Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~L~737~
Backqround of the Invention
This patent relates to a body implant which softens and/or
swells when implanted in the body, a novel composition use-
ful in preparing said implant and a method of preparing said
implant. It further relates to methods of inserting a sof-
tening and/or swelling catheter or cannula into a blood
vessel of an animal.
Body implants, in particular tubular implants inserted
into the body such as catheters, cannulae, endotracheal
tubes or the like need to be relatively stiff for ease of
insertion in the body. Commercially available cannulae, for
example, are typically made of fluorinated ethylene-
propylene copolymer, polytetrafluoroethylene,
poly(chlorotrifluoroethylene), or the like. When such com-
mercial cannulae or catheters of such materials are left in
the body for relatively long periods of time, trauma to the
surrounding tissue may result.
Various compositions which soften in contact with water
are known and some have been suggested for use as body
implants. The following are illustrative.
U.S. Patent No. 3,822,238, Blair et al, discloses water
absorptive polyurethane polymers prepared from resins having
a low ratio of carbon to oxygen to nitrogen or having ionic
quaternary ammonium or salt groups in the resin backbone and
a low amount of isocyanate. It is suggested that the poly-
mers can be used as coatings or membranes or shaped by
~737;~0
casting or machining to make body implants. There is no
suggestion as to which polymers of the many disclosed would
be useful for forming body implants. Nor is there any indi-
cation of what properties polymers for use as body implants
should have.
U.S. Patent No. 3,975,350, Hodgin et al, discloses
hydrophilic crosslinked polyurethane systems useful as
carrier systems for slow release of medication, as coatinqs
or for body implants, such as catheters and cannulae. Again
there is no suggestion as to which polyurethane systems
would be useful for body implants.
U.S. Patent No. 4,279,795, Yamashita et al, relates to
materials capable of forming a hydrogel and having improved
anti-thrombogenic properties. The polymer is a graft copo-
lymer having a hydrophilic polymer backbone, e.g. a poly-
methacrylate, with hydrophobic moieties, e.g. polystyrene,
granted onto it. The hydrogel is said to be self-
reinforcing and capable of being shaped into a tube, film,
rod, etc.
U.S. Patent No. 4,371,686, Yamamoto et al, discloses
implanting into jugular and femoral veins of animals, tubes
of a polyurethane containing polyoxyethylene and polyoxypro-
pylene blocks. The polymers are said to be highly elastic
and to posses~ anti-thrombogenic properties.
U.S. Patent No. 4,359,583, Gould et al, discloses a
polyurethane diacrylate composition which forms a h~drogel
73730
on immersion in water. The compositions are said to have a
variety of uses including use in body implants such as
catheters and cannulae.
U.S. Patent No. 4,454,309, Gould et al, discloses a com-
position comprising a hydrophilic polyurethane resin and a
polyene selected from polyalkyl esters and polyacrylic acid
esters. The compositions are said to have a variety of uses
including use in body implants such as catheters and can-
nulae.
U.K. Patent No. 1,511,563, Ciba-Giegy AG, relates to
water-insoluble hydrophilic copolymers, preferably a
methacrylate copolymer. The copolymers are primarily useful
as drug delivery systems. It is disclosed that the copoly-
mers can be fashioned into substitute blood vessels or
extracorporeal shunts.
Japanese Kokai Sho 52-9087 to Nakashima et al, discloses
block and or graft copolymers made of hydrophilic polymer
segments and hydrophobic polymer segments and having a
phase-separated microstructure with one of the segments,
preferably the hydrophilic, forming a continuous phase and
the other a dispersed phase. The copolymers are said to
have good anti-thrombogenic properties. The copolymers are
said to be useful for medical and therapeutic equipMent that
may come into contact with blood, such as, for example,
vascular catheters, cannulae, shunts, etc.
In summary, while a great number of compositions are
disclosed in each of these patents, there is no indication
373()
of which particular compositions would be suitable for use
as a body implant, such as a catheter or cannula. Where a
particular composition has been made into a catheter or can-
nula, the composition selected is one which does not provi-
de a body implant having desired properties.
