Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ 16271Y
-- 1 --
BACKGROUND_OF INVENTION
The invention in its broad aspects relates to
carboxyalkyl dipeptides and derivatives thereof which
are useful as converting enzyme inhibitors and as anti-
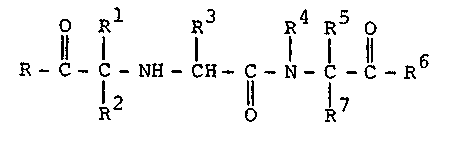
hypertensives. The compounds of this invention can beshown by the following formula:
o 71 R3 R IR5 O
R- C- C- NH- C~I- C- N-C- C- R
R2 R7
I
wherein:
R and R6 are the same or different and are hydroxy or
loweralkoxy;
Rl is a substituted lower alkyl wherein the sub-
stituent is phenyl or halophenyl;
R2 and R7 are hydrogen;
R3 is lower alkyl amino;
R is lower alkyl;
R5 is lower alkyl;
R4 and R5 may be connected together to form an alkylene
bridge of from 2 to 4 carbon atoms;
and the pharmaceutically acceptable salts thereof.
The lower alkyl groups except where noted
otherwise represented by any of the variables include
straight and branched chain hydrocarbon radicals from
one to six carbon atoms, for example, methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, t-butyl, pentyl,
isopentyl, hexyl or vinyl allyl, butenyl and the like.
The R substituted lower alkyl moieties are
exemplified by groups such as
~CH2--
:' ~ .
.
. ' ~ :, .
.. . .
.
,: . .. - .
.
: : ~ . :
-
' ' , ' '
~7~i~5~)
- ~ - 16271Y
R4 and R when joined through the carbon and
nitrogen atoms to which they are attached form a 4 to 6
membered ring. Preferred ring has the formula:
r~
-N
COOH
S Preferred are those compounds of Formula I
wherein:
R is hydroxy;
R6 is hydroxy;
R2 and R7 are hydrogen;
R3 is amino lower alkyl;
R4 and R5 are joined to form the preferred ring as
defined above;
Rl is as defined previously.
Still more preferred compounds are those pre-
ferred compounds of Formula I wherein further
Rl is a substituted lower alkyl wherein the alkyl
group has 1-4 carbon ato~s and the substituent
i5 phenyl.
Most preferred are compounds of Formula I
wherein:
R is hydroxy;
R6 is hydroxy;
R2 and R7 are hydrogen;
R is ~mino lower alkyl;
R4 and R5 are joined through the carbon and nitrogen
atom to form proline;
Rl is a substituted lower alkyl wherein the alkyl
group has 1-4 carbon atoms and the substituent
is phenyl~
: : :
;
.. ..
,
~: .. - . . : . ..
.. , ~ .. . , . : :
- .: . .. , : . ..
- . ~ . ,: , -. . , - . . :
~, , :: ' ': . .
~%~
~ 3 - 16271
The preferred, more preferred and most pre-
ferred compounds also include the pharmaceutically
accepta~le salts thereof.
The products of Formula I and the pre:~erred
5 subgroups can be produced by one or more of the me~hods
and subroutes depicted in the following equations:
O Rl R3 0 R4 R5 0
R C 1 - NH - 1H - C - N -1 - C - R6
2 l7
~ . . . . .
_ ~
-:, , : ... : :
.
. . . . , . :
~! 2~;i3~iÇD
~ 4 ~ 16271Y
As will be evident to those skilled in the art
and as demonstrated in the Exa~ples, reactive groups not
involved in the condensa~ions, such as amino, carboxy,
mercapto, etc., may be protected by methods standard in
peptide chemistry prior to the coupling reactions and sub-
sequently deprotected to obtain the dPsired products.
~ethod I, Route 1 tR2 = H)
_ _
", R30 R4 RS 6 NaBH3CN
R-C-C = 0 + H2NCHC - N - C - C - R
II III
Keto acid (or ester, amide or hydroxamic
acid) II is condensed with dipeptide III in aqueous
solution, optimally near neutrality, or in suitable
organic solvent (CH3C~ for example) in the presence of
sodium cyano borohydride to give I (R = H). Alter-
natively the intermediate Schiff base, enamine,or aminol
may be catalytically reduced to yield produc_ I, for
example, by hydrogen in the presence of 10~ palladium on
1~ carbon or of Raney nickel. The ratio of diasterivm~ric
products formed may be altered by choice of catalyst.
If R and R6 are carboxy protecting groups
such as alkoxy or benzyloxy or the like, they can be
converted by well-known methods such as hydrolysis
or hydrogenation to (I), where R and R6 are hydroxy.
This is ~rue in all the following methods where the
above situation exists.
- . . : . - :
~ . ~' . - ' ' : -
~.,27~
16271Y
~ lternatively II can be condensed with an
amino acid IV
__
R3 0 Rl ~3
H2NCH-COOH + II Na~H3CN~ R-C-CHNHCHCOOH
IV
under the same conditions to yield amino acid V. Sub-
s~quent coupling by known methods with amino acid
derivative VI gives I
The ~nown methods encompass reactive group pro-
tection during the coupling reaction, for example, by
N-formyl, N-t-butoxycarbonyl and N-carbobenzyloxy groups
followed by their removal to yield I. Furthermore, the R
functi~n may include removable ester groups such as benzyl,
ethyl, or t-butyl. Condensin~ agents in this synthetic
route are typically those useful in peptide chemistry such
as dicyclohexylcarbodiimide (DCC) or diphenylphosphoryl
azide (DPPA) or V may be activated via the intermediacy of
active esters such as that derived from l-hydroxybenzotri-
azole.
: R4 ~5
V ~ HN - C - CO - R6 DCC ~
~7 (DCC = Dicyclohexylcarbodiimide)
or
(~I) DPPA
(DPPA = Diphenylph~sphoryl azide)
~ .
.
: ~ , : - . ~ .: .
-. - ~ . . .. . . .
- ' ', -,
ii3S;~
16271
O Rl R30 R4 R5
~ 6
R-C - C - NH2 + O=C-C - N - C - CO - R
~2 R7
V VIII
Amino acid (or ester, amide or hydroxamic acid~ VII
is condensed with ketone VIII under conditions
described for Route I to give I.
Alternatively the synthesis can be performed
in a step-wise fashion by condensing VII with keto acid
IX.
R3 Rl R3
.. . .
VII ~ O = C - COO~ 3 RC - C - N~CH COOH
R2
lX X
t~ yield amino acid X. By known methods as indicated
above under R~ute 1, X can be condensed with amino acid
derivative VI to give I.
R4 R5 0 Rl R O R4R O
~; n ~ ~ n t ~ n L
X + HN - C CO - R ~ R - C~ C - NHCHC-N-C-C-RV
R7 R2 R7
VI
_
In the special case of Rl bearing an Q-amino
substituent, the carbonyl and amino groups can be convenient-
ly protected as a ~-lactam function.
.
:
.
. . .
.
' " ~: . ' ' '
~275~
16271
Method 2 Route 1
_
R3 o R4 P~5 Rl
H2N - CH - C - N - C - COR6 ~ X-C - COR
R7 R2
III XI
O Rl R3 R4 R5
R-C - C - NH - CH - C - N - C - CORV
~2 R7
The dipeptide III is alkylated with the
appropriate Q-haloacid (ester or amide) or a-sulfonyloxy
acid (ester or amide) XI under basic conditions in water
or an organic solvent.
X is chlorine, bromine, iodine or alkyl sulfonyl-
oxy or