-
~atlon from methAnol. Th~ cls and tran~ omer~ have m.p 178-81C
and 139-42C respectiv01y, and the following percentage compo-
sitions:
Carbon Hydrogen
Found for cls-iAomer 83.13 7.43
Calculated for trans-l~omer 83.28 7.42
Calculated for C23H2 402 83.10 7.28
The dlstillation i,3 continued and gives a fractioD bo 5 260-350C
whlch is dissol~ed ln ether, concentrated and diluted with 40-60C
petroleum-ether to give a mixture of 296 and 2,7-bi~3-(4-methoxy-
carbonyl-l-methyl-cyclohex-l-yl)-aslthracene m.p 176-214C.
Carbon Hydrogen
Found 78.83 7.91
Calculated for C3 2~13 a4 78.98 7.87
Exa~ple 6
Into 3.2 parts 2-~5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene,
; 2.6 parts ~tannic chloride and O.lS parts trifluoromethane sulphonicacid ln 25 parta oE p-xylene at 100C is pas~ed isobutylene ga6 for
1 hour with stirring. The reaction mixture i~ poured into 250 parts
of water and the organic phase i~olated with ether. Di~tillation
glves a fraction bo 5 190-220C which, aft~r dilution with 40-60C
petrolPum-ether and ~torage at -20C yield~ a mlxture of 2-tert-
butyl-6 and 7-(5-methoxycarbonyl-2-methyl-pent-2-yl)-anthracene m.p
81-9C.
Carbon Hydrogen
Found 83.27 8.60
Calculated for C26H3202 82.94 8.57
,~
,:
~1~3~
- 17 -
Example 7
1305 part~ of 2-(5-methoxycarbonyl-2-methyl-pent--2-yl) anthracerle in
100 parts o~ ether are added to a ~olutlon of methyl magn~lum
iodide prepared from 3.0 parts magnesium and 18.0 parta of methyl
iodide in 150 part~ of ether. On completing the addition the
reaction mixture is stirred for 3 hour~ before being treated with
dilute hydrochloric acid. The ether phase i~ separated, washed with
water, and evaporated to leave 2-(6-hydroxy-2,6-dimethyl-hept-2-yl~-
anthracene with m.p 117-8C after crystallisation from 60-80C
petroleum-ether.
Carbon ~ydrogen
Found 86.48 8~94
Calculated for C23H2aO 86.20 8.81
Example 8
1.1 part~ of 2-(5-methoxycarbonyl 2-pent-2-yl)-anthracene and
1.0 part~ of lithium aluminium hydride in SO parts of ~odium dried
ether sre stirred at room temperature overnight and then treated
with dilute hydrochloric acid. The ether phase is ~epa~ated, washed
with water, evaporated, and the residue cry3talli~ed from 4~-60C
petroleum-ether containing a little scetone to give 2-(6~hydroxy-
2-methyl-hex-2-yl~-anthrscene m.p 123-5C.
Ca~bon ~ydrogen
Found 86.31 8.25
Calculated for C21H240 86.26 8.27
Treatment of the alcohol with acetic anhyd~ide give~ 2-(6-acetoxy
2-methyl-hex-2-yl)-anthracene with m.p. 58-9C after cry3tallisation
from 40-60C petroleum-ether at O~C.
Carbon Hydrogen
Found 82.01 7.84
Calculated for C21H260 82.60 7.84
"
~L;2~73~
~xamplo 9
1.0 part~ of 2-(5-methoxycarbonyl-2-methyl-pent-2-yl) anthracene arld
3.0 psrts of n-butylamine are ~eal~d into a gla3~ vial an~ heated flt
120C for 24 hour~. The exceas amtne i~ evaporated and the re~idue
crystalli~ed from ether at 0C to glve 2-(5-N-n-butylcarbamoyl-2-
methyl-pent-2-yl)-anthracene m.p 111-3C.
Carbon Hydrogen Nitrogen
Found 83.04 8.o6 3.69
Calculated for C25H31N0 83.06 8.64 3.87
Example 10
1.0 Parts of 2.6-bis-(5 methoxycarbonyl-2-methyl-pent-2-yl~-
anthracene and 1.0 parts of lithium aluminium hydride in 25 parts of
~odium drled ether are reacted and worked up as described in
Exampl~ 3. From the reduction i8 obtained 2,6-bis-(6-hydroxy-2-
methyl-hex-2-yl)-anthracene m.p. 174-6C after cry~tallisation from
acetone.
Carbon Hydrogen
Found 82.62 9.28
Calculated for C28H3~3O2 82.71 9.42
Example 11
0.25 Parts of 2~6 bia-(5-methoxycarbonyl-2-methyl-pent-2-yl)-
anthracene and 2.0 parta of n-butylsmine are reacted together and
worked up a6 de~cribed in Exa~ple 9.
2,6-Bis-(5-N-n-butylcarbamoyl-2-methyl-p~nt-2~yl?-anthracen2 i8
crystalll~ed fro~ ether and has m.p 153-5C.
Carbon Hydrogen Nitrogen
Found 79.05 9.72 4.91
Calculated for C36Hs2NzO2 79.36 9.62 5.14
`` ~ ~ ~ ~ 3
~ 19 -
Application Examples
A solution consisting of 10 g of an indu~trial epoxy-
cresol novolak ~epoxy content 4.5 eq/kg), 0.25 g (7 x 10 4 mol~ of
(~6-toluene) (n5-cyclopentadienyl)iron(II) hexafluorophosphate
[prepared according to Bull.Soc.Chim. France, 2572 (1975)], 1.3 x
10 3 mol of 2,6-Bis-(5-methoxycarbonyl-2-methylpent-2-yl)-anthracene
and 4 g of 1-acetoxy-2-ethoxyethane i8 applied to a copper-coated
epoxy board with a 25 ~ wire doctor. The initlally wet film is dried
at 80C (dry film thickness approx. 15 ~). The photo-polymeric board
prepared in this way is exposed with a 5000 Watt high-pressure
mercury discharge lamp located 50 cm from the sample bench, a
21 step Stoufer ~en3itivity guide being used a~ the pattern (cf.
W.S. DeForest, Photoresist, McGraw-~lll Bood Comp., N.Y., page 109
et 6eq. ) . The exposure time is 10, ~0 a~d 30 seconds snd the exposed
board is then hardened for 2 minutes at 110C. It i3 developed in
1-acetoxy-2-ethoxyethane, the unexposed part~ being dissolved~ Thi3
results in a relief image, no.'s 3, 4 and 6 being the last step of
which an image i8 formed.
The procedure of Example I is followed using 1.3 x 10 3
mol 2,7-Bis-(5-methoxycarbonyl-2-methylpent-2-yl)-anthracene instead
of the 2,6-isomer. Expo~ure time i8 10, 20 and 30 seconds and the
lhst steps of which images are formed are no.'s 3, 4 and 6.