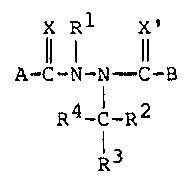Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~73~
INSECTICIDAL N-~OPTIONALLY SUBSTITUTED)-
N ' -SUBSTITUTED N ,N ' -DISUBSTITUTEDHYDRAZINES
Back~ound of the Invention
This invention relates to N-(optionally substituted)-
N'-substituted-N,N'-disubstitutedhydrazines, which are
useful as insecticides, compositions containing those
compounds and methods of their use. The disclosed
hydrazines are new compounds.
The search for compounds which have a combination of
excellent insecticidal activity and low undesirable
toxicity is a continuing one because of factors such as
the desire for compounds exhibiting greater activity,
better selectivity, low undesirable environmental impact,
low production cost and effectiveness against insects
resistant to many known insecticides.
Compounds of the present invention are particularly
suitable for controlling plant-destructive insects in
crops of cultivated plants, ornamentals and forestry.
Certain hydrazine derivatives have been disclosed in
the literature.
In 25 Aust. J._Chem., 523-529 (1972), several N,N'-
dibenzoylhydrazine derivatives are disclosed includlng
--1--
7~ ;33
N'-1-propyl-; N'-n-propyl~ 2-methylpropyl)-;
N ' -( 3-methylbutyl)-i N ' -benzyl- and N'-phenyl-
N,N'-dibenzoylhydrazine in which one or both nitrogen
atoms are alkylated or phenylated. No biological activity
is disclosed for those compounds.
In 61 Helv. Chim. Acta, 1477-1510 (1978), several
N,N'-dibenzoylhydrazine and hydrazide derivatives
including N'-t-butyl-N-benzoyl-N'-(4-nitrobenzoyl)-
hydrazine are disclosed. No biological activity is
disclosed for those compounds.
In 44 J.A.C.S., 2556-2567 (1922), isopropylhydrazine
(CH3)2CH NH-NH2, symmetrical dlisopropylhydrazine,
dibenzoylisopropylhydrazine and certain derivatives are
disclosed. No biological activity is disclosed for ~hose
compounds.
In 44 J~A~CoS~ ~ 1557-1564 ( 1972), isopropyl, menthyl
and bornyl semicarbazides are disclosed. No biological
activity is disclosed for those compounds.
In 48 J.A.C.S., 1030-1035 (1926), symmetrical
di-methylphenylmethylhydrazine and certain related
compounds including 1,2-bis-methylphenylmethyl-4-phenyl-
semicarbazide are disclosed. No biological activity is
disclosed for those compounds.
In 27 Bull. Chem. Soc. Japan, 624 627 (1954), certain
hydrazine derivatives including alpha,beta-dibenzoyl-
phenylhydrazine are disclosed. No biological activity is
disclosed for those compounds.
In J. Chem_ Soc. (C), 1531-1536 (1966), N,N'-
dibenzoylphenylhydrazine and N-acetyl-N'-benzoyl-p-nitro-
phenylhydrazine are disclosed. No biological activity is
disclosed for those compounds~
In 56B Chem. 8erichte, 954-962 (1923), symmetrical
di-isopropylhydrazines, s~nmetrical diisobutyl- and
certain derivatives including N,N'-diisobutyldibenzoyl
3~33
hydrazine are disclosed. No biological activity is
disclosed for those compounds.
In 590 Annalen der Chemie, 1~36 (1954), certain N,N'-
dibenzoylhydrazine derivatives are disclosed including
N'-methyl- and N'-(2-phenyl)-isopropyl-N,N'-dibenzoyl-
hydrazine. No biological activity is disclosed for those
compounds~
In J. Chem. Soc., 4191-4198 (1952), N,N'-di-n-propyl~
hydrazine, N,N'-dibenzoylhydrazine and bis-3,5-dinitro-
benzoyl hydrazine are disclosed No biological activity is
disclosed for those compounds.
In 32 Zhur. Obs. Khim., 2806-2809 (1962), N'-2,4-
methyl-2,4-pentadiene-N,N'-dibenzoylhydrazine is
disclosed. No biological activity is disclosed.
In 17 Acta. Chim. Scand., 95-102 (1963~, 2-benzoyl-
thiobenzhydrazide (C6H5-CS-NHNH-CO-C6~5) and certain
hydrazone and hydrazine derivatives are disclosed
including 1,2-dibenzoyl-benzylhydrazine. No biological
activity is disclosd for tho e compounds~
In 25 Zhur. Obs. Khim, 1719-1723 (1955), N,N'-bis-
_
cycloh xylhydrazine and N,N'-dibenzoylcyclohexylhydrazine
are disclosed. No biological activity is disclosed for
those compounds.
In _. Chem. Soc., 4793-4800 (1964), certain
dibenzoylhydrazine derivatives are disclosed including
tribenzoylhydrazine and NIN'-dibenzoylcyclohexyl-
hydrazine. Wo biological activity is disclosed for those
compounds.
In 36 J Prakt Chem., 197-201 (1967), certain
dibenzoylhydrazine derivativPs including N'-ethyl-; N'-n-
propyl~; N' isobutyl-; N'-neopentyl-; N'-n~heptyl-; and
N'-cyclohexylmethyl-N,N'-dibenzoylhydrazines are
disclosed. No biological activity i~ disclosed for those
compounds~
--3--
In 26 J.O.C., 4336-4340 (1961) N'-t butyl-N,N'-di-(t-
butoxycarbonyl)hydrazide is disclosed. No ~iological
activity is di SG losed.
In 41 J~O.C., 3763-3765 (1976), N'-t-butyl-N-
(phenylmethgxycarbonyl)~N'-(chlorocarbonyl)hydrazide is
disclosed. No biological activity is disclosed,
In 94 J.A.C.S., 7406-7416 (1972) N'-t-butyl-N,N'-
dimethoxycarbonylhydra2ide is disclosed. ~o biological
activity is disclosed.
In 43 J.O.C., 808-815 (1978), N'~t-butyl-N-ethoxy-
carbonyl-N'-phenylaminocarbonylhydrazide and N'-t-butyl-N~
ethoxycarbonyl-N'-methylaminocarbonylhydrazide are
disclosed. No biological activity is disclosed for those
compounds.
In 39 J. Econ. Ent~, 416-417 (1946), certain
.
N-phenyl-N'-ac~lhydrazines are disclosed and evaluated for
their toxicity against codling moth larvae.
The N-(optionally substituted)-N'-substituted-N,N'-
disubstitutedhydrazines of the present invention differ
from known compounds primarily by their N and N'
substituents.
Compounds of the present invention are also
distinguished by their excellent insecticidal activity,
particularly against insects of the orders Lepidoptera and
Coleoptera, and most particularly a~ainst insects o~ the
order Lepidoptera, without material adverse impact on
benefioial in~ects.
SummarY of the Invention
In accordance with the presen~ invention, there are
provided novel insecticidal compounds having the formula:
--4--
i~ '
~ ~3~i33
X Rl X'
Il 1 11
. A-C-N-N - C-B
R4-C-R2
l3
S wherein
X and X' are the same or different O, S or NR;
~1 is hydrogen; (Cl-C6)alkyl, (Cl C6)al y y
having independently the stated number of carbon
atoms in each alkyl group; (Cl-C6)alkylthioalkyl
10 . having independently the stated number of carbon
atoms in each alkyl group; (C2-C6)alkenyl;
(C2-C6)alkynyl; or phen-(Cl-C4)alkyl where the
phenyl ring is unsubstituted or substituted with
one to three of the same or different halo,
cyano, nitro, hydroxy, (Cl-C4)alkyl, (Cl-C4)-
haloalkyl (cl-C4)alkoxY~ (Cl-C4)haloalko~Yi
carboxy, (Cl-C~)alkoxycarbonyll (Cl-C4)-
alkanoyloxy or NZZ';
R2 and R3 are the same or different hydrogen or
(Cl-C~)alkyl;
; R4 is (Cl-C4)alkyl substituted with l to 4 fluoro;
straight chain (C2-C4)alkenyl; carboxyl;
(Cl-C3)alkoxycarbonyl; cyano; cyano substituted
(Cl-C4)alkyl; ~Cl-C~)trialkylsilyl having
independently the stated number of carbon atoms
in each alkyl group; or (Cl-C2)trialkylsilyl~
methyl having independently the stated number of
carbon atoms in each alkyl group; provided that
R2 and R3 are both alkyl when R4 is carboxyl or
~0 alkoxycarbonyl; and
A and B are the same or different unsubstituted or
substituted naphthyl where the substituents can
--5--
` ~7~36~.~3
be Erom one to three of the same or dif~erent
halo; nitro; ~Cl-C4)alkoxy; (Cl-C4)alkyl or
NZZ';
unsubstituted or substituted phenyl where the
substituents can be from one to five of the same
or different halo; nitroso; nitro; cyanoi
hydroxy; (Cl-C6)alkyl; (Cl-C6)haloalkyl;
(Cl-C6)cyanoalkyl; (Cl-C6)hydroxyalkyl; (C1-
C6)alkoxy; (Cl-C6)haloalkoxy; (Cl-C6)alkoxyalkyl
having independ0ntly the stated number of carbon
atoms in each alkyl group; (Cl-C6)alkoxyalkoxy
having independently the stated number of carbon
atoms in each alkyl group; (Cl-C6)alkylthio-
alkoxy (-ORSR') having independently the stated
number of carbon atoms in each alkyl group; (C
C6)alkoxycarbonyloxy (-OC02R); hydroxycarbonyl-
oxy (-OC02H); (Cl-C6)alkanoyloxyalkyl having
independently the stated number of carbon atoms
in each alkyl group; (C2-C6)alkenyl optionally
~0 substituted with halo, cyano, (Cl-C~)alkyl,
(Cl-C4)haloalkoxy, (Cl-C4)alkylthio or (Cl-
C4)alkoxy; (Cl-C4)alkadienyl; (C2-C6)alkenyloxy;
(C2-C6)alkenylcarbonyl; (C2-C6)alkenyloxy-
carbonyloxy; (C2-C6)alkynyl optionally
substituted with halo, cyano, nitro, hydroxy,
(Cl C4)alkoxy, (Cl-C4)haloalkyl, (Cl-C~)alkyl-
thio or (Cl-C4)alkyl; carboxy; (Cl-C6)carboxy-
alkyl having independently the stated number o~
carbon atoms in each alkyl group (-RC02~');
vO -COR; -COH; (Cl-C6)haloalkylcarbonyl; (Cl~C6)-
alkoxycarbonyl (-C02R); (Cl-C6)haloalkoxy-
carbonyl; (Cl-C6)alkanoyloxy (-OCOR); (Cl-C6)-
alkoxycarbonylalkoxy ( ORC02R') having
~ ~7~36~3
independently the stated number of carbon atoms
in each alkyl group; amino (-Nzæ~ ); amino
substituted with hydroxy, ~Cl-C4)alkoxy or
(Cl-C4)alkylthio; carboxamido (-CONZZ');
(C2-C6)alkenylcarbonylamino; (Cl-C6)-
hydroxyalkylaminocarbonyl; aminocarbonyloxy
(-OCONZZ'); amido (-NZCOZ'); alkoxycarbonylamino
(-NZCO2Z'); thiocyanato; isothiocyanato;
(Cl-C6)thiocyanatoalkyl; (Cl-C6)alkylthio;
(Cl-C6)haloalkylthio; sulfoxide (-S(O)Z);
sulfonyl (-SO2Z); alkylsulfonyloxy (-OSO2R);
-OSO2H; sulfonamide (-SO2NZZ'); alkylthio-
carbonyl (-CSR); -CSH; alkylcarbonylthio
(-SCOR); -SCOH; thioamido (-NZCSZ');
unsubstituted or substituted phenyl having one
to three of the same or different halo, cyano,
nitro, hydroxy, (Cl-C4)alkyl, (Cl-C4)haloalkyl,
(C1-C4)alkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
(Cl-C4)alkanoyloxy, amino, (Cl-C4)alkylamino or
(Cl-C4)dialkylamino having independently the
stated number of carbon atoms in each alkyl
group; phenoxy where the phenyl ring is
unsubstituted or substituted with one to three
o~ the ~ame or different halo, cyano, nitro,
hydroxy, (Cl-C4)alkyl, (Cl-C4)haloalkyl,
(Cl-C4)alkoxy, carboxy, (Cl-C4)alkylcarbonyl,
(Cl-C4)alkanoyloxy, amino, (Cl-C4)alkylamino or
( Cl-C4 ) dialkyl amino having independently the
stated number of carbon atoms in each alkyl
3d group; benzoyl where the phenyl ring is
unsubstituted or substituted with one to three
of the same or different halo, cyano, nitro,
y y, (Cl C4)alkyl, (Cl-C4)haloalkyl, (Cl-
C4)alkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
3 ~ ~
(Cl-C4)alkanoyloxy, amino, (Cl-C4)alkylamino or
(Cl-C4)dialkylamino having independently the
stated number of carbon atoms in each alkyl
group; benzoyloxy(Cl-C6)alkyl; phenylthio-
S (Cl-C6)alkyl where the phenyl ring is
unsubstituted or substituted with one to three
of the same or different halo, cyano, nitro,
hydroxy, (Cl-C~)alkyl, (Cl-C4)haloalkyl,
(Cl-C4)alkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
(Cl-C4)alkanoyloxy, amino, (Cl-C4)alkylamino or
(Cl-C4)dialkylamino having independen~ly the
stated number of carbon atoms in each alkyl
group; imino (-CR=N-R"') where R"' is hydroxy,
(Cl-C4)alkyl, (Cl~C4)alkoxy, amino (-NZZ'),
phenylamino, -COR, -COH or benzoyl; (C2-C6)-
oxiranyl; acetylthiosemicarbazone; pyrrolyl;
oxazolyl, unsubstituted or substituted with one
or two me~hyl groups; or when two adjacent
positions on the phenyl ring are substituted
with alkoxy groups, these groups may be joined
to form together with the carbon atoms to which
