Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ 0732 CAN 6A
--1--
METABOLITES OF TRIAZOLO[1,5-c]PYRIMIDINES
.
The present invention relates to metabolites of
certain tria~olo[1,5-c]pyrimidines, and more specifically
to metabolites of 2,5-diethyl-7-(4-thiomorpholino)-1,2,4-
triazolo[1,5-c]pyrimidine. The pharmacological use of the
compounds of the invention as bronchodilators and
pharmaceutical compositions comprising the compounds are
also within the scope of the invention.
Some 1,2,4-triazolo[1,5-c]pyrimidines are known to the
art. Certain 1,2,4-triazolo[1,5-c]pyrimidines are
disclosed as being bronchodilators in the patents discussed
below, the compounds being re~erred to therein as
triazolo[2,3-c]pyrimidines:
United Kingdom Patent No. 859,287 discloses
2-amino-1,2,4-triazolo[1,5-c]pyrimidines which are
substituted on the pyrimidine ring at the 5, 7 and 8
positions by certain combinations of substituents selected
from hydrogen, alkyl, halogen-substituted alkyl,
hydroxy-substitu~ed alkyl, alkoxy-substituted alkyl,
alkenyl, cycloalkyl, amino, alkylamino, dialkylamino,
phenyl, alkylthio, alkoxy and halogen substituents.
United Kingdom Patent No. 873,223 discloses 2-amiro or
2-acetamido-1,2,4-triazolo[1,5-c]pyrimidines which are
substituted on the pyrimidine ring at the 5, 7 and 8
positions by certain combinations of substituents selected
from nydrogen, alkyl, halogen-substituted alkyl,
alkoxy-substituted alkyl, alkenyl, cycloalkyl, alkylthio
and halogen substituents.
United Kingdom Paten~ No. 897,870 discloses
2-alkylamino-1,2,4-triazolo[1,5-c]pyrimidines,
2-dialkylamino-1,2,4-triazolo[1,5^c]pyrimidines, and
1,2,4-triazolo[1,5-c]pyrimidlnes containing a piperidino or
morpholino substituent bonded at the 2-position ~hrough the
nitrogen atom, which compounds are substituted on the
p~
~ ~ 7 ~
pyrimidine ring at ~he 5, 7 and 8 positions by certain
combinations of substituents selected from hydrogen, alkyl,
halogen-substituted alkyl, hydroxy-substituted alkyl,
alkenyl and halogen substituents.
The following article discloses the synthesis o~
certain 1,2,4-triazolo[1,5-c]pyrimidines as potential
bronchodilators:
G.W. Miller et al., J. Chem. Soc., 1963, 3357,
discloses 1,2,4-triazolo[1,5-cJpyrimidines (referred to
therein as triazolo[2,3-c]pyrimidines) which are
substituted at the 2-position by hydroxy, halogen, alkoxy,
amino or substituted amino substituents and on the
pyrimidine ring by alkyl substi~uents, or alkyl and
halogen-substituted alkyl substituents.
Still other 1,2,4-triazolot1,5-c]pyrimidines are
disclosed-in the following patent.
French Patent No. 1,205,444 discloses compounds which
are purportedly 7-methyl-1,2,4-triazolo[4,3-c]-pyrimidines
having, for example, an am1no or heterocyclic amino
substituent in the 5-position. However, it is known that
the compounds actually obtained when the examples are
followed are the corresponding 7-methyl-1,2,4-triazolo-
[1,5-c]pyrimidines.
Finally, the following patent and applications which
are owned by the assignee of the instant application relate
to triazolopyrimidines which are bronchodilators.
European Patent Application 84301383.0 (published on
September 10, 1984 as 0 121 341) describes various
triazolotl,5-c]pyrimidines including 2,5-diethyl-7-(4-
thiomorpholino)-1,2,4-triazolo[1,5-c]pyrimidine of which
the instantly claimed compounds are metabolites.
This application also discloses 2,5-diethyl-7-
t4-(1-oxothiomorpholino)]-1,2,4-triazolo-[1,5-cJpyrimidine
and 2,5-diethyl-7-[4~(1,1-dioxothio-morpholino)]-1,2,4-
triazolotl,5-c]pyrimidine.