It has now been discovered that a body implant having
desirable properties can be made from a novel multiple phase
polymeric composition having a non-hydrophilic phase and
hydrophilic phase, the relative amounts of the non-
hydrophilic and hydrophilic components being adjusted,
depending on the particular materials employed, to provide a
composition having certain properties. The body implant and
the composition from which it is made softens and/or swells
when in the body and is sufficiently soft when in the body
to reduce trauma to the surrounding tissue. Swelling of the
implant permits insetion of a smaller device and/or can
result in pressure around a wound site reducing bleeding and
bacterial invasion into the wound. The implant is suf-
ficiently strong and tough when in the body to maintain its
desired shape, to resist deformation and to be capable of
removal without tearing and leaving any undesired fragments
in the body. For certain applications, the implant has a
high initial stiffness for insertion yet softens when in the
body to become pliable.
Summary of the Invention
One aspect of this invention comprises a body implant,
at least a portion of which is comprised of a multiple phase
polymeric composition comprising:
12~373~
a) a first phase which comprises a substantially non-
hydrophilic polymeric component; and
b) a second phase which comprises a hydrophilic poly-
meric component;
said composition (i) being capable of absorbing water to an
extent that it softens with a softening ratio of at least
about 3:1 and/or swells with a swelling ratio of at least
about 1:1.5; and (ii) when substantially completely
hydrated, having an energy to break of at least about 700
N-cm/cm3 and a 2.5% Secant modulus of less than about 7,000
N/cm2 .
Another aspect of the invention comprises a novel
multiple phase composition comprising:
a) a first phase which comprises a substantially non-
hydrophilic polymeric component; and
b) a second phase which comprises a hydrophilic poly-
meric component;
said composition (i) being capable of absorbing water to an
extent that it softens with a softening ratio of at least
about 3:1 and/or swells with a swelling ratio of at leact
about 1:1.5; and ~ii) when substantially completely
hydrated, having an energy to break of at least about 700
N-cm/cm3 and a 2.5~ Secant modulus of less than about 7,000
N/cm2 .
~ ~7373~)
A further aspect of the invention comprises a method
of making a shaped article comprising:
1) selecting a mixture comprising a multiple phase
polymeric composition comprising:
a) a first phase which is continuous and which
comprises a substantially non-hydrophilic polymeric
component; and
b) second phase which comprises a hydrophilic poly-
meric component
said composition (i) being capable of absorbing
water to an extent that it softens with a softening
ratio of at least about 3:1 and/or swells with a
swelling ratio of at least about 1:1.5; and (ii)
when substantially completely hydrated, having an
energy to break of at least about 700 N-cm/cm3 and a 2.5%
Secant modulus of less than about 7,000 N/cm2;
2) forming the mixture of 1) into a shaped article.
Another aspect of this invention comprises a method of
inserting a catheter or cannula into a blood vessel of an
animal, said catheter or cannula having a tubular portion
and a hub portion, one end of the tubular portion being
attached to the hub portion, said method comprising:
a) selecting a tubular article the walls of which are
made of a composition which upon hydration has a sof-
373~
tening ratio of at least about 3:1; a swelling ratio ofat least about 1:1.5; and a tensile energy to break when
substantially completely hydrated of at least about 700
N-cm/cm3;
b) positioning a portion of the tubular article over a
needle such that the inner diameter of the tubing is
slightly greater than the outer diameter of the needle,
the sharp end of the needle being distal to the hub por-
tion and the attached end of the tubular portion;
c) inserting the needle into the desired blood vessel of
the animal such that both the needle and tubular article
are inserted; and
d) retracting the needle leaving a portion of the tubu-
lar article in the blood vessel.
Yet another aspect of this invention comprises a method
- of inserting a catheter or cannula into a blood vessel of an
animal, said method comprising:
a) selecting a tubular article the walls of which are
made of a composition which upon hydration has a sof-
tening ratio of at least about 3:1; a swelling ratio of
at least about 1:1.5; and a tensile energy to break when
substantially completely hydrated of at least about 700
N-cm/cm3;
b) inserting a needle having an inner diameter slightly
larger than the outer diameter of the tubular article
into the desired blood vessel;
~1 ~73~
c) positioning a portion of the tubular article through
the needle and then into the blood vessel for a prede-
termined distance; and
d) retracting the needle leaving a portion of the tubu-
lar article in the blood vessel.
Brief Description of the Drawinqs
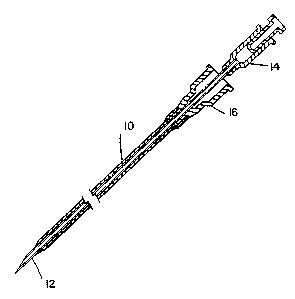
Fig. 1 is a side elevation view of an IV catheter or
cannula of this invention arranged over a needle for inser-
tion into a blood vessel of an animal.