they are attached a 5 or 6 membered dioxolano or
dioxano heterocyclic ring;
unsubstituted or substituted (Cl-C10)alkyl
having one to four of the same or different
halol cyano, nitro, hydroxy, (Cl-C4)alkoxy, (Cl-
C4)haloalkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
(Cl C4)alkanoyloxy, phenyl or ~NZZ';
unsubstituted or substituted (C3-C8)cycloalkyl
or unsubstituted or substituted (C3-C8)cyclo-
alkyl(Cl-C4)alkyl having one to four of the same
or different halo, cyano, nitro, hydroxy, (Cl-
- ~7~633
C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4)alkoxy,
(Cl-C4)haloalkoxy, carboxy, (cl~c4)alkanoyl~
(Cl-C4)al~oxycarbonyl, tC1-C4)alkanoyloxy or
-NZZ';
unsubstituted or substituted (C2-C8)alkenyl or
unsubstituted or substituted (C2-C8)alkadienyl
having a furyl, thienyl or pyridyl or having one
to four of the same or different halo, cyano,
nitro, hydroxy, (Cl-C4)alkyl, (C3-C6)cycloalkyl,
(Cl-C4)haloalkyl, (Cl-C4)alkoxy, (Cl-C4)halo-
alkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
(Cl-C4)alkanoyloxy or -NZZ';
unsubstituted or substituted (C3-C~)cycloalkenyl
or unsubstituted or substituted (C3-C8)cyclo-
alkadienyl havin~ one to four of the same or
different halo, cyano, nitro, hydroxy, (Cl-C4)-
alkyl, (Cl-C4)haloalkyl, (Cl-C4)alkoxy,
(Cl-C4)haloalkoxy, carboxy, (Cl-C4)alko
carbonyl, (Cl-C4)alkanoyloxy or -NZZ';
unsubstituted or substituted (C2-C8)alkynyl
having one to four of the same or different
halo, cyano, nitro, hydroxy, (Cl-C4)alkyl,
~Cl-C4)haloalkyl, (Cl-C4)alkoxy, (Cl-C4)halo-
alkoxy, carboxy, (Cl-C4)alkoxycarbonyl, (Cl-C~
alkanoyloxy, phenyl or -NZZ';
phenalkyl having one to four carbon atoms in the
alkyl group and the alkyl group is unsubstituted
or substituted with one to three of the same or
different halo, cyano~ hydroxy, (Cl-C4)alkoxy,
(Cl-C4)alkoxycarbonyl or -NZZ',
_9~
~ ~ ~73~
and the phenyl ring is unsubstituted or
substituted with one to three of the same or
different halo, cyano, nitro, hydroxy, (Cl-
C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4)cyanoalkyl,
(C1-C4)alkoxy, (Cl C4)haloalkoxy, carboxy, (Cl-
C4)alkoxycarbonyl~ (Cl-C4)alkanoyloxy, (C2-
C6)alkenyl, (C2-C6)haloalkenyl, (C2-C6)alkynyl
or -NZZ';
phenalkenyl having two to six carbon atoms in
the alkenyl group and the alkenyl group is
unsubstituted or substituted with one to three
of the same or different halo, cyano, hydroxy,
(C1-C4)alkyl~ (Cl-C4)haloalkyl, (Cl-C4)alkoxy,
(Cl-C4)haloalkoxy, (Cl-C4)alkoxycarbonyl or
lS -NZZ', and the phenyl ring is unsubstituted or
substituted with one to three of the same or
different halo, cyano, nitro, hydroxy, (Cl-
C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4)alkoxy, (Cl-
C4)haloalkoxy, carboxy, (Cl-C4)alkoxycarbonyl,
(cl-c4)alkanoyloxy or -NZZ';
unsubstituted or substituted five-membered
heterocycle selected from furyl, thienyl,
triazolyl, pyrrolyl, isopyrrolyl, pyrazolyl,
isoimidazolyl, thiazolyl, isothiazolyl, oxazolyl
and isooxazlyl where the substituents can be
from one to three of the same or different halo;
nitro; hydroxy; (Cl-C6)alkyl; (Cl-C6)alkoxy;
carboxy; (Cl-C6)alkoxycarbonyl;
(Cl C~)carboxyalkyl (-RCO2H'); (Cl-C6)alkoxy-
carbonylalkyl (-RCO2R') having independently the
stated number of carbon atoms in each alkyl
group; carboxamido (-CONZZ'); amino (-NZZ');
--10--
~73~
amido (-NZCOZ'); (Cl-C6)alkylthio; or
unsubstituted or substituted phenyl having one
to three of the same or different halo, nitro,
(Cl-C6)alkyl, (Cl-C6)haloalkyl, (Cl-C6)alkoxy,
(Cl-C6)haloalkoxy, carboxy, (Cl-C4)alkoxy-
carbonyl or amino (-NZZ'); or
unsubstituted or substituted six-membered
heterocycle having one, two, three or four
nitrogen atoms and two to five nuclear carbon
atoms where the substituents can be from one to
three of the same or different halo; nitro;
hydroxy; (Cl-C6)alkyl; (Cl-C6)alkoxy; carboxy;
(Cl-C6)alkoxycarbonyl; (Cl-C6)carboxyalkyl
(-RCO2H); (Cl-C6)alkoxycarbonylalkyl (~RCO~R')
having independently the stated number of carbon
atoms in each alkyl group; carboxamido
(-CONZZ'); amino (-NZZ'); amido ( NZCOZ');
(Cl-C6)alkylthio; or unsubstituted or
substituted phenyl having one to three of the
same or different halo, nitro, (Cl-C6)alkyl,
(Cl-C6)haloalkyl, (Cl-C6)alkoxy, (Cl-C6)halo-
alkoxy, carboxy, (Cl-C4)alkoxycarbonyl or amino
(-NZZ');
where R, R' and R" are (Cl-C6)alkyl; Z and Z'
are hydrogen or (Cl C4)alkyl; and
agronomically acceptable salts thereof.
Also in accordance with the present invention, there
are provided novel insecticidal compositions and methods
of using such compositions wherein the compositions
comprise an agronomically acceptable carrier and an
insecticidally effective amount of, or from about 0O0001%
to about 99% by weight of the composition, a compound of
Formula I above.
--1 1--
3~j33
Detailed Descri~tion of the Inventlon
The term "halo" should be understood as including
chloro, fluoro, bromo and iodo. The term "alkyl" by
itself or as a part of another substituent, unless
otherwise stated, includes straight or branched chain
groups such as methyl, ethyl, n-propyl, isopropyl,
n-butyl, t-butyl, isobutyl, neopentyl and the like and
where indicated higher homologues and isomers such as
_-octyl, isooctyl and the like. The term "haloalkyl" by
itself or as part of another substituent is an alkyl group
of the stated number of carbon atoms having one or more
halo atoms bonded thereto such as chloromethyl, 1~ or 2-
bromoethyl, trifluoromethyl and the like. Analogously,
"cyanoalkyl" by itself or as part o~ another group is an
alkyl group of the stated number of carbon atoms having
one or more cyano groups ~onded thereto; "haloal~oxy" by
itself or as part of another group is an alkoxy yroup of
the stated number of carbon atoms having one or more halo
atoms bonded thereto such as difluoromethoxy 9
trifluoromethoxy, 2-fluoroethoxy, 2,2,2-trifluoroethoxy
and the like. "~lkenyl" and "alkynyl" by themsalves or as
part of another substituent comprise straight and branched
chain groups of the stated number of carbon atoms.
"Alkadienyl" is a straight or branched chain alkenyl group
comprising two carbon-carbon double bonds that can be
conjugated such as l,3-butadienyl, cumulated such as 1,2-
propadienyl or isolated such as 1,4-pentadienyl.
Repre~entative examples of five-membered heterocycles
includes 2-furyl; 3-furyl; 2-thisnyl; 3-thienyl; ~-(1,2,3-
triazolyl); 3-(1,2,4-triazolyl); 5-(1,2,4-tria201yl); 2-
pyrrolyl; 2-oxazolyl; and the like.
Because of their anticipated good insecticidal
activity, compounds of the present invention for use in
the insecticidal compositions and formulations include
those where, independently
-12-
i
X and X' are O or 5;
R1 is hydrogen (C1-C~)alkyl; (C1-C4)alkoxyalkyl having
independently the stated number of carbon atoms
in each alkyl group; methylthiomethyl; (C2-C5)-
alkenyl; ~C2-C5)alkynyl; or phen(C1-C2)~lkyl where the
phenyl ring is unsubstituted or substituted with one or
two halo, nltro, (C1-C4)alkyl or (C1-C4)alkoxy;
R2 and R3 are the sæme or diff~rent hydrogen or
(Cl-C3)alkyl;
R4 is (Cl-C3)alkyl substituted with one to four
fluoro; straight chain (C2-C4)alkenyl; carboxyl;
~Cl~C2)alkoxycarbonyl; cyano; cyano substituted
(cl-c3)alkyl; (Cl-C2)trialkylsilyl having
independently the stated number of carbon atoms
in each alkyl group; or (Cl-C2)trialkylsilyl-
; me~hyl having independently the stated number of
carbon atoms in each alkyl group; provided that
R2 and R3 are both alkyl when R4 is carboxyl or
alkoxycarbonyl; and
A and B are the sxme or different
unsubstituted naphthyl;
unsubstituted or substituted phenyl where the
substituents can he from one to three of the
same or different halo; nitro; cyano;
~5 (Cl-C4)alkyl; (cl-c4)haloalkyl;
(Cl-C4)cyanoalkyl; (Cl~C4)alkoxy;
(Cl-C4)alkoxyalkyl having independently the
stated number of carbon atoms in each alkyl
group; -COZ; carboxy; (Cl-C4)alkoxycarhonyl;
(Cl-C4)alkanoyloxy; (C2-C6)alkenyl;
(C2-C6)alkynyl; amino (-NZ2'); thiocyanato;
(Cl-C4~alkylthio; alkylthiocarbonyl ( -CSR);
-CSH; unsubstituted or substituted phenyl having
-13-
~ ~3~33
i, .~
Y .,
f one to two of the same or diferent halo, nitro,
(Cl-C4)alkyl, (C1-C~)alkoxy, carboxy, -~ZZ';
ph~noxy where the phenyl ring is unsubstituted
or substituted with one or two of the same or
different halo, nitro, (Cl-C4)alkyl,
(Cl-C4)alkoxy, carboxy, -NZZ'; or when two
adjacent positions on the phenyl ring are
substituted with alkoxy groups, these groups may
be joined to form a 5- or 6-membered dioxolano
or dioxano heterocyclic ring;
unsubstituted or substituted (Cl~C8)alkyl having
one to three of the same or different halo,
cyano, (Cl-C4)alkoxy, phenyl, (Cl-
C4)alkoxycarbonyl or (Cl-C4)haloalkoxy;
unsubstituted or substituted (C3-C6)cycloalkyl
or unsubstituted or substituted (C3-
C6)cycloalkyl(Cl-C4)alkyl having one or two of
the same or different halo, (Cl-C4)alkyl, (Cl-
C4)haloalkyl, (Cl-C4)alkoxy, (Cl-C4)alkanoyl or
2Q (Cl-C4)haloalkoxy;
unsubstituted or substituted (C2-C6)alkenyl
having a furyl or one to three of the same or
different (C1-C4)alkyl, (Cl-C4)haloalkyl, (Cl-
C4)alkoxy or (Cl-C4)haloalkoxy;
unsubstituted or substi~.uted (C3-C6)cycloalkenyl
having one or two o~ the same or differen~ halo,
(Cl-C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4)alkoxy or
(Cl-C4)haloalkoxy;
unsubstituted or phenyl substituted alkynyl;
-14~
- ' ' " ' : :
73~33
phenalkyl having one or two carbon atoms in the
alkyl group and the alkyl group is unsubstituted
or substituted with one or two of the same or
different halo, (Cl-C4)alkyl, (Cl-C4)haloalkyl,
S (Cl-C4)alkoxy or (Cl-C4)haloalkoxy and the
phenyl ring is unsubstituted or substituted with
one or two of the same or different halo, cyano,
(Cl C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4~alkoxy or
(Cl-C4)haloalkoxy;
phenalkenyl having two or three carbon atoms in
the alkenyl group and the alkenyl group i5
unsubstituted or substituted with halo, (Cl-
C4)alkyl, (Cl-C4)haloalkyl, (Cl C4)alkoxy or
(Cl-C4)haloalkoxy and the phenyl ring is
unsubstituted or substituted with one or two of
the same or different halo, cyano, (Cl-C4)alkyl,
(Cl-C4)haloalkyl, (Cl-C4)alkoxy or (Cl-
C4)haloalkoxy;
unsubstituted or substituted five-membered
heterocycle selec~ed from furyl, thienyl,
triazolyl, pyrrolyl, and oxazolyl where the
substituents can be one or two of the same or
different halo; nitro; (Cl-C~)alkyl; (Cl-C4)-
alkoxy; -NZZ'; or unsubstituted or substituted
phenyl having one or two of the same or
different halo, nitro, (Cl-C4)alkyl,
(Cl-C4)haloalkyl, (Cl-C4)alkoxy, (Cl-C4)halo-
alkoxy~ carboxy or -NZZ'; or
unsubstituted or substituted six-membered
heterocycle having one or two nitrogen atoms and
-15-
:: ~V~73~j3;~
4 to 5 nuclear carbon atoms whcre the
substituents can be one or two of the same or
different halo; nitro; (ClC4)alkyl; (Cl-C4)-
alkoxy; (Cl-C4)thioalkoxy; -NZZ'; or
unsubstituted or substituted phenyl.havIng one
or two of the same or different halo, nitro,
(Cl-C4)alkyl, (Cl-C4)haloalkyl, (Cl-C4)alkoxy,
(Cl-C4)haloalkoxy, carboxy or -NZZ';
where R and R' are (Cl-C4)alkyl; Z and Z' are
hydrogen or (Cl-C4)alkyl; and
ag-onomically acceptable salts.