The present invention relates to 1,2,4-triazolo-
7 4
--3--
[1,5-c]pyrimidines which are bronchodilators. The
invention also relates to a method for induciny
bronchodilation in a mammal us~ng a 1,2,4-tr~azolo[1,5-c]-
pyrimidine of the invention, and to pharmaceutical
compositions comprising an effective amount of a
1,2,4-triazolo[1,5-c]pyrimidine of khe invention and a
pharmaceutically acceptable carrier.
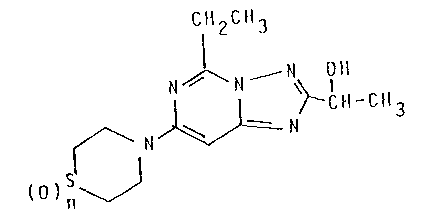
Specifically, the present invention relates to
compounds of the Formula I
1 CH2CH3
I
N ~ N - N OH
~ N ~ ~-~H-CH3
()Sn ~
wherein n is 1 or 2; and pharmaceutically acceptable
acid-addition salts thereof.
The compound of Formula I wherein n is 1 is named
5-ethyl-2-(1-hydroxyethyl)-7-[4-(1-oxothiomorpholino)]-1,2,
4-triazolo[1,5-c]pyrimidine, and the compound of Formula I
wherein n is 2 is named 5-ethyl-2~ hydroxyethyl)-7-
[4-(1,1-dioxothiomorphol;no)]-1,2,4-triazolo[1,5-c]-
pyrimi~ine.
The carbon atom substituted by the hydroxyl moiety is
asymmetric in the compounds of the invention. Both
compounds have been determined to be levorotatory, the
specific rotations in ethanol being []27= -20.6 for
5-ethyl-2-(1-hydroxyethyl)-7-[4-(1-oxothiomorpholino)]-
1,2,4-triazolo[1,5-c]pyrimidine and [a]2D7= -18.2 for
5-ethyl-2-(1-hydroxyethyl)-7-[4-(1,1-dioxothiomorpholino)]-
1,2,4-triazolo[1,5-c]pyrimidine. It is unders~ood that the
specific rotation will depend upon a variety of factors
including purity of the compound.
The bronchodilator activity of the compounds of
Formula I was assessed by the measurement o~ effects on
isolated tracheal spirals. This is a well-known and long
established ln vitro test method. The bronc~odilator
ac~ivity was determined according to the following
procedure: Female guinea pigs were sacrificed and each
trachea removed and cut into a spiral strip. This s~rip
was mounted in a constant temperature (37 C) muscle bath
having a volume of approximately 15 ml. The bathing medium
was Krebs-Henseleit solution. Movement of ~he tracheal
lG strip was measured by means of an isome~ric transducer
connected to an electric recorder. The bath was aerated
with a mixture of 95~ carbon dioxide and 5% oxygen.
Contractions were induced in the strips by the addition of
histamine, acetylcholine or barium chloride in an amount of
0.4, 0.5 or 250 micrograms per ml, respectively. The
amount of a given compound of Formula I (measured in
micrograms per ml) required to provide greater ~han 75%
relaxation of drug-induced contraction is considered an
effectiYe concentration. For comparison, a well known
standard bronchodilator, aminophylline, requires
concentrations of 50 micrograms per ml versus histamine,
100 micrograms per ml versus acetylcholine and 10
micrograms per ml versus barium chloride to provide greater
than 75~ relaxation.
Bo~h of the compounds of Formula I were active in the
in vitro test, and could be tested in vivo ;n the guinea
pig for oral activi~y in the so-called histamine aerosol
method descrlbed in U.S. Patent 3,248,292. This test is
modified slightly 1n that a 0.1% aqueous solution of
histamine is used as the agent for inducing bronchial
constriction. Oral doses are measured in mg/kg of body
weight of the guinea pig.
The compounds of Formula I may be administered to
mammals in order to ob~ain bronchodilation. The oompounds
may be administered orally, parenterally or by lnhalation.