Fig. 2 is a side elevation view of an IV catheter or
cannula of this invention and a hollow needle through which
the catheter or cannula can be inserted after the needle has
been inserted in a blood vessel of an animal.
Detailed Description of the Invention
The body implant of this invention comprises a multiple
phase polymeric composition comprising a first phase which
comprises a substantially non-hydrophilic polymeric com-
ponent and a second phase which comprises a hydrophilic
polymeric component. The relative amounts of these com-
ponents are selected, depending on the particular polymeric
materials employed, to provide a composition having the
desired properties, as discussed more fully below.
Preferably the non-hydrophilic polymeric component forms
a continuous phase. The hydrophilic polymeric component
~3~
g
can form a co-continuous phase with, or a dispersed phase
in, the non-hydrophilic polymer phase.
The non-hydrophilic polymeric component comprises a
polymer which does not substantially absorb or attract
water. Preferably, the non-hydrophilic polymer is capable
of absorbing in an amount of no more than about 20%, more
preferably no more than about 10%, and most preferably no
more than about 5%, by wei~ht, based on the weight of the
non-hydrophilic polymer.
The non-hydrophilic polymer can be for example, a
polyurethane such-as an aliphatic polyurethane, a polyether
polyurethane, a polyester polyurethane; and ethylene copo-
lymer such as ethylene-vinyl acetate copolymer or ethylene-
ethyl acrylate copolymer; a polyamide, in particular a
polyamide of low crystallinity; aliphatic polyesters; or the
like. A particularly preferred non-hydrophilic polymer is a
polyurethane, especially an aliphatic polyurethane.
The hydrophilic polymer preferably is a polymer that
absorbs at least about 50% water, more preferably about 100%
and most preferably at least about 250%, by weight based on
the weight of the hydrophilic polymer. The hydrophilic
polymer preferably forms a hydrogel on absorption of water.
The hydrophilic polymer is preferably polyvinyl alcohol,
poly(ethylene oxide), polypropylene oxide, polytethylene
glycol) polypropylene glycol, polytetramethylene oxide,
~737;3~)
--10--
polyvinyl pyrolidene, polyacrylamide, poly(hydroxy ethyl
acrylate), poly(hydroxyethyl methacrylate), or the like.
The multiple phase composition can be prepared by mixing
the polymeric components or by forming a block or graft
copolymer containing the polymeric components. A mixture of
. the components can be prepared using, for example, a two-
i B roll mill, an internal mixer, such as a Brabender~or Banbury~mixer, and extruder, e.g. twin-screw extruder, or the like.
Block and graft copolymers can be prepared by appropriate
methods depending on the particular nature of the components
used. Typical preparatory methods can be found, ~or
example, in "Block and Graft Copolymerization", R.J. Ceresa
(Zd), 1973, Vol. 1 6 2, Wiley-Interscience, New York and
"Block Copolymers", D.C. Allport and W.H. Jane, 1973, Wiley,
New York.
Generally, the ratio of non-hydrophilic polymeric com-
ponent to hydrophilic polymeric component is 0.65:1 to 9:1.
Preferably the ratio of the polymeric component is 1~1.
The polymeric components are selected to provide a
multiple phase system. Generally, the polymeric comonents
each have a molecular weight of at least about 3,000 pre-
ferably at least about 5,000 and most preferably at least
about 10,000.
As stated above, the relative amounts of non-hydrophilic
and hydrophilic polymeric components are selected, depending
on the particular materials employed, to provide the desired
~ Tr~Q~ k
~ ~37~a)
properties. Due to the presence of the hydrophilic poly-
meric component, the composition is capable of being
hydrated by the absorption of water. As water is absorbed
by the composition, it may soften with a softening ratio of
at least about 3:1, preferably at least 6:1, more preferably
at least about lO:l, most preferably at least about 20:1,
and in particular at least about 40:1. The term "softening
ratio" is used herein to refer to the ratio of the 2.5%
Secant modulus values of the compositicn, in the form of a
tubular article, when substantially non-hydrated, to the
2.5% Secant modulus of the composition when substantially
completely hydrated. The term "substantially non-hydrated"
refers to the state of the composition under conventional
ambient conditions, i.e. room temperature, 50-80~ relative
humidity atmospheric pressure. The term "substantially
completely hydrated" refers to the state of the composition
when it is in equilibrium with an exces~ of water under
ambient conditions of temperature and pressure.