Insecticidal compounds o~ the present invention
believed to have very good activity for use in the
insecticidal compositions and formulations of the present
invention include those where, independently,
x and X' are O or S;
Rl is hydrog~n; (Cl-C4)alkyl; ~Cl-C4)alkoxyalkyl
having independently the stated number of carbon
atoms in each alkyl group; (C2-C5)alkenyl;
(C2-C5)alkynyl; or benzyl where the phenyl ring is
unsubstituted or substituted with one halo, nitro,
: (Cl-C4)alkyl or (Cl-C4)alkoxy;
R2 and R3 are the same or different hydrogen or
(Cl-C2)alkyl;
R4 is (Cl-C3)alkyl substituted with l to 4 fluoro;
straight chain (C2-C4)alkenyl; methoxycarbonyl;
cyano; cyano substituted (Cl~C2)alkyl; (Cl-
C2)trialkylsilyl having independently the stated
number of carbon atoms in each alkyl group; or
(Cl-C2)trialkylsilylmethyl having independently
the stated number of carbon atoms in each alkyl
group; provided that R~ and R3 are both alkyl
when R4 is methoxycarbonyl; and
-16~
~i
~ ~"t~3633
A and B are the same or different
unsubstituted naphthyl;
unsubstituted or substituted phenyl having one
to three of the same or different halo; nitro;
cyano; (Cl-C4)alkyl; (Cl-C4)haloalkyl; (Cl-C4)-
cyanoalkyl; (Cl-C4)alkoxy; (Cl-C4)alkoxyalkyl
: having independently the stated number of carbon
atoms in each alkyl group; -COZ; (Cl-C4)alkoxy-
carbonyl; (C1-C4)alkanoyloxy; thiocyanato;
-NZZ'; (C1-C4)alkylthio; alkylthiocarbonyl
(-CS2, -CS2Z); unsubstituted or substituted
phenyl having one or two of the same or
different halo, nitro, (C1-C4)alkyl,
(Cl-C4)alkoxy, carboxy, -NZZ'; or phenoxy where
the phenyl ring is unsubstituted or substituted
with one or two of the same or different halo,
nitro, (Cl-C4)alkyl, (Cl-C4)alkoxy, carboxy,
-NZZ' or when two adjacent positions on the
phenyl ring are substituted with alkoxy groups,
these groups may be joined to form a 5- or 6-
membered dioxolano or dioxano heterocyclic ring;
unsubstituted or substituted (Cl-C6)alkyl having
one to three of the same or different halo,
cyano, nitro, carboxy, (Cl-C4)alkoxy, phenyl,
(Cl-C4)alkoxycarbonyl or (Cl-C4)haloalkoxy;
unsubstituted or substituted (C3-C6)cycloalkyl
or unsubstituted or substituted (C3-C6)cyclo-
alkyl(Cl-C4)alkyl, where the substituent is
halo, (Cl-C4)alkyl, (Cl-C4~alkanoyl, (Cl-C4)-
alkoxy or (Cl-C4)haloalkoxy;
~,~7;~3
unsubstituted or substituted (C2-C6)alkenyl
having one or two of the same or different halo,
~Cl-C4)alkyl, (Cl-C4)alkoxy or (Cl-C4)halo-
alkoxy;
unsubstituted or substituted (C4-C6)cycloalkenyl
where the substituent is halo (Cl-C4)alkyl, (C
C4)alkoxy or (Cl-C4)haloalkoxy;
unsubstituted or phenyl substituted alkynyl;
benzyl where the phenyl ring is unsubstituted or
lo substituted with one or two of the same or
difference halo, methyl or ethyl;
unsubstituted or substituted five-membered
heterocycle selected from furyl, thienyl,
pyrrolyl and oxazolyl where the substituents can
be one or two of the same or different halo,
nitro, (Cl-C4)alkyl or (Cl-C4)alkoxy; or
unsubstitutad or substituted six-membered
heterocycle having one or two nitrogen atoms and
four to five nuclear carbon atoms where the
substituents can be one or two of the same or
different halo, nitro, (Cl-C~)alkyl,
(Cl-C4)alkoxy or (Cl-C4)thioalkoxy;
where Z and Z' are hydrogen or (Cl C4)alkyl; and
agronomically acceptable salts thereof.
Because of their anticipated excellent insecticidal
activity, preferred compounds of the present invention for
use in the insecticidal compositions and formulations of
the~ presen~ invention include those where, independently,
-18-
~ 3~33
X and X' are O;
Rl is hydrogen; methyl; ethyl; (Cl-C2)alkoxyalkyl
having independently the stated number of carbon
atoms in each alkyl group (C2-C5)alkenyl;
s (C2-C5)alkynyl; or benzyl where the phenyl ring
is unsubstituted or substituted with halo;
R2 and R3 are the same or different hydrogen or
(Cl-C2)alkyl;
R4 is (Cl-C2)alkyl substituted with l to 4 fluoro;
13 straight chain (C2-C4)alkenyl; cyano; cyano
substituted (Cl-C2)alkyl; (Cl-C2)trialkysilyl
having independently the stated number of carbon
atoms in each alkyl group; or (Cl-C2)trialkyl-
silylmethyl having independently the stated
number of carbon atoms in each alkyl group; and
A and B are the same or different phenyl or
substituted phenyl where the substituents can be
from one to three of the same or different halo,
nitro, (Cl-C4)alkyl, (Cl-C~)alkoxy or (Cl-
C4)haloalkyl;
unsubstituted or ~ubstituted (Cl-C6)alkyl having
one to two of the same or different halo, phenyl
or cyano;
unsubst.ituted (C4-C6)cycloalkyl;
unsubstituted or substituted (C2-C6)alkenyl
having one to three of the same or different
halo or (Cl-C4)alkyl;
unsubstituted (C4-C6)cycloalkanyl;
--19--
33
phenalkyl having one or two carbon atoms in the
alkyl group and the phenyl ring is unsubstituted
or substituted with one or two of the same or
different halo, (Cl-C4)alkyl or (Cl-C4)alkoxy;
and unsubstituted or substi~uted pyridyl or 2,5-
pyrazinyl where the substituent can be halo,
t o, (Cl C4)alkyl, (Cl-C4)alkoxy or (C1-C4)-
thioalkoxy; or
unsubstituted furyl or thienyl or an
unsubstituted or substituted pyrrolyl where the
substituent can be (Cl-C4)alkyl; and
agronomically acceptable salts thereof.
Because of their anticipated outstanding insecticidal
activity, more preferred compounds of the present
invention for use in the insecticidal compositions and
formulations of the present invention include those where,
independently,
X and X' are O;
~1 is hydrogen; methyl; methoxymethyl; (C2-C4)-
alkenyl; ~C2-C5)alkynyl; benzyl; or 4-
halobenzyl;
R2 and R3 are the same or different hydrogen, methyl
or ethyl;
R4 is trifluoromethyl, 2,2,2-trifluoroethyl; straight
chain (C2-C4)alkenyl; cyano; cyanomethyl;
(Cl-C2)trialkylsilyl having independently the
stated number of carbon atoms in each alkyl
group; or trimethylsilylmethyl; and
A and B are the same or different phenyl or
substituted phenyl where the substituents can be
one, two or three of the same or different
chloro, fluoro, bromo, iodo, nitro, methyl,
ethyll methoxy or trifluoromethyl;
-20-
~ 73~i~33
(C2-C3) substituted alkenyl having one to three
of the same or dif~'erent chloro, bromo, methyl
or ethyl;
cyclohexenyl;
benzyl;
unsubstituted or substituted pyridyl or 2,5-
pyrazinyl where the substituent can be halo,
methyl, ethyl or methoxyi or
unsubstituted furyl or thienyl or an
unsubstituted or substituted pyrrolyl where the
substituent can be ethyl or methyl; and
agronomically acceptable sal's thereof.
Those N'-substituted-N,N'-diacylhydrazines of Formula
I which possess a~idic or basic functional groups may be
further reacted to form novel salts with appropriate bases
or acids. These salts also exhibit pesticidal activityO
Typical salts are the agronomically acceptable metal
salts, ammonium salts and acid addition saltsO Among the
metal salts are those in which the metal cation is an
alkali metal cation such as sodium, potassium, lithium or
the like; alkaline earth metal cation such as calcium,
magnesium, barium, strontium or the like; or heavy metal
cation such as zinc, manganese, cupric, cuprous, ferric,
ferrous, titanium, aluminum or the like. The ammonium
salts include those in which the ammonium cation has the
formula NR5R6R7R8 wherein each of RS, R6, R7 and R8 are
independently hydrogen, hydroxy, (Cl-C4)alkoxy~ (Cl-C20~-
- alkyl, (C3 C8)alkenyl, (C3-C8)alkynyl, (C2-C8)hydroxy-
alkyl, (C2-C8)alkoxyalkyl, (C2-C6)aminoalkyl, (C2-C6)-
-21-
~ ;~73~
haloalkyl, amino, (Cl-C4)alkyl- or ~Cl-C4)dialkylamino,
substituted or unsubstituted phenyl, substituted or
unsubstituted phenylalkyl, having up to four carbon atoms
in the alkyl moiety, or any two o~ R5, R6, R7 or R8 can be
taken together to form with the nitrogen atom a 5- or 6-
membered heterocyclic ring, optionally having up to one
additional hetero atom (e.g., oxygen, nitrogen, or sulfur)
in the ring, and preferably saturated, such as piperidino,
morpholino, pyrrolidino, piperazino or the like, or any
three of R5, R6~ R7 or R8 can be taken together to form
with the nitrogen atom a 5- or 6-memberad aromatic
heterocyclic ring, such as piperazole or pyridine. When
R5~ R6, R7 Or R8 Substituent in the ammonium group is a
substituted phenyl or substituted phenylalkyl, the
substituents on the phenyl and phenalkyl will generally be
selected from halo, (C1-C8)alkyl, ~Cl-C4)alkoxy, hydroxy,
nitro, trifluoromethyl, cyano, amino, (Cl-C4)alkylthio and
the like. Such substituted phenyl groups pre~erably have
up to two such substituents. Representative ammonium
cations include ammonium, dimethylammonium, 2-ethylhexyl-
ammonium, bis(2-hydroxyethyl)ammonium, tris(2-hydroxy-
ethyl)ammonium, dicyclohexylammonlum, t-octylammonium,
2-hydroxyethylammonium, morpholinium, piperidinium,
2-phenethylammonium, 2-methylbenzylammonium, n-hexyl-
ammonium, triethylammonium, trimethylammonium,tri(n-butyl)-ammonium, methoxyethylammonium,
diisopropylammonium, pyridinium, dialkylammonium,
pyrazolium, propargylammonium, dim~thylhydrazinium,
octadecylammonium, 4-dichlorophenylammonium, 4-nitro-
3~ benzylammonium, benzyltrimethylammonium, 2-hydroxy-
ethyldimethylo~tadecylammonium, 2-hydroxyethyldiethyl-
octylammonium, decyltrimethylammonium/ hexyltriethyl-
ammonium, 4-methylbenzyltrimethylammonium, and the like.
Among the acid addition salts are those in which the anion
~ ~7~3~3
is an agronomically acceptable anion such as hydro-
chloride, hydrobromide, sulfate, nitrate, perchlorate,
acetate, oxalate and the like.