Preferably they are administered orally in tablets or
7 ~ ~a3
--5--
capsules, The estimated eFfective human dose will be in
the range of 0.1 to 5 mg/kg of body weight.
Salts of compounds of Formula I are generally prepared
by reaction with an equimolar amoun~ of a relatively strong
acid, preferably an inorganic acid such as hydrochloric,
sulfuric or phosphoric acid, in a polar solvent. Isolation
of the salt is facilitated by the addition of a solvent in
which the salt is insoluble. An example of such a solvent
is diethyl ether.
The compounds of Formula I, either as the free base or
in the form of a pharmaceutically acceptable acid-addition
salt, can be combined with conventional pharmaceutical
diluents and carriers to form such dosage forms as tablets,
capsules, suspension, solutions, suppositories and the
like.
The pharmaceutical carrier ~mployed may be, for
example, either a solid or liquid. Examples of solid
carriers are lactose, terra alba, sucrose, talc, gelatin,
agar, pectin, acacia, magnesium stearate, stearic acid, and
the like. Liquid carriers include syrup, peanut oil, olive
oil, water and the like. Similarly, the carrier or diluent
can include a time delay material well known to the art,
such as glyceryl monostearate or glyceryl distearate, these
being employed alone or, for example, in combination w~th a
wax.
T~e compounds of Formula I are obtained by
administering the bronchodilator compound 2,5 diethyl-7-(4-
thiomorpholino)-1,2,4-triazolo[1,5-c]pyrimidine to a
mammal, collecting urine from the mammal and separating the
compounds of Formula I from the urine. The compound
2,5-diethyl-7-(4-thiomorpholino)~ ,4-triazoloC1,5-c]-
pyrimidine may be prepared as described in U. S. Patent
No. 4,572,910.
Suitable laboratory mammals from which the me~abolites
may likely be obtained are dogs and rats.
Standard methods may be used for the separation of the
,
7 ~
-6-
metabolites from urine such as extraction, chromatography,
precipitation and the like. The specific procedure
utilized in the present invention was to extract the urine
of a dog to which the compound 2,5 diethyl-7-
(4-thiomorpholino)-1,2,4-triazolo[1,5-c]pyrimidine had been
administered. Chloroform was used as the extraction
solvent. The organic extracts were evaporated to provide a
solid which was flash chromatographed on silica gel to
pro~ide separation of the two metabolites. Recrystalliza-
tion followed by spectral analysis (i.e., nuclear magnetic
resonance spectrometry, infrared spec~rometry, or mass
spec~rometry), elemental analysis and comparison to known
compounds of similar structure established the structures
of the compounds of Formula I.
Pharmacological evaluation of the metabolites isolated
as described herein demonstrated that both metabolites were
quite active as bronchodilators in both in vitro and in
vivo models.
The metabolites of Formula I are also quite useful for
studying the metabolism of the parent compound,
2,5-diethyl-7-(4-thiomorpholino)-1,2,4-triazolo[1,5-c]-
pyrimidine.
Although it has not been established experimentally,
it is presumed that the compounds of Formula I would also
be metabolites of the compounds
2,5-diethyl-7-~4-(1-oxothiomorpholino)]-1,2,4-trlazolo-
~1,5-c]pyrimidine and 2,5-diethyl-7-[4-(1,1-dioxothiomor-
pholino)]-1,2,4-triazoloC1,5-c~pyrimidine.
The following examples are provided to illustrate the
methods used in the invention. They are no~ intended to
limit the invention.
Example 1
A dosage formulation was prepared containing 35.7% by
weight of 2,5-diethyl-7-(4-thiomorpholino)-1,2,4-
triazolo[1,5-c]pyrimidine granulated with lactose. This
~ 74 ~
formulation was weighed into gelatin capsules, which were
used to dose each of two beagle dogs at a rate of 51
mg/kg/day of the drug, split into two doses per day, for a
two-day period. Dog (A) tl2.6 kg) was dosed 4 times with
900 mg per dose of the formulation of 2,5-diethyl-7-(4-
thiomorpholino)-1,2,4-triazoloLl,5-c]pyrimidine for a total
dose of 1.29 g. Dog (B) (14.4 kg) was dosed 4 times with
1030 mg per dose of ~he formulation and received a total
dose of 1.47 9. Urine was collected from both dogs from
the time of first dosing to 19 hours after the final dose.