The composition may swell on absorption of water with a
swelling ratio of at least about 1.5:1, preferably at least
about 2:1 and most preferably at least about 2.5:1. The
term "swelling ratio" refers to the ratio of the volume of a
given sample of the composition when substantially comple-
tely hydrated to its volume when substantially non-hydrated.
Preferably the composition both softens and swells when
hydrated.
When substantially completely hydrated the composition
has a tensile energy to break of at least about 700 Newton-
7~)
-12-
centimeters per cubic centimeter (N-cm/cm3), preferably at
least about 1,400 N-cm/cm3 and most preferably about 1,700
N-cm/cm3. The term ~Itensile energy to break" (TEB) is
defined in ASTM-D882 as the area under the stress-strain
curve or
~ r
TEB = J Sd
o
where S is the stress at any strain, ~,; and E r is the
strain at rupture. The tensile energy to break provides an
indication of the toughness of the hydrated composition and
its ability to withstand the conditions it will be subjected
to in use.
It will be readily appreciated that when a tubular pro-
duct such as a catheter or cannula is withdrawn from the
body it is extremely important that it does not tear or
break leaving pieces remaining inside the body. Neither
tensile strength nor elongation to break are good indicators
of toughness. Brittle materials and notch sensitive
materials can have high tensile strengths. Extremely weak
materials can have high elongation but not the strength to
be extracted. TEB i5 a measure of the energy required to
break and is a combination of these two important criteria.
Th2 ultimate elongation of the multiple phase com~
position should be at least about 10%, preferably at least
about 25% and most preferably at least about 50%.
~ ~7;37~t3
To provide the desired benefits, the composition should
become sufficiently soft once implanted in the body to mini-
mize trauma to the surrounding tissue. The composition when
substantially completely hydrated has a 2.5% Secant modulus
of less than about 7,000 Newtons/square centimeter (N/cm2),
preferably less than about 3,500 N/cm2 and most preferably
less than about 2,000 N/cm2. When substantially completely
hydrated the 2.5% Secant modulus can be as low as about 30
N/cm2 but preferably above about 60 N/cm2 and most pre-
ferably above about 120 N/cm2.
The 2.5% Secant modulus of the composition when substan-
tially non-hydrated is not critical and varies depending on
the proposed use of the composition or implant. Typically
the 2.5% Secant modulus of the composition is at least about
20,000 N/cm2 when used as an over the needle catheter as
described more fully below. Preferably the 2.5~ Secant
modulus of the composition is at least about 28,000 N/cm2
for this particular use. For other proposed uses of the
composition a lower modulus may be desirable. For example,
for use as a catheter in a through the needle device, as
described more fully below,, the 2.5% Secant modulus of the
composition can be in the range of about 7,000 to about
20,000 N/cm2.
The composition may be crosslinked if desired.
Crosslinking of the composition gives the polymeric com-
position strength above the melting or softening points of
the polymeric components permitting sterilization of a
device utilizing the composition at above that temperature.
~37~3~
-14-
This is particularly advantageous if the polymeric component
of continuous phase has a relatively low melting or
softening point. Crosslinking of the composition may also
be used to adjust the 2.5~ Secant modulus of the composition
to bring it to the desired value for the proposed use of the
composition. When the composition comprises a physical
mixture of the non-hydrophilic and hydrophilic components,
crosslinking of the mixture can control the tendency of the
hydrophilic co~ponent to leach out of the composition when
it is in extended contact with water or body fluids.
Crosslinking may also improve the toughness (TEB) of the
composition in the hydrated state.
Crosslinking of the composition can be effected by use
of an appropriate crosslinking agent or by irradiation, pre-
ferably in the presence of a crosslinking promoter, such as
triallyl isocyanuate, or the like. In a preferred embodi-
ment the composition is crosslinked by high energy radiation
in an electron accelerator. The amount of irradiation
should be in the range of about 0.5 to about 30 Megarads
(Mrads) preferably about 0.5 to about 15 Mrads and most pre-
ferably about 0.5 to about 10 Mrads.
Either or both components of the composition may contain
additional ingredients such as stabilizers, antioxidants,
radiopacifiers, medicaments, fillers or the like. For
certain applications it may be advantageous to incorporate a
water soluble or water dispersible medicament which can
leach from the composition of the implant when it contacts
body fluids. Such medicaments include antithombogenic
, ~
~X~37~
-15-
agents, antibiotics, anti-viral agents, anticoagulants,
anti-inflammatory agents or the like.