The above-identified preferred embodiments are based
on the 34 examples which were made, tested and disclosed
infra and the disclosure in the following six related Canadian
applications:
Canadian Patent Application No. 520,380, of Adam Chi-Tung Hsu
et al, filed October 14, 1986;
Canadian Patent Application No. 529,977, of Raymond A. Murphy
et al, filed February 18, 1987;
Canadian Patent Application No. 526,935, of Raymond A. Murphy
et al, filed January 8, 1987;
Canadian Patent Application No. 530,163, of Adam Chi-Tung Hsu
et al, filed February 20, 1987;
Canadian Patent Application No. 534,372, of Adam Chi--Tung Hsu
et al~ filed April 10, 1987; and
Canadian Patent Application No. 534,506, of Adam Chi-Tung Hsu
et al, filed April 13, 1987.
These related
applications disclose nearly 1,000 active compounds in
which the presently disclosed R2, R3 and R4 are methyl and
A and B are various unsubstituted and substituted moieties
including alkyl, phenyl and 5- and 6-membered heterocyclic
rings.
The present inventors have determined that a critical
feature of the structure which leads to activity is not
the t-butyl substituent of the above-mentioned related Canadian applications
(R2~R3=R4=methyl) but that one of the nitrogens be
substituted with a bulky substituent. This bulky
substituent i5 preferably a trisubstituted alpha carbon or
a trisubstituted beta carbon. ~owever, the bulky
substituent may also be a trisubstituted gamma carbon or a
disubstituted alpha carbon.
Compounds of the present invention which were made
and used in insecticidal compositions and formulations
include those where, independently,
X and X' are O;
Rl is hydrogeni
R2 and R3 are hydrogen, methyl and ethyl;
-23
3~ 3
R4 is trifluoromethyl, cyano, allyl, methoxycarbony:L,
and trimethylsilyl; and
A and B are the same or different unsubstituted or
substituted phenyl having one or two of the same
or different chloro, fluoro, nitro, methyl and
ethyl; or
unsubstituted thienyl.
Since one of ordinary skill in the art would
anticipate homologues to be active, the present invention
also includes compounds for use in insecticidal
compositions and formulations where, independently,
X and X' are O;
Rl is hydrogen or methyl;
R2 and R3 are hydrogen or (Cl-C3)alkyl;
lS R4 is trifluoromethyl, 2,2,2-trifluoroethyl, cyano,
cyanomethyl, straight chain (C2-C4)alkenyl,
carboxyl, methoxycarbonyl, (Cl-C2)trialkylsilyl
having independently the stated number of carbon
atoms in each alkyl group or (Cl~C2)trialkyl-
silylmethyl having independently the stated
number of carbon atoms in each alkyl group; and
A and B are the same or different thienyl or
unsubstituted phenyl or substitu~ed phenyl where
the substituents can be one to three of the same
or different chloro, fluoro, nitro, methyl,
ethyl or propyl; and
agronomically acceptable salts thexeof.
The compounds of this invention or their precursors
can be prepared according to the following processes.
Process A can be used when preparing compounds according
to Formula I where X and X' are both oxygen and A and B
axe the same (for example, both A and B are phenyl or 4-
chlorophenyl) or different (for example, A is 4-methyl-
phenyl and B is 4-chlorophenyl).
-~4-
3~i3~
Process A:
O R2 R2
A-C-Cl + NHRlN~l - R3 Base ~ A C-NRl-NHC ~ R3
I Solvent
R4 R4
II III IV
O R2 O O R2~ 1 ,R4
A-C-NR1-NHC--R3 + B-1~C1 BaSe ~ A-1-NR1--N 1-B
I Solvent
R4
IV V
where Rl, R~, R3, R4, A and B are as defined above for
Formula I and X and X' are oxygen.
~hen R4 is cyano the intermediate IV may be made as
~ollows:
Step la
O O
R2-1-R3 + M-CN + A-C-NRlNH2 _ HCl
Solvent
VIIa VI
O R2
A-C_NRl NHC~R3
CN
IVa
where M = K, Na
-25~
~ ~3~33
Process B can be used when preparing compounds
according to Formula I where X and X' are oxygen, and Rl,
R3, R4, ~ and B are as defined above for Formula I.
Process B:
Method 1
Step 1
Q O 0 ~3
¦1 1 3 11 4 Catalyst
A-C-NR NH2 + R -C-R (optional) ~ A-C-NR N=C
Solvent R4
VI VII VIII
Stap 2
O R3 R3
Il / Reducina Aqent
A-C-NRlN=C Solvent A-C-NR1NHCH
\ 4 Catalyst (optional) \ 4
R
VIII IX (IV)
Ste~ 3
R \ H R4
O R3 0 0 C O
Il / 11 11 1 11
A-C-NRlNHCH ~ B-C-Cl Base ~ A-C-N-N-C-B
\ R4 Solvent 11
IX (IV) V X (I)
As can be seen above, the intermediate product of Step 2,
the compounds of Formula IX, corresponds to the compounds
of Formula IV. In addition, the compound of Formula X
corresponds to the compounds of Formula I where X and X'
are oxygenO
~ ~3~3~3
Method 2
O Rl O
A-C-W +~N - N - C-B Solvent
R2--C-R4
13
XI XII
O O
Il 11
A-C-N N - C-B
R f3 R
R
I
where Rl, R2, R3, X4, A and B are as def ined above for
Formula I and W is a good leaving group such as halo, for
example, chLoro; an alkoxy, for example, ethoxy~ methyl
sulfonate (-OSo2cH3); or an ester~ for example, acetate
(-OC(O)C~3).
Process C can be used when preparing compounds
according to Formula I where A, B and Rl are as def ined
for Formula I and one or both X and X' are sulfur.
Process C:
Step 1
X R2 X Rl
Il l 11 1
A-C-Y + NHNHC-R3 Base . A-C - N- - NH
R1 R4 R4~C-R2
13
XIII III XIV
-27-
3 ~ 3
Ste 2:
X X' X X'
¦¦ R ' ll ll Rl ll
A-C-N---NH + B-C-Y Base . A-C-N---N---C-B
¦ Solvent ~ l
R4-C--R2 R4--C-R2
13 13
R R
XIV XV
where Rl, R2, R3, R4, A and B are as defined above for
Formula I and one or both X and X' are sulfur, and Y is a
good leaving group such as carboxyalkylthio (for example,
carboxymethylthio, -SCH2C02H); alkylthio (for example,
methylthio); or halo (for example, chloro).
Process D can be used when preparing compounds
according to Formula I where X and X' are oxygen and Rl, A
and B are as defined above for Formula I.
Process D
Step l
R2 R2
NHNHC--R3 + (Z-ot~c=o Base ~ Z-o-c-NRlNHl--R3
R1 R4 Solvent 14
III XVI XVII
Step 2
O R2 o oo
Il 1 1 3
Z-O-C-NR NHC - R + B-C-Cl Base ~ Z-O-C-NR N - C -
R4 Solvent ~4_1_R2
l3
XVII V XVIII
28-
Step 3
~
O O o
ll 1 11 Rl ll
Z-O-C-NR - N ~C-B Acid ~ NHN C-B
R4-C-R2 Solvent R4_¦_R2
13 13
XVIII XIX
Step 4
O o O o
A~C-Cl + NHN C-B Base _ ~ A-C-~ - N - C-B
R4-C-R2 Solvent R4-C-R2
13 R3
II XIX
wherein Rl, R2, R3, R4, A and B are as defined above for
Formula I and Z is t-butyl; ethyl; phenyl; or benzyl.
The starting materials for each process are generally
commercially available, or can be prepared by generally
customary and known methods.
In process ~, a compound of Formula II is reacted
with a monosubstituted hydrazine of Formula III or a
corresponding acid addition salt such as the hydrochloride
salt or the like in the presence of a base in an inert or
substantially inert solvent or mixture of solvents to
afford an intermediate product of Formula IV which can be
isolated or further reacted with a compound of Formula V
in the presence of a base in an inert or substantially
inert solvent or mixture of solvents to afford the desired
product of Formula I.
When A and B are the same, for example, both A and B
are 4-chlorophenyl, two equivalents of a compound o
-29-
~73~33
Formula II or V are reacted with a monosubstituted
hydrazine o~ Formula III in the presence of a base in an
inert or substantially inert solvent or mixture of
solvents to a~ford the desired product of Formula I~
Examples of the compounds of Formula II and/or
Formula V which can be used in the above processes include
benzoyl chloride, 4-chlorobenzoyl chloride, 4-methyl-
benzoyl chloride, 3,5-dichlorobenzoyl chloride, 2-bromo-
benzoyl chloride, 3-cyanobenzoyl chloride, 3-toluoyl
chloride, 4-toluoyl chloride, 4-ethylbenzoyl chloride, 2-
nitrobenzoyl chloride, 2,3~dimethylbenzoyl chloride, 2-
nitro-5-toluoyl chloride and the like. The compounds of
Formula II and/or Formula V are generally commercially
available or can be prepared by known procedures.
Examples of the compounds of Formula III which can be
used in the above processes include l,l-dimethyl-3-
butenylhydrazine, (trimethylsilylmethyl)hydrazine, (1,1,1-
tri~luoro-2-propyl)hydrazine, (2,2,2-trifluoroethyl)-
hydrazine, (l-cyano-l-methyl)ethylhydrazine and the
like. The compounds of Formula III are generally
commercially available or can be prepared by known
procedures. For example, the Grignard reagent addition
product of acetone azine in diethyl ether is hydrolyzed by
the adition of an acid (such as oxalic acid), in a
suitable solvent or mixture of solvents (such as ethanol
and diethyl ether, 1:1) to afford the monosubstituted
hydrazine of Formula III.
Suitable solvents for use in the above processes
include water; alcohols such as methanol, ethanol,
isopropanol and the like; hydrocarbons such as toluene,
xylene, hexane, heptane and the like; glyme; tetrahydro-
~uran; acetonitrile; pyridine; or haloalkanes such as
methylene chloride or mixtures of these solvents.
-3~-
~ ~73~i3;3
Pre~erred solvents are water, toluene, methylene
chloride or a mixture of these solvents.
Examples of bases for use in the above processes
include tertiary amines such as triethylamine; pyridine;
potassium carbonate; sodium carbonate; sodium bicarbonate;
sodium hydroxide; or potassium hydroxide. Preferred bases
are sodium hydroxide, potassium hydroxide or triethyl-
amine.
In Process B, Method 1, a compound of Formula VI is
reacted with a ketone or aldehyde of Formula VII in an
inert or substantially inert solvent or mixture of
solvents and optionally in the presence of a catalyst to
a~ford an intermedia~e product of Formula VIII. The
intermediate product of Formula VIII is then further
reacted with a reducing agent in an inert or substantially
inert solvent or mixture of solvents to afford a second
intermediate product of Formula IX (IV) which can be
isolated or further reacted with a compound of Formula V
in the presence of a base in an inert or substantially
inert solvent or mixture o~ solvents to afford the desired
product of Formula X (I).
Examples of the compounds of Formula VI which can be
used in the above Process B, Method 1, include
~enzoylhydrazine, 4-chlorobenzoylhydrazine, 2-
methylbenzoylhydrazine, 4-methylbenzoylhydrazine, 3,5-
dichlorobenzoylhydrazine and the like. The compounds of
~ormula VI are generally commercially available or can be
prepared by known procedures,
Examples of the compounds of Formula VII which can be
used in the above Process B, ~ethod 1, include trifluoro-
acetone, methacrolein, ethyl pyruvate and the like. The
compounds of Formula VII are generally commercially
available or can be prepared by known procedures.
~.~736~
Optionally, a catalyst may be used in Step 1, Method
l of of Process ~. Suitable catalysts generally include
organic acids such as acetic acid, trifluoroacetic acid,
oxalic acid and the like; mineral acids such as
hydrochloric acid, sulfuric acid, nitric acid and the
like; arylsulfonic acids such as toluenesulfonic acid; or
pyridinium ~oluenesulfonate~ Preferred catalysts are
organic acids or arylsulfonic acids. Most preferred
catalys~s are acetic acid or trifluoroacetic acid.
Suitable solvents for use in the above Process B,
Method l, Step l, include alcohols such as methanol,
ethanol, isopropanol and the like; hydrocarbons such as
toluene, benzene; ethers such as tetrahydrofuran (THF),
glyme and the like; or dimethylformamide. Preferred
solvents are alcohols and hydrocarbons. Most preferred
solvents are alcohols such as methanol or ethanol.
Examples of suitable reducing agents for use in the
above Process ~, Method l, Step 2, include hydrides such
as sodium borohydride and derivatives thereof such as
sodium cyanoborohydride, lithium aluminum hydride and
derivatives thereof and the like; or diborane~ Preferred
reducing agents are sodium borohydride and derivatives
thereof or lithium aluminum hydride and derivatives
thereof. ~ost preferred as - reducing agents are borane
and diborane.
~ptionally, in Process B, Method 1, Step 2, a
catalyst may be included. Examples of suitable catalysts
include organic acids such as acetic acid, trifluoroacetic
acid; or mineral acids such as hydrochloric acid, sulfuric
acid and the like. Preferred catalysts are organic acids
or hydrochloric acid. Most preferred catalysts are acetic
aci~, tri~luoroacetic acid or hydrochloric acid.
~uitable solvents for use in the above Process B,
Method l, Step 2, include alcohols such as methanol,
-32-
..
~; ,.
7~3~i33
ethanol, isopropanol and the like; ethers such as
tetrahydrofuran (THF), diethylether, glyme and the like;
or halohydrocarbons such as methylene chloride, chloroform
and the like. Preferred solvents are alcohols and most
S preferred are methanol or ethanol.
Step 3 of Process B, Method 1 corresponds to Step 2
o~ Process A. Consequently, those bases and solvents
suitable for use in Step 2 of Process A are suitable for
use in Step 3, Method 1 of Process B including the
preferred bases and solvents described above.
In Process ~, Method 2, and N'-substituted-N'-
benzoylhydrazine of Formula XII is reacted with a compound
o$ Formula XI in the presence of a base in an inert or
substantially inert solvent or mixture of solvents to
af~ord the desired pro~uct of Formula I.
The compounds of Formula XI are generally
commercially available or can be prepared from
commercially available compounds by procedures well known
to those skilled in the art as described below.
Examples of the compounds of Formula XII which can be
used in the a~ove Process B, Method 2, include N'-(l,l-
dimethyl-3-butenyl)-N'-benzoylhydrazine, N'-(trimethyl-
silylmethyl)-N'-(4-meth~lbenzoyl)hydrazine, N'-(l,l,l-
trifluoro-2-propyl)-N'-(2,4-dichlorobenzoyl~hydrazine, N'-
(1-cyano-1-methyl)ethyl-N'-(2-methylbenzoyl)hydraæine and
the like.
Suitable solvsnts for use in the above Process B,
Method 2, include water; hydrocarbons such as toluene,
xylene, hexane, heptane and the like; alcohols such as
methanol, ethanol, isopropanol, and the like; ~lyme;
tetrahydrofuran; acetonitrile; pyridine; or haloalkanes
such as methylene chloride; or mixtures of these
solvents. Preferred solvents are water, toluene,
methylene chloride or a mixture of these solvents.
~:7~ i33
Examples of bases suitable for use in the above
Process C includes tertiary amines such as triethylamine;
pyridine; potassium carbonate; sodium carbonate; sodium
bicarbonate; sodium hydroxide; or potassium hydroxide.
Pre~erred bases are sodium hydroxide, or triethylamine.
The compounds of Formula XI are commercially
available, such as nicotinoyl chloride hydrochloride,
lsonicotinOyl chloride hydrochloride and ethyl picolinate
or can be prepared from commercially available materials
by procedures known to those skilled in the art.
The compounds of Formula XII can be prepared by
procedures known to those skilled in the art from
commercially available reactants. By way of example, a
suitably substituted hydrazine (such as (l,1,1-trifluoro-
2-propyl)hydrazine) is reacted with an aldehyde or ketone
(such as acetone) in the presence o~ a base (such as
triethylamine) to afford a hydrazone which is then reacted
with a benzoyl chloride in an inert or substantially inert
solvent or mixture of solvents in the presence of a base
(such as sodium hydroxide) to afford an N'-substituted-N'-
~enzoylhydrazone which is then reacted with an acid (such
as hydrochloric acid) to afford the compound of ~ormula
XII. Alterna~ively, a suitable substituted hydrazine
(such as (trimethylsilylmethyl)hydrazine) is reacted with
di-tert-butyldicarbonate in an inert or substantially
inert solvent or mixture of solvents (~uch as
toluene/water) to afford an N'-(trimethylsilylmethyl)-N-t-
butoxycarhonylhydrazine which is then reacted with a
benzoylchloride in an inert or substantially inert solvent
or mixt~re of solvents to afford an N'-
(trimethylsilylmethyl)-N'-benzoyl N-t-butoxycarbonyl-
hydrazine which is th~n reacted with an acid to afford the
aesired compound of Formula XII.
-34-
`:'
"
3 6 ~ 3 3
In Process C, a compound of Formula XIII is reacted
with a monosubstituted hydrazine of Formula III or a
corresponding acid addition salt such as the hydrochloride
salt or the like in the presence of a base in an inert or
substantially inert solvent or mixture of solvents to
afford an intermediate compound of Formula XIV which can
be isolated or further reacted with a compound of Formula
XV in the presence of a base in an inert or substantially
inert solvent or mixture of solvents to afford the desired
product of Formula I.
In Process D, a monosubstituted hydrazine of Formula
III or a corresponding acid addition salt, such as the
hydrochloride salt or the like, is reacted with a compound
of Formula XVI in the presence of a base in an inert or
lS substantially inert solvent or mixture of solvents to
afford an intermediate product of Formula XVII. The
intermediate product of Formula XVII is then further
reacted with a compound of Formula V in the presence of a
base in an inert or substantially inert solvent or mixture
of solvents to afford a second intermediate product of
Formula XVIII. The second intermediate product of Formula
XVIII is then further reacted with an acid in an inert or
substantially inert solvent or mixture of solvents to
afford a third intermediate product of Formula XIX. The
third intermediate product of Formula XIX is then further
reacted with a compound of Formula II in the presence of a
base in an inert or substantially inert solvent or mixture
of solvents to afford the desired product of Formula I.
Examples of the compounds of Formula XVI which can be
used in the above Process D include di-t-butylcarbonate,
diethylcarbonate, diphenylcarbonate, dibenzylcarbonate and
the like. The compounds of Formula XVI are generally
commercially available or can be prepared by known
procedures.
-35-
3~3;3
Suitable solvents for use in the above Process D,
Steps 1, 2 and 4 include water; tetrahydrofuran; dioxane;
toluene; alcohols such as methanol, ethanol and
isopropanol; hexane; acetonitrile; pyridine; and
haloalkanes such as methylene chloride; or mixtures of
these solvents.
Preferred solvents are dioxane; toluene; tetrahydro-
furan; pyridinei methylene chloride or water.
Most preferred solvents are dioxane; water or
toluene.
Examples of the bases for use in the above Process D,
Steps 1, 2 and 4 include tertiary amines such as triethyl-
amine; pyridine; potassium carbonate~ sodium carbonate;
sodium bicarbonate; sodium hydroxide; and potassium
hydroxide.
Preferred bases are sodium hydroxide; potassium
hydroxide; pyridine or triethylamine.
Suitable solvents for use in the above Process D,
Step 3 include alcohols such as methanol, ethanol and
isopropanol; water; tetrahydrofuran; dioxane; and aceto-
nitrile.
Preferred solvents are methanol or ethanol.
Examples of acids for use in the above Process D,
Step 3 include concentrated hydrochloric acid or
concentrated sulfuxic acid.
When A and B are the same, for example, both A and B
are unsubstituted phenyl, two equivalents of a compound
Formula XIII or XV are reacted with a monosubstituted
hydrazin~ of Formula III in the presence of a base in an
inert or substantially inert solvent or mixture of
solvents to afford the desired product of Formula I.
Examples of the compounds of Formula XIII and/or
Formula XV which can be used in the above Process C
include 3-methyl-methylthio-thiobenzoate, 4-chloro~
-36-
~ ~73~j~33
methylthio-thiobenzoate, 4-methyl-methylthio-thiobenzoate,
carboxymethylthio-thiobenzoate and the like. The
compounds of Formula XIII and/or Formula XIV are generally
commercially available or can be prepared by known
procedures.
Suitable solvents for use in the above Process C are
generally polar high-boiling solvents such as dimethyl-
formamide (DMF); glyme; tetrahydrofuran (THF); and
pyridine. The preferred solvent is pyridine.
Suitable bases for use in the above Process C include
tertiary amines such as triethylamine; and pyridine. The
preferred base is pyridine.
The above Processes A and B, Method 1, can be carried
out at temperatures between about -20C and about 100C.
Preferably, these reactions are carried out between about
-5C and about 50C.
The above Process B, Method 2, can be carried out at
temperatures between about -50C and about 150C.
Preferably when W is a halo radical, the reaction is
carried out between about 0C and about 30C. When W is
alkoxy, the reaction is preferably carried out between
about 100C and about 150C. When W is methyl sulfonate,
the reaction is preferably carried out between about -20C
to about 20C. When W is an ester, the reaction is
preferably carried out between about 0C and about 50C.
Process C can be carried out at temperatures between
about 10C and 200C. Preferably, this reaction is
carried out between about 70C and about lO~QC.
Pro~ess D can be carried out at temperatures between
about 0C and 100C. Preferably, these reactions are
carried out between about 0C and abou~ 50C.
Preparation of the compounds of the present invention
by processes A, B, C and D are preferably carried out at
about atmospheric pressure, although higher or lower
pressures can be used if desired.
-37-
!
~ ~73~33
Substantially equirnolar amounts of reactants are
preferably used in processes A, B and C, although higher
or lower amounts can be used if desired.
Generally, about one equivalent of base is used per
e~uivalent of starting material of Formula II, V, XI
and/or XIII. Where the acid addition salt of the mono-
substituted hydrazine of Formula III is used, one
additional equivalent o~ base is used. For example, in
Process A, when substituents A and B are the same and a
monosubstituted hydrazine is used, about two equivalents
of base are used since about two equivalents of a suitably
substituted benzoyl chloride of Formula II or V are
employed. In Process A, when substituents A and B are
different and an acid addition salt o~ the monosubstituted
hydrazines o~ Formula III is used, about two equivalents
of base are used in Step 1 and about one e~uivalent of
base is used in Step 2.
Modifications to the above processes may be necessary
to accommodate reactive functionalities of particular A
and/or B substituents. Such modifications would be
apparent and kno~n to those skilled in the art.
It will be appreciated by those skilled in the art
that electronic forces may give rise to more than one
isomer o~ the compounds of Formula I such as enantiomers,
Zs conformers and the like. There may be a dif~erence in
properties such as physical characteristics and degree of
biological activity between such isomers. Separation of a
speci~ic isomer can be accomplished by standard techniques
well known to those skilled in the art such as silica gel
chromatography.
The agronomically acceptable salts embraced by
Formula I of the invention can be prepared by reacting a
metal hydroxide, a metal hydride or an amine or ammonium
salt, such as a halide, hydroxide or alkoxide with a
-38-
~73~3;3
compound of Formula I having one or rnore hydroxy or
carboxy groups or reacting a quaternary ammonium salt,
such as chloride, bromide, nitrate or the like with a
metal salt of a compound of Formula I in a suitable
solvent. When metal hydroxides are used as reagents,
useful solvents include water; ethers such as glyme and
the like; dioxane; tetrahydrofuran; alcohols such as
methanol, ethanol, isopropanol and the like. When metal
hydrides are used as reagents, useful solvents include
nonhydroxylic solvents, for example, ethers such as
dioxane, glyme, diethylether and the like; tetrahydro-
furan; hydrocarbons such as toluene, xylene, hexane,
pentane, heptane, octane and the llke; dimethylformamide,
and the like. When amines are used as reagents, useful
solvents include alcohols, such as methanol or ethanol;
hydrocarbons, such as toluene, xylene, hexane and the
like; tetrahydrofuran; ~lyme; dioxane; or water. When
ammonium salts are used as reagents, useful solvents
include water; alcohols, such as methanol or ethanol;
glyme; tetrahydrofuran; or the like. When the ammonium
salt is other than a hydroxide or alkoxide, an additional
base, such as potassium or sodium hydroxide, hydride, or
alkoxide is generally used. The particular choice of
solvent will depend on the rela-tive solubilities of the
starting materials and the resultant salts, and slurries
rather than solutions of certain reagents may be used to
obtain the salts. Generally, equivalent amounts of the
starting reagents are used and the salt-forming reaction
i~ carried o~t at about 0C to about 100C, preferably at
about room temperature.
The acid addition salts of the present invention can
be preparad by reacting hydrochloric, hydrobromic,
sulfuric, nitric, phosphoric, acetic, propionic, ben~oic
or other suitable acid with a compound of Formula I having
-39-
73~3
a basic functional group in a suitable solvent. Useful
solvents include water, alcohols, ethers, esters, ketones,
haloalkanes and the like. The particular choice of
solvent will depend on the relative solubilities of the
starting materials and the resulting salts and slurries
rather than solutions of certain reagents may be used to
obtain the salts. Generally, equivalent molar amounts of
starting materials are used and the salt-forming reaction
is carried out at from about -10C to about 100C,
preferably at about room temperature.
Tha following examples will further illustrate this
invention but are not intended to limit it in any way. In
Table I, some N'-substituted-N,N'-diacyl hydrazines of the
present invention that have been made are listed. The
lS structure of these compounds was confirmed by NMR and in
some cases by IR and/or elemental analysis. Specific
illustrative preparation of the compounds of Examples 8,
15 and 28 are described after Table I.
-40-
3~;i;33
, .
TABLE I
O H O
Il l 11
A -C -N ~ N C ~ B
R4 ~1 ~ R2
13
Ex No. R2 R3 R4 A B
1 H Me CF3 C6H3Me2-2,3 C6H3C12-2,4
2 H Me CF3 C6H3Me2-2,3 C6H3Me2-3,5
3 H Me CF3 C6H3Me2-2,3 C6H3N02Me-2,5
4 H Me CF3 C6H5 C6H5
H H CF3 C6H3C12-3,4 C6H3C12-3,4
6 H H CF3 C6H4Cl-4 C6H4Cl-4
7 H H CF3 C6H5 C6H5
Me ~e CN C6H5 C6H5
Me Me CN C6H5 C6H4Cl-4
10 Me Me CN C6H44-Me C6H4Me-3
11 Me Me CN C6H5 C6H4Me-3
12 Me Me CN C6H4Cl-4 C6H4Cl-4
13 Me Me CN C6H4Me-4 C6H3Me23,5
14 Me Me CN C6H'LMe-4 C6H4F 4
lS Me Me CN C6H~Me-4 C6H4Me-4
16 Me Et CN C6H5 C6H5
17 Me Me CN S C6H4Me 3
18 Me Me CN C6H4Et-4 C6H3Me2-3,5
19 Me Me CN C6H4Me 2 C6H3Me~-3,5
2~ Me Me CN C6H4Cl-4 C6H4Me-3
21 Me Me CN C6~3F2-2,6 C6H4Cl-4
22 Me Me CN C6H4Me 2 C6H4~e-3
23 Me Me CN C6N4N02-2 C6H4Me-3
24 Me Me CN C6H4C1 2 C6H4Me-3
-41-
: "`'' ....;.~.: ~''"'''' " "'
,.:
3~7~ 3
. .
Me Me 02Me C6H5 C6H5
26 Me Me CH2CH=CH2 C6H4Me-4 C6H4Me-4
27 Me Me CH2CH=CH2 C6H4Me-3 C6H4Me-3
28 Me ~e C~2CH=CH2 C6H5 C6H5
29 H H SiMe3 C6H4Et-4 c6H3No2Me-2 ,5
H H SiMe3 C6H4Cl-4 C6H4Cl--4
31 ~ H SiMe3 C6Hg~Me-2 C6H4Me 2
32 H H SiMe3 C6H4N02-2 C6H4N02-2
3 3 H H SiMe3 C6H5 C6H5
34 H ~i SiMe3 C6H4Et-4 C6H3Me2-3 ~ 5
--42--
73~;3~
EXAMPLE NO. 8 - Preparation of N'~ cyano-l-methyl)e~hyl-
N,N'-dibenzoylhydrazine
To a suspension of benzoylhydrazine (13.6 g, 0.1
mole) in deionized water (50 ml) at 5C with stirring was
dropwise added concentrated hydrogen chloride (9.8 9, 0.1
mole). To the resulting clear solution was added sodium
cyanide (5.2 9, 0.1 mole) and acetone (6.5 g, 0.11
mole). A white and thick precipitate appeared. The
cooling bath was removed and the reaction flask was
stoppered tightly. The reaction mixture was stirred for
18 hours. The precipitate was collected by suction-
filtration and was washed with a small amount of water to
give 17.5 g (86.2% yielded) of desired intermediate, N'-
(l-cyano-l-methyl)ethyl-N-benzoic acid hydrazide, as the
lS starting material for the next stepO (m.p. 82-92C)
To the solution of N'-(l-cyano-l-methyl)ethyl-N-
benzoic acid hydrazide (2 g, 0.01 mole) in dry methylene
chloride (25 ml) under nitrogen at room temperature with
stirring was added benzoyl chloride (2.02 g, 0.014
mole). To the above mixture was dropwise added
triethylamine (1.31 g, 0.013 mole). The reaction mixture
was stirred at room temperature for 5 hours~ The reaction
mixture was diluted with methylene chloride (50 ml),
washed with water and brine. The organic layer was dried
over MgS04 and the solvent was evaporated at reduced
pressure to give a residue. The residue was treated with
ethyl acetate/hexane mixture (1:1) a~fording a crude
solid-formed product which was collected by suction-
filtration (1 g, 33% yield). An analytical sample was
obtained by crystallization from ethyl acetate/hexane
(4:1), m.p. 202-204C. NMR and IR spectra showed the
desired product.
-43-
73~33
Elemental Anal~sis
C H N
Calc.(%)70.34 5.58 13.67
Found(%)70.20 5.67 13.40
S EXAMPLE NO. 15 - Preparation of N'~ cyano-l-
meth l)eth l-N N'-di-4-toluovlhvdrazine
Y _ Y
To the suspension of 4-toluic acid hydrazide (15.0 g,
0.1 mole) in water (150 ml) and ethanol (20 ml) at 5C
with stirring was dropwise added concentrated hydrogen
chloride (10 g, 0.1 mole)O To the above still suspension
was carefully added sodium cyanide (5.3 g, 0~1 mole) and
acetone (6.6 g, 0.11 mole). The reaction flask was
stoppered tightly and the cooling bath was removed. The
resulting viscous reaction mixture was stirred at room
temperature for more than 24 hours. The precipitate was
collected by suction-filtration and was washed with a
small amount of diluted hydrogen chloride and water
affording 14.6 9. (64.8%) of N'-(l-cyano-l-methyl)ethyl 4-
toluic acid hydrazide as the starting material for the
next step. Analytical sample was obtained by
crystallization from ethyl acetate/hexane (3:1), m.p. 146
148Co
To the solution of N'-(l-cyano-l-methyl)ethyl 4-
toluic acid hydrazide (2.17 9, 0.01 mole) in dry methylene
~5 chloride (65 ml) under nitrogen with magnetic stirring was
added 4-dimethylaminopyridine catalyst (1.34 g, 0.U11
mole) followed by 4-methylbenzoyl chloride (2.52 g, 0.017
mole). To the above mixture was dropwise added
triethylæmine (1.1 g, 0.011 mole). The reaction mixture
~0 was slightly exothermic. After stirring at room
temperature or 40 minutes the reaction mixture was
diluted with methylene chloride (50 ml), washed with
diluted HCl solution (2X50 ml), dil NaOH (50 ml), H~O (50
~ ~73~3~3
. .
ml) and brine. The organic layer was dried over MgSO~,
and the solvent was evaporated under reduced pressure to
give a residue. The residue was treated with ethyl
acetate/hexane mixture (1:1) and the resulting solid was
collected by suction-f.iltration and affording 2.75 9 (82%
yield) of almost pure product. (m.p. 192-198C) NMR and
IR spectra showed the desired product.
By following substantially the procedures in Example
8 (without catalyst) or 15 (with catalyst) and using the
reactants shown below in Table II, the products of
Examples 8 to 24 were prepared.
TPELE II
Ex. Ccmpound of Ccmpound of Compound of
No. Fonmula VI Fonmula VIIa Fonmula V
8 benzoylhydrazine acetone benzoyl chloride
9 ben2oyIhydrazine acetone 4-chlorobenzcyl chloride
10 4 toluoylhydrazine acetone 3-methylbenzoyl chloride
11 benzoylhydrazine acetone 3,methylbenzoyl chloride
12 4-chlorobenzoyl- acetone 4-chlorokenzoyl chloride
hydrazine
13 4 toluoylhydrazine a oe tone 3,5-dimethylkenzoyl chloride
14 4-toluoylhydrazine acetone 4-fluorobenzoyl chloride
15 4 toluoylhydrazine acetone 4~methylbenzoyl chloride
16 benzoylhydrazine methylethylketone benzoyl chloride
17 thienoylhydrazine acetone 3-methylbenzoyl chloride
18 4-ethylbenzoyl- acetone 3,5-dimethylbenzoyl chloride
hydr æine
19 2-toluoyLhydrazine acetone 3,5-dimethylbenzoyl chloride
20 4-chlorobenzoyl- acetone 3-methylbenzoyl chloride
hydrazine
'73~3
Ex. ~und of G~x~nd of Ccmpound of
No. FormuLa VI Formula VIIaFormula V
~1 2,4-difluorobenzoyl- acetone4-chlorobenzoyl chloride
hydrazine
~2 2-toluoylhydrazine acetone3-methylbenzoyl chloride
23 2-nitrobenzoyl- acetone3-methylbenzoyl chloride
hydrazine
24 ~-chlorobe~oyl- acetone3-methylbe~oyl chloride
hydrazine
EXAMPLE NOO 2~ Pre~aration of N'-(1,1=dimethvl-3-
butenvl)-N,N'-dibenzoylhydrazine
To a gently refluxing solution of allyl magnesium
bromide (3~0 ml of lM solution) was addad acetone azine
(2~ 9) dissolved in diethyl ether (200 ml). The solution
was re~luxed ~or three days. Upon cooling~ a saturated
solution of ammonium chloride (50 ml) was added. The
aqueous layer was separated and washed twice with diethyl
ether (2~0 ml). The combined ether extracts were dried
over anhydrous magnesium sulfate, filtered and the ether
removed at reduced pressure. The product was distilled
through a vigreux column at 3.1 torr and collected in a
dry ice/acetone cooled receiving flask. The boiling point
was 60-65C. lS g o~ product was collected.
oxalic acid (16.7 g) was dissolved in a solution of
ethanol:diethyl eth~r (1:1) (150 ml) and water (3.3 9) was
a~ded. To this acid solution was added the hydrazone (13
g) dissolved in diethyl ether (75 ml). The solution was
stirred for 24 hours then filtered. The solid is washed
once with diethyl ether. The filtrate was concentrated
and combined with the solid to afford a 71% yield (17.2 9)
of the hydrazine oxalate.
The l,l-dimethyl-3-butenylhydrazine oxalate (2 9) was
dissolved in toluene and neutralized with 50~ aqueous
sodium hydroxideO To this solution was added benzoyl
-46-
~3~ 3
chloride (2.8 9) and sodium hydroxide (50~ Aq. solution)
(3.2 g) at 25C. The reaction mixture was warmed to room
temperature and stirred. The mixture was diluted with
hexane and filtered to afford an oily product which
solidified upon standing. (m.p. 105-112C)
Examples 26 and 27 were made generally in accordance
with the procedures for Example 29 abova.
The (trimethylsilylmethyl)hydrazine, trifluoroalkyl
hydrazine 2nd(2-carbomethoxy -2-propyl)hydrazine were made
generally in accordance with the procedures from Noll,
J.E~; Sprier, J.L.; Daubert, a.F.; JACS 73, 3867, 1951;
~ung, S.C.; Le; Breton, G.C.; JOC 46, 5413, 1981; and
organic Synthesis Vol 5, pg. 43, respectively, From these
starting materials Examples 1-7, 25 and 29-34 were made
generally in accordance with the procedure for Example 28
above.
The react`ants shown below in Table III were used to
prepare Examples 1-7 and 25 34.
TAELE III
Ex. Compound o~Co~x~nd of Cc0pound of
No. FoDmula IIFonmula III Fornula V
1 2,3-dimathyl(1-methyl-2,2 t2- 2,4~dichloro
benzoylchloride trifluoroethyl) benzoyl~oride
hydrazine
~ 25 2 2,30d ~ ~ 1(1~me~1-2,2,2- 3,5-d~thyl
; . benzoylchloridatrifluoroethyl) benzoyl ~ oride
h~dr~ine
3 2,3-d ~ thyl(1-methyl-2,2,2- 2-nitro-5-
benzoylchloride trifluoroethyl) tolu~yl~loride
hydrazine
4 benzoylchloride (1-me ~ 1-2,2,2- benzoylchloride
tri~luoroethyl)
hydrazine
-47-
~.~
~.2736~
E~c. Con~o~md o$ Cc~ound of Ca~und o~
No. Formula II Formula III ro:A.. l~ V
3,4-dichloro (2,2,2-trifluoroett~l) 3,4-dichloro
benzoylchlori~e hydrazine benzoylchloride
6 4-chlorobenzoyl (2,2,2-trifluoroethyl) 4chlorobenzoyl
chloride h~drazine chloride
7 benzoylchloride (2,2,2- trifluoroethyl) benzoylchloride
hydrazine
benzoylchloride (l~car~cmethc~y-l- benzoylchloride
nethylethyl)
- hydræir~
26~toluoylchloride (l,l-dim~thyl~3- 4-toluo5~1chloride
bute~l)h~drazine
273-toluoylchloride (l,l~d~msthyl-3- 3-toluoylchloride
butenyl)hydræine
28 benzoylchloride (1,1-d~methyl-3- benzoylchloride
h~ter~l)hydrazine
29 4-ethylbenzoyl (trimethylsilyln~thyl) 2-nitro-5-toluoyl
chloride hydrazine chloride
3(1 4-chlorobenzoyl (trimethylsilylmethyl) 4-chlorobenzoyl
chloride hydrazine chloride
31 ;2-toluoyl (tri~thylsilylmethyl) 2-toluoyl
shlori~:le hydræine chloride
32 ~-nitrobenzoyl (trimechylsilylsnethyl) 2-nitrobenzoyl
chloride ~drazine chloride
33 ben~oyl ~tr~nethylsilylmethyl) benzoyl
chloride hy~ræine c~loride
34 4ethylbenzoyl (tr~3thylsilylmethyl) 3,5-dimethyl
chloride hydrazine benzoyl~loride
It will b~ appreciated by those skilled in the art
that compound~ of Fonnula I can be used as precursors ~or
preparing other compounds of Formula I by procedures well
known to those skil lad in the art. For example, a
suitable comE~ound of Formula I can be reduced, alkylated,
substituted, es'cerified, hydrolyzed or the like.
--48--
i~
736~33
As previously noted, the cornpounds of the present
invention exhibit excellent insecticidal activity and are
most active against insects of the orders Lepidoptera and
Coleoptera.
In general, for the control of insects in agricul-
ture, horticulture and forestry, the compounds of the
present invention may be used at a dosage corresponding to
from about 10 grams to about 10 kilograms of the active
substanc~ per hectare and from about 100 grams to about 5
kilograms per hectare of the active substance ls
preferred. The exact amount of dosage for a given
situation can be routinely determined and depends on a
variety of factors, for example, the substance used, the
kind of insect, the formulation used, the state of the
crop infested with the insect and the prevailing weather
conditions. The term "insecticidal" as employed in the
specification and claims of this application is to be
construed as any means which adversely affects the
existence or growth of the target insects at any stage in
their life cycle. Such means can comprise a complete
killing action, eradication, arresting in growth,
inhibition, reducing in number, reproductive inhibition
(such as ovicidal or chemisterilant) or any combination
thereof. The term "control" as employed in the
~5 specification and claims of this application is to be
construed as meaning "insecticldal" or protecting plants
from insect damage. By "insecticidally effective amount"
is meant that dosage of active substance sufficient to
exert insect "control."
The compounds of the present invention, for
practical applications, can be utilized in the form of
compositions or formulations. Examples of the preparation
of compositions and formulations can be found in the
American Chemical Society publication "Pesticidal
-49-
~7~
,,
Formulation Research," (1969), Advances in Chemistry
Series No. 86, written by Wade Van Valkenburg; and the
Marcel Dekker, Inc. publication "Pesticide Formulations,"
(1973), edited by Wade Van Valkenburg. In these
compositions and formulations, the active substance or
substances are mi~ed with conventional inert agronomically
acceptable (i.e., plant compatible and/or pesticidally
inert) diluents or extenders such as solid carrier
material or liquid carrier material, of the type usable in
conventional compositions or formulations. By
agronomically acceptable carrier is meant any substance
which can be used to dissolve, disperse or diffuse the
active ingredient in the composition without impairing the
active ingredient's effectiveness and which by itself has
no significant detrimental effect on the soil, equipment,
desirable plants or agronomic environment. If desired,
conventional adjuvants such as surfactants, stabilizers,
antifoam agents and antidrift agents may also be added.
Examples of compositions and formulations according
to the invention are aqueous solutions and dispersions,
oily solutions and oil dispersions, pastes, dusting
powders, wettable powders, emulsifiable concentrates,
flowables, granules, baits, invert emulsions, aerosol
compositions and fumigating candles.
Wettable powders, pastes, flowables and emulsifiable
concentrates are concentrated preparations which are
diluted with water before or during use.
Baits are preparations generally comprising a ~ood
or other substance attractive to the target pest, that
includes at least one lethal or non-lethal toxicant.
Lethal toxicants kill the pest upon ingesting the bait
while non-lethal toxicants change the behavior, feeding
habits and physiology of the pest for the purpose of
control.
-50-
3~
The invert emulsions are mainly used for air
application, where large areas are treated with a
comparatively small amount of preparation. The invert
emulsion may be prepared in the spraying apparatus shortly
before, or even during, the spraying operation by
emulsifying water in an oil solution or an oil dispersion
of the active substance.
Compositions and formulations are prepared in a
known manner, for instance by extending the active
compounds with conventional dispersible liquid diluent
carriers and/or dispersible solid carriers optionally with
the use of carrier vehicle assistants, e.g., conventional
sur~ace-active agents, including emulsifying agents and/or
dispersing agents, whereby, for example, in the case where
water is used as diluent, organic solvents may be added as
auxiliary solvents. The following may be chiefly
considered for use as conventional carrier vehicles for
this purpose: aerosol propellants which are gaseous at
normal temperatures and pressures, such as halogenated
hydrocarbons, e.g., dichlorodifluoromethane and trifluoro-
chloromethane, as well as butane, propane, nitrogen and
carbon dioxide; inert dispersible liquid diluent carriers,
including inert organic solvents, such as aromatic
hydrocarbons (e.g., benzene, toluene, xylene, alkyl
naphthalenes, etc.), halogenated, especially chlorinated,
aromatic hydrocarbons (e.g., chlorobenzenes, etc.),
cycloalkanes (e.g., cyclohexane, etc.), parafEins (e.g.,
petroleum or mineral oil fractions), chlorinated aliphatic
hydrocarbons (e.g., methylene chloride, chloroe-thylenes,
etc.), vegetable oils (e.g., soybean oil, cottonseed oil,
corn oil, etc.), alcohol~ (e.g., methanol, ethanol,
propanol, butanol, glycol, etc.) as well as ethers and
esters thereof (e.g., glycol monomethyl 0ther, etc.),
amines (e.g., ethanolamine, etc.), amides (e.g., dimethyl
-51-
~,~d~73~i~33
formamide, etc.), sulfoxides (e~gO, dimethyl sulfoxide,
etc.), acetonitrile, ketones (e.g., acetone, methyl thyl
ketone, methyl isobutyl ketone, cyclohexanone, isophorone,
etc.), and/or water; solid carriers including ground
natural minérals, such as kaolins, clays, talc, chalk,
quartz, attapulgite, montmorillonite or diatomaceous
earth, and ground synthetic minerals, such as highly-
dispersed silicic acid, alumina and silicatesi solid
carriers for granules include crushed and fractionated
natural rocks such as calcite, marble, pumice, sepiolite
and dolomite, as well as synthetic granules of inorganic
and organic meals, and granules of organic material such
as sawdust, coconut shells, corn cobs and tobacco
stalks. The following may be chiefly considered for use
as conventional carrier vehicle assistants: emulsifying
agents, such as cationic and/or nonionic and/or anionic
emulsifying agents (e.g., polyethylene oxide esters of
fatty acids, polyethyIene oxide ethers of fatty alcohols,
alkyl sulfates, alkyl sulfonates, aryl sulfonates, alburnin
hydrolysates, etc~, and especially alkyl arylpolyglycol
ethers, magnesium stearate, sodium oleate, etc.); and/or
dispersing agents, such as lignin, sulfite waste liquors,
methyl cellulose, etc.
Adhesives such as carboxymethylcellulose and natural
and synthetic polymers in the form of powders, granules or
latices, such as gum arabic, polyvinyl alcohol and
polyvinyl acetate, can be used in the for~ulations.
If desired/ it is possible to use coloran~s in
compositions and formulations containing compounds of the
present invention such as inorganic pigments, for example,
iron oxide, titanium oxide and Prussian Blue, and organic
dyestuffs, such as alizarin dyestuffs, azo dyestufs and
metal phthalocyanine dyestufs, and trace nutrients such
as salts of iron, manganese, boron, copper, cobalt,
molybdenum and zinc.
-52-
~ ~'7~;33
The active compounds of the present invention may be
employed alone or in the form of mixtures with one another
and/or with such solid and/or liquid dispersible carrier
vehicles and/or with other known compatible active agents,
esp0cially plant protection agents, such as other
insecticides, arthropodicides, nematicides, fungicides,
bactericides, rodenticides, herbicides, fertilizers,
growth-regulating agents, synergists, etc., if desired, or
in the form of particular dosage preparations for specific
application made therefrom, such as solutions, emulsions,
suspensions, powders, pastes, and granules which are thus
ready for use.
As concerns commercially marketed preparations,
these generally contemplate carrier composition mixtures
in which the active compound is present in an amount
substantially between about 0.1% and 99% by weight, and
preferably between about 1% and 75% by weight, of the
mixture. Carrier composition mixtures suitable for direct
application or field application generally contemplate
those in which the active compound is used in an amount
substantially between about 0.0001% and 5%, preerably
between about 0.001% and 3%, by weight of the mixture.
Thus the present invention contemplates overall formula-
tions and compositions which comprise mixtures of a
conventional dispersible carrier such as (1) a dispersible
inert finely divided carrier solid, and/or (2) a
dispersible carrier liquid such as an inert organic
solvent and/or water, preferably including a surface
active effective amount of a carrier vehicle assistant
(e.g., a surface-active agent, such as an emulsifying
agent and/or a dispersing agent), and an amount of the
active compound generally, between about 0.0001~ and about
99~ by weight of the composition, preferably between about
0.001% and about 90% by weight of the composition, and
3~i~ 3
more pref-erably between about 0001% and about 75% b~
weight of the composition, which is effective for the
purpose in question.
The active compounds can be applied as sprays by
methods commonly employed, such as conventional high-
gallonage hydraulic sprays, low gallonage sprays, ultra-
low-volume sprays, airblast spray, aerial sprays, and
dusts. If low volume applications are desired, a solution
of the compound is usually used. In ultra-low-volume
applications, a liquid composition containing the active
compound is usually applied as a spray (e.g., mist) by
means of atomizing equipment in finely divided form
(average particle size of from about 50 to about 100
microns or less) using airplane crop spraying tech-
niques. Typically only a few liters per hectare are
needed and often amounts up to about 15 to 1000 g/hectare,preferably about 40 to 600 g/hectare are sufficient. With
ultra-low-volume, it is possible to use highly concen-
trated liquid compositions with said liquid carrier
vehicles containing from about 20 to about 95% by weight
of the active compound.
Furthermore, the present invention contemplates
methods of killing, combatting or controlling insects,
which comprises contacting insects with a correspondingly
combative or toxic amount (i.e., an insecticidally
effective amount) of at least one active compound of the
invention alone or together with a carrier vehicle
(composition or formulation) as noted above. The term
"contacting" as employed in the specification and claims
of this application is to be construed as applying to at
least one of (a) such insects and (b) the corresponding
habitat thereof (i.e., the locus to be protected, for
example, to a growing crop or to an area where a crop is
to be grown) the active compound of this invention alone
-54-
~ ~.,73~i~3
or as a constituent of a composition or formulation. The
instant formulations or compositions are applied in the
usual manner, for instance by spraying, atomizing,
vaporizing, scattering, dusting, watering, squirting,
S sprinkling, pouring, fumigating, dry dressing, moist
dressing, wet dressing, slurry dressing, encrusting and
the like.
It will be realized, of course, that the concen-
tration of the particular active compound utilized in
1~ admixture with the carrier vehicle will depend upon such
factors as the type of equipment employed, method of
application, area to be treated, types of pests to be
controlled and degree of infestation. Therefore, in
special cases it is possible to go above or below the
aforementioned concentration ranges.
Granular preparations are produced for example, by
taking up the active substance in a solvent and by using
the resulting solution, as the case may be in the presence
of a binder, to impregnate a granular carrier material,
such as porous granules (for example, pumice and
attaclay~, or chopped tobacco ~tems or the like.
A granular preparation (frequently termed a
"pellet") may alternatively be produced by compressing the
active substance together with powderéd minerals in the
presence of lubricants and binders and by disintegrating
and straining the composite to the desired grain size.
Dusts are obtainable by intimately mixing the active
substance with an inert solid carrier material in a
concentration of from about 1 to about 5~% by weight.
Examples of suitable solid carrier materials are talc,
kaolin, pipe clay, diatomaceous earth, dolomite, gypsum~
chalk, bentonite, attapulgite and colloidal SiO2 or
mixtures of these and similar substances. Alternatively
organic carrier materials such as, for example, ground
walnut shells may be used.
~55-
73~3
Wettable powders and flowables are produced by
mixing from about 10 to about 99 parts by weight of a
solid inert carrier such, for example, as the
aforementioned carrier materials with from about 1 to
about 80 parts by weight of the active substance
optionally dissolved in a volatile solvent such as
acetone, from about 1 to about 5 parts by weight of a
dispersing agent such, for example, as the lignosulfonates
or alkylnaphthalene sulfonates known for this purpose and
preferably also from about 0.5 to about 5 parts by weight
of a wetting agent, such as fatty alcohol sulfates, or
alkylarylsulfonates of fatty acid condensation products.
In the case of flowables, a liquid inert carrier such as
water is also included.
To produce emulsifiable concentrates the active
compound is dissolved or finely divided in a suitable
solvent which preferably is poorly miscible with water, an
emulsifier being added to the resulting solution.
Examples o suitable solvents are xylene, toluene, high-
boiling aromatic petroleum distillates, for example
solvent naph~ha, distilled tar oil and mixtures of these
liquids. Examples of suitable emulsifiers are alkyl-
phenoxypolyglycol ethers, polyoxyethylene sorbitan esters
of fatty acids or polyoxyethylene sorbitol esters of fatty
acids. The concentration of the active compound in these
emulsi~iable concentrates is not restricted within narrow
limits and may vary between about 2% and about 50% by
weight depending upon toxicant solubility. A suitable
liquid highly concentrated primary composition other than
an emulsifiable concentrate is a solution of the active
substance in a liquid which is readily miscible with
water, for example, acetone, to which solution a
dispersant and, as the case may be, a wetting agent are
added. When such a primary composition is diluted with
-56-
d ~3~;~3
water shortly before or during the spraying operation an
aqueous dispersion of the active substance is obtained.