Total volumes of urine collected were 450 ml (Dog A)
and 457 ml (Dog B). About 225 ml of urine was put on each
of four extraction columns, (ExtubeTM, Analytichem
International, Harbor City, CA) WhiCh were disposable
extraction columns, capacity 300 ml. The columns were
eluted first with three 200 ml portions of diethyl ether,
then with eight 200 ml portions of chloroform. The ether
fractions were discarded. Chloroform fractions of 400 ml
were collected and combined with the same fraction from the
other columns. Evaporation of the first three chloroform
fractions provided a solid residue which was combined to
provide 1.88 9 of off-white solid.
The off-white solid was placed on a one-liter flash
chromatography column, and eluted first with two liters of
1:9 ethanol:dichloromethane and then with two liters of
15:85 ethanol:dichloromethane. The first 150 ml of eluate
was combined and evaporated to provide 0.96 9 of white
solid (designated hereinafter as C). The next 1275 ml of
eluate was discarded. The next 1750 ml of eluate was
combined and evaporated to provide 0.85 9 of white solid
(designated hereinafter as D).
Solid C was recrystallized with treatment with
decolorizing charcoal from a mixture of 75 ml of ethyl
acetate and 25 ml of hexanes. After cooling, the solid was
separated by filtration, washed with hexanes and dried to
give 0.69 g of white fluffy needles, m.p. 168-169 C.
~ ~ 7 ~
Infrared, nuclear magnetic resonance and mass spectral
analyses indicated the structure of the compound ~o be
5-ethyl-2-(1-hydroxyethyl)-7-[4-(1,1-dioxothiomorpholino)]-
1,2,4-triazoloC1,5-c]pyrimidine Analysis: Calculated for
C13H1gN503S:%C, 48.0; %H, 5.9; %N, 21.5; Found: %C, 47.6;
~H, 5.9; %N, 21.3.
Solid D was recrystallized with treatment with
decolorizing charcoal from a mixture of 50 ml of
dichloromethane and 15 ml of hexanes. After cooling, the
solid was separated by filtration, washed with hexanes and
dried to provide 0.46 9 of fluffy off-white solid, m.p.
192-193 C. Infrared, nuclear magnetic resonance and mass
spectral analyses indicated the structure of the compound
to be 5-ethyl-2-(1-hydroxyethyl)-7-[4-(1-oxothiomor-
pholino)]-1,2,4-triazolo[1,5-c]pyrimidine. Analysis:
Calculated for C13H1gN502S:%C, 50.5; %H, 6.2; %N, 22-6;
Found: ~C, 50.4; %H, 6.1; %N, 22.8.
Example 2
A solution of each of the compounds 5-ethyl-2-(1-
hydroxyethyl)-7-[4-(1,1-dioxothiomorpholino)]-1,2,4-tri-
azolo[1,5-c]pyrimidine (C) and 5-ethyl-2-(1-hydroxyethyl-7-
~4-(1-oxothiomorphol;no)]-1,2,4-~riazolo~1,5-c]pyrimidine
(D) in 0.1 N hydrochloric acid was prepared by gentle
heating.
Each of these solutions was diluted to provide various
concentrations of the compound and the bronchodilator
activity was measured by its effect in the isolated
tracheal spiral model described hereinabove. The activity
is shown below:
7 ~ 3
TABLE I
Percent Relaxation of Contraction
at Indicated Concentration of
Compound of the lnventlon ~~g/ml)
Compound
of the Agonist
InYention Employed 1.25 2.5 5 10 25 50
Compound D Histamine 55% 80% 85% 100% -- --
Acetylcholine 5% 15g30% 100% --
Barium chloride 25% 40%70% 100g - --
Compound C Histamine -- 80% 100~
Acetylcholine -- -- 25~ 15% 55% 60%
Barium chloride -- -- 40% 60% 50g 85%