As ~entioned above the compositions of this invention
are particularly useful for making articles suitable for use
as body implants. The body implant can take any desired
shape such as a film, rod, fiament, tube or the like. The
composition can be formed into a shaped article can be
crosslinked, if desired.
In particular the compositions of this invention are
useful for making catheters for use in intravenous ~IV)
devices. Tubular articles of this invention can be used in
both over the needle and through the needle techniques of
introducing an IV device into the blood vessel of an animal.
The term animal is used to refer to any member of the animal
kingdom which may be treated using an IV technique and in
particular refers to mammals such as horses, cattle, dogs,
cats and humans.
The use of a catheter of this invention in an over the
needle technique for administering a fluid intravenously is
illustrated in Fig. 1. In Fig. 1 catheter, 10, of this
invention fits snugly over a needle, 12, and at the distal
end, the catheter wall is tapered to a feathered edge. Both
the needle and the catheter have a female luer fitting, 14
and 16, respectively, at the proximal end. The
needle/catheter assembly is advanced through the skin and
into the blood vessel ~vein). The needle is then withdrawn
leaving the catheter in place. An IV line is connected to
the catheter by means of the luer fitting.
~3
--16--
The tubular article used as the catheter in this appli-
cation is of a composition which softens and swells as
defined above. The 2.5~ Secant modulus of the catheter
prior to insertion should be at least about 20,000 N/cm2, it
should soften with a softening ratio of at least about 3:1,
should swell with a swelling ratio of at least about 1.5:1
and should have a 2.5% Secant modulus while it remains in
contact with the blood of the vein of less than about 7,000
N/cm2. The catheter should retain its hardness during its
insertion. The catheter should not swell or soften appre-
ciably during the time it is being inserted. It has been
found that the time for the catheter to swell 50% of its
fully swollen volume should be at least about 15 seconds,
preferably at least about 60 seconds~
Another insertion technique is the through the needle
technique and is illustrated in Fig. 2. With this insertion
technique, the venipuncture is made using either a conven-
tional needle, 22, fitted with luer fitting, 24. After the
vein entry, a catheter, 20, that is preconnected to an IV
line, 26 through luer fitting 28, is threaded through the
needle 22 into the vein (not shown). In this technique, a
catheter which swells to 50% of its fully swollen volume in
not less than 30 seconds, preferably in not less than 60
seconds, is preferably used. Once the catheter is in place,
the needle is retracted. If a conventional needle is used,
it is left on the catheter and placed in a protective
plastic sheath. If a break-away needle is used, the needle
is split into two along its axis and discarded.
~37;~ L)
-17-
Because the catheter of this invention becomes relati-
vely soft after insertion, it tends to cause less irritation
to the intima (lining of the vein) and to the insertion site
and, therefore, is less likely to contribute to mechanical
phlebitis. The catheter may contain a slow release medica-
ment. The softness of the catheter permits it to float in
the vein rather than lie on the point where inserted and
consequently the medication is delivered evenly helping to
avert chemical phlebitis.
Because the catheter swells, the gauge size of the
catheter used can be ~maller than that of a non-swelling
catheter for a given flow requirement. This allows access
to smaller veins in the limb extremities and easier inser-
tion into the vein. In addition, the swelling of the
catheter seals the wound site more effectively and helps
prevent catheter slip out, a common cause for changing
catheters prematurely.
- The following examples illustrate typical compositions
of this invention and their uses in preparing shaped
articles useful as body implants.
Examples 1 to 7 - Blends
For each Example, the ingredients and the amounts
thereof, in parts by weight, indicated in the Table I, were
mixed together on a heated (150-160C) mill, about 20-30%
of the polyurethane being added first, then about 30% of the
polymer capable of forming a hydrogel then about 20-30% of
~LX~;~7~)
-18-
the polyurethane, then the remainder of the polymer capable
of forming a hydrogel and finally the remainder of the
polyurethane. The mixture was stripped from the mill and
pressed into slabs 6" x 6" and about 25 mils thick. The
slabs were tested after they had been irradiated (i.e.
exposed to electrons in a radiation beam) to a dose of 10
Mrad. Data for modulus at 100% elongation for 25 Mrad
samples are also given. The secant modulus for the dry
state was measured by the procedure of ASTM D-882. Each
sample was immersed in water at body temperature t37C) for
between 10 minutes and 3 hours to hydrate the blend. The
hydrated secant modulus was then determined by the same pro-
cedure. The percent swell was also measured.