An aerosol preparation according to the invention is
obtained in the usual manner by incorporating the active
5 substance or a solution thereof in a suitable solvent in a
volatile liquid suitable for use as a propellant such, for
example, as a mixture of chlorine and fluorine derivatives
of methane and ethane~
Fumigating candles or fumigating powders, i.e.,
preparations which when burning are capable of emitting a
pesticidal smoke, are obtained by taking up the active
substance in a combustible mixture which may, ~or example,
comprise a sugar or a wood, preferably in the ground form,
as a fuel, a substance to sustain combustion such, for
example, as ammonium nitrate or potassium chlorate, and
furthermore a substance for retarding combustion, for
example kaolin, bentonite and/or colloidal silicic acid.
A bait preparation comprises a food or other
substance attractive to pests, a carrier, the toxicant and
may optionally include other substances commonly used in
preparations of this kind, such as, a preservative to
inhibit bacterial and fungal growth, a waterproofing agent
to prevent disintegration under wet conditions and dyes or
colorants as described above.
In addition to the aforementioned ingredients, the
preparations according to the invention may also contain
other substances commonly used in preparations of this
kind.
For example, a lubricant, such as calcium stearate
or magnesium stearate, may be added to a wettable powder
or to a mixture to be granulated. Fur~hermore, there may,
for example, be added "adhesives" such as polyvinyl-
alcohol cellulose derivatives or other colloidal
~3~ 3
materials, such as casein, to improve the adherence of
this pesticide to the surface to be protected.
Representative preparation of compositions and
formulations including the compounds of the present
S invention are set forth below as Examples A through I by
way of illustration but not limitation.
Example A
Granular
Ingredient %/wt.
Toxicant and toxicant impurities ~.25
Triton~ X-305 (binder) 0.25
(Octylphenyl-30-ethylene
oxide ethanol)
Agsorb~ 24/48 (diluent)99.50
(Montmorillonite clay)
Preparation: The toxicant and Triton~ X-305 are
dissolved into methylene chloride and the mixture is added
to the Agsorb~ with continuous mixing. The methylene
chloride is then allowed to evaporate.
Example B
Dust
Ingredie,nt %/wt.
Toxicant and toxicant impurities 1.0
Talc gg.o
Preparation: Toxicant is dissolved in excess acetone
and the mixture is impregnated onto the talc. The acetone
is then permitted to evaporate.
-58
~ ~ ~ 3
Exarnple C
Wettable Powder
Ingredient ~/wt.
Toxicant and toxicant impurities 31.3
Duponal3 WA ~ry (wetter) 2.0
(Sodium lauryl sulfate)
Reax~ 45A (dispersant) 5.0
(Sodium lignin sulfonate)
Barden clay (diluent) 31.7
HiSil~ 233 (diluent) 30.0
(Sodium silica~
Preparation: The toxicant, optionally dissolved in a
volatile solvent, is absorbed onto the Barden clay and
HiSil~ carriers. The Duponal~ and Reax~ are then added
and the entire dry mixture blended until homogeneous. Th0
composition is then micronized to a fine particle size.
Example D
Emulsifiable Concentrate
Ingredient %/wt.
Toxicant and toxicant impurities 15.0
Sponto~ 232T (emulsifier) 6.0
(Anionic and nonionic blend of the
following surfactants: calcium
dodecyl benzene sulfonate; and
ethoxylated alkylphenol)
Sponto~ 234T (emulsifier) 4.0
(Anionic and nonionic blend of the
following surfactants: calcium
dodecyl benzene sulfonate; and
aO ethoxylated alkylphenol)
Cyclohexanone (solvent) 22.5
-59-
~L~73~3
Tenneco~ 500-100 (solvent) 5Z.5
(Aromatic solvent mixture
principally comprising xylene,
cumene and ethyl benzene having
a boiling point range of 290-345F)
Preparation: All ingredients are mixed together with
continuous agitation until a homogeneous clear solution is
obtained.
Example E
Aerosol
Ingredient ~/wt.
Toxicant and toxicant impurities 0.5
"Freon 12"* (dichlorodifluoromethane) 99.5
Preparation: The components are mixed and packaged
under pressure in a suitable container equipped with a
release spray valv~.
Example F
Fumiqatinq Candle or Fumigating Powder
In~redient %/wt.
Toxicant and toxicant impurities 1.0
Wood dust 96.0
Starch 3.0
Preparation: Toxicant, wood dust, and starch are
blended together and then molded into a candle using a
small amount of water to activate the starch.
* Trademark
-60-
~.'i
3~i;33
Example G
Balt
Method A
Ingredient %/wt.
Toxicant and toxicant impurities 1.00
Wheat Bran (carrier and attractant) 89.95
Corn Syrup (attractant) 7.00
Corn Oil (attractant) 2.00
Kathon~ 4200 (preservative) 0O05
(2~n-octyl-4-isothiazolin-3-one)
Preparation: The corn oil and corn syrup are added to
the wheat bran with adequate mixing. The toxicant and
Kathon~ are premixed with excess acetone and this solution
is added to the wheat bran base with continued mixing.
The acetone is then permitted to evaporate.
Method B
In~redient
Toxicant and toxicant impurities 0.06
Granulated Sugar (carrier and attractant) 99.94
Exam~e H
Pellet
Same as Example G, Method A, with this addition: the
bait composition is formed into l/4" diameter by 3/8" long
pellets using a suitable die and press apparatus.
Flowable
In~redient %/wt.
Toxicant and toxicant impurities 31.3
Duponal~ WA Dry (wetter) 2.0
(Sodium lauryl sulfate)
-61-
~3~3
... I
Reax~ ~5A (dispersant) 5~0
~Sodium lignin sulfonate)
HiSil~ 233 (diluent) 30~0
(Sodium silica)
Kelzan~ (thickener) 0.5
(Xanthan gum)
Water 31.2
Preparztion The toxicant is a~sorbed onto the HiSil~
carrier The Duponal~ and Reax~ are then added and the
entire dry mixture blended until homogeneous~ The
composition is then micronized to a fine particle sizeO
The resulting powder i5 suspended in water and the Kelzan~
addedl.
Compositions and fonmulations according to the
present invention may also include known pesticidal
compounds. This expands ~he spectrum of activity of the
preparations and may ~ive rise to synergism.
The following known insecticidal, fungicidal and
acarlcidal compounds are suitable for use in such a
combined preparation.
Insecticides such as:
Chlorinated hydrocarbons, for example, 2,2-bis~-
chlorophenyl~ trichloroethane and
hexachloroepoxyoctahydrodimethanonaphthalene;
Carbamates, for example, N methyl-l-n~phthylcarbamates;
Dinitrophenol~, for example, 2-methyl-4,6-dinitrophenol
and 2-(2-butyl)-4,6-dinitrophenyl-3,3-dimethyl--
acrylate;
Organic phosphorus compounds, such as dimethyl-2-methoxy-
3-carbonyl-1-methylvinyl phosphate, O,O-diethyl-O~e-
nitrophenylphosphorothioa~e: N-monomethylamide of
O,O-dimethyldithiophosphorylacetic acid;
-62-
"` ~;c:73~
Diphenylsulfide~, for example, ~-chlorobenzyl or p-chloro-
phenyl sulfide and 2,4,4',5-tetrachlorodiphenyl-
sulfide;
Diphenylsulfonates, for example, ~-chlorophenylbenzene-
sulfonate;
Methylcarbinols, for example, 4,4-dichloro-1-trichloro-
methylbenzhydrol;
Quinoxaline compounds, such as methylquinoxaLine dithio-
carbonate;
Amidines such as N'-(4-chloro-2~methylphenyl) N,N-
dimethylformamidine;
Pyrethroids such as Allethrin;
Biologicals ~uch as Bacillus thuringiensis preparations;
Organic tin compounds such as tricyclohexyltin hydroxide;
Synergists such as piperonyl butoxide;
Insect growth regulators such as N-benzoyl-phenyl ureas,
for example, diflubenzuron.
Fungicides such as:
Organic mercury compounds, for example, phenylmercury-
acetate and methylmercurycyanoguanide;
Organic tin compounds, for example, triphenyltin hydroxide
and triphenyltin acetate;
Alkylenebisdithiocarbamates, for example, zinc ethylene-
bisthiocarbamate and manganese ethylenebisdithio-
carbamate; and
2,~-dinitro 6-(2-octyl-phenylcrotonate), l-bis(dimethyl-
amino)phosphoryl-3-phenyl-5-amino~1,2,4-triaæole, 6-
methylquinoxaline 2,3-dithiocar~onate, 1,4-dithio-
anthraquinone-2,3-dicarbonitrile, N-triclloromethyl-
~ thiophthalimide, N-trichloromethylthiotetrahydro-
phthalimide, N-(1,1,2,2-tetrachloroethylthio)-tetra-
hydrophthalimide, N-dichlorofluoromethylthio-N-
phenyl N' dimethylsulfonyldiamide and tetrachloroiso-
phthalonitrile.
-63-
~ 7~36~3
Biological Activi~
It has been found by biological evaluation that
compounds according to the present invention have
pesticidal activity and are capable of controlling larvae
and adult forms o~ pests, especially insects ~rom the
orders Lepidoptera and Coleoptera and most especlally
insects from the order Lepidopter~. One skilled in the
art will know how to determine the activity of a given
compound against a given insect and the dosage required to
obtain general or selective insecticidal effects. The
compounds of the present invention in part affect the
normal development of insects, particularly insects from
the order Lepidoptera, by directly and/or indirectly
influencing the moulting process.
As previously noted, the compounds of the Qresent
invention are particularly suitable for controlling plant
destructive insects in crops of cultivated plants, such
as, but not limited to, cotton, vegetables, corn and other
cereals and the like; forestry, such as, but not limited
.o, bir_h, spruce, pine, fir and the like; and ornamental
plants, Llowers and trees. Compounds o~ the present
invention ara also particularly suitable for controlling
insects destructive to stored commodities such as seeds
and the like; ~ruit crops, such as, but not limited to
fruit and/or citrus trees, raspberry bushes and the like;
and turf, such as, but not limited to, lawns, sod and the
like.
In evaluating the pesticidal activity o~ the
compounds of this invention, the ollowing test procedures
were employed.
A test solution containing 600 parts per million
(ppm) was made by dissolving the tast compound in a
solvent (acetone:methanol, l:l), adding water to give an
-64-
~L,q~36~;3
acetone:methanol:water system of 5:5:90 and then a
surfactant. A 1:1 mixture of an alkylarylpolyetheralcohol
(sold under the trademark Triton~ X-155) and a modified
phthalic glycerol alkyl resin (sold under the trademark
Triton~ B-1956) was utilized at the equivalent of 1 ounce
per 100 gal. of test solution as a surfactant.
Initial evaluations were made on one or more of the
following pests:
Code
Symbol Common Name Latin Name
SAW Southern Armyworm Spodoptera eridania
MBB Mexican Bean Beetle Epilachna varivestis
For the foliar bean beetle and armyworm tests,
individual bean (Phaseolus limensis var. Woods' Prolific)
leaves are placed on moistened pieces of filter paper in
Petri dishes. The leaves are then sprayed with the test
solution using a rotating turntable and allowed to dry.
The dishes are infested with 10 third instar larvae of
Southern armyworm or Mexican bean beetle. The dishes are
then covered.
The percent mortality for the bean beetle and
armyworm evaluations are determined 96 hours a~ter
treatment. Evaluations are based on a scale of 0-100
percent in which 0 equals no activity and 100 equals total
kill.
The rotating turntable consists of a fixed,
continuously operating spray no~zle under which targets
are rotated at a fixed speed and distance. If the target
is a Petri dish (such as for the armyworm), the distance
from the nozzle is 15 inches. If the target is a Mason
jar, the distance between the screened lid and the nozzle
is 6 inches (10 inches from the base of the jar to the
noz.zle). The nozzle is located 8 inches from the rotating
-65-
33
shaft. The targets on individual platforms revolve around
the shat at l revolution per 2U seconds but only a brief
portion of this time occurs in the spray path. Targets
pass only once under the nozzle and then are removed to
drying hoods.
The nozzle used is a 1/4 JCO Spraying Systems
(Wheaton, Illinois) air atomizing nozzle equipped with a
No. 2850 fluid cap and No. 70 air cap. At the lO psig air
pressure used and with liquid siphon feed 0.5 GPH (gallons
per hour) are delivered in a round spray pattern with a
21 spray angle. Targets are misted with spray droplets
to the point that the droplets coalesce to form a uniform
thin film insufficient to drown test organisms.
All treatments are maintained at 75-80F under
continuous fluorescent light in a well-ventilated room.
The results of the initial insecticidal evaluations
are given in Table IV.
-66-
TABL E IV
- Initlal Biologica-l Evaluations
Fo 1 i ar Ap pl i ca t i on
Test Species
ExamPle No. SAW MBB
100
2 100
100
100
O
8 100 40
9 100 60
100 30
11 100 30
12 100 20
13 100 0
14 100 20
100 0
16 100 50
17 50 0
18 10 0 0
19 100 0
100 20
21 100 0
22 100
23 100 30
24 100 40
26 40
--67--
Fo ~ on
_ Test Species _
Example No. _SAW BB
27 lO0
28 lO0
39 lO0
0
31 lO0 _
32 lO0
33 20
34 0
It should be understood that the instant
specification and examples are set forth by way of
illustration and not limitation, and that various
modifications and changes may be made without departing
from the spirit and scope of the present invention as
defined by the appended claims.
68-
' :,., " .. :,