In the Table I below, the polymers are identified by
their trade names; they have either been described already,
in the earlier part of this specification, or are ~urther
described below.
~37~1) ,~ '`
~j I I I O ~ ~ ~ ~ I
-w U~
au ~1 1 1 0 I I
~uo
'!~ u7 ~
1~
1.
I o I I ~ O ~ O
In
~n
1 1 I
.
U~
E~ _~
¢ ~ I II o ~ o ~ U~ I ~ O
C" ~~
~;
~ I ~~ o
~D;
CO
O I ~ I I o ~ o
~ g
o
o I I
O OD 5 -- -- ~ C D ~ ~
~ t ~~ O
a ~ s _~
h t~t-1 t-l C ~ C C
~ ~ U U -J ~ O O
_~ 4 t~ ~ t :~ O O
~i ~
~ ~0 'O
~ O O O O O O ~ ~ ~ O
0~ ~ ~ Q~ ~ ~ ~ ~ C~
~37~
--19--
Example 8 Cannula Tubing
A blend of 3 grams of Sartomer 295, 13~ grams of
Tecoflex~60D, and 59 grams of Polyox~WSR3154 (Example 7)
was compounded in 200 gram batches on the mill as described
earlier. The blend was chopped on a Nelmor~chopper; the
tubing was extruded on a Wayne 3/4" extruder; and irradiated
at 10 Mrads.
The irradiated tubing, having an I.D. of .035" and an
O.D. of .050", experienced a swell of 3 mlls in the I.D. and
6 mils in the O.D., for a 3 mil wall swell t40%) by uptake
of water during a 24 hour, 37C hydration in Ringer's
Solution.
Tubing samples were conditioned for 1 hour at 37C in
Ringers's Solution to provide specimens in the hydrated
state. Ringer's Solution is a sterile solution for paren-
teral administration containing 147 milliequivalents tmeq~
Na+ per liter, 4 meq K+/l, 4 meq Ca+2/1 and 155 meq Cl-/l.
The solution closely simulates body fluid electolytes.
Tubing made from PTFE as a control was similarly
treated.
Example 9
Four blends were compounded in 2Q0 gram batches on the
mill as described earlier. These blends were then chopped
on a Nelmor chopper; then extruded into tubing on a 3/4"
~ f~ k
3~3V
-20-
Wayne crosshead extruder; and then irradiated with a 10
Megarad dosage.
The formulations (% weight) were:
Sample l 2 3 4
Polyethylene Oxide 29.i23.7 29.1 34.0
(Union Carbide WSR301)
B Polyurethan _ _ _ _ _ _ 63.1
I (Thermedic ~ -60D)
Polyurethan ~with 20~ Barium Sulfate 68.0 _ __ _ _ _
(Thermedic -60D-20B)
Polyurethane with 40% Barium Sulfate _ _ 73.4 _ _ _ _
(Thermedic~'EG-60D-40B)
Polyurethane with 20% Bismuth Subcarbonate _ _ _ _68.0 _ _
(Thermedic ~ -60D-20HC)
Titaniu~ Dioxide 1.5 1.5 1.5 1.5
(Tipu 101) _
Butylated Hydroxy Toluene 3 3 3 3
Butylated Hydroxy Anisole 3 3 3 3
Pentaerythritol Tetraacrylate 8 8 8 8
100.O 100.O 100.O 100.O
T~e ~~n~-
-21-
The irradiated tubing, having an I.D. of approximately
.041 inches and an O.D. of approximately .055 inches was
tested for tensile properties, energy to break and swell
both in the dry state and after 24 hour immersion in 37C
distilled water.
The following results are for these four samples (1-4)
and samples of commonly used medical catheter materials when
submitted to the same test procedure.
Dry 23C Hydrated Volume Softening Energy
2.5 ~ant 37C 2.5% SwellRatio in-lbs/in3
Modulus Secant Ratio
(DSi ) ~dulus (~Si )
Sample 1 45,009 4092.8:1 110 4,560 .
2 33,300 429 2.7:1 77 5,064
3 33,643 562 2.3:1 60 4,493
4 30,006 468 2.6:1 64 6,485
FEP Catheter 50,300 39,113 1:1 1.3 14,188
mat'l #A
FEP Catheter 58,179 51,223 1:1 1.1 18,905
mat'l #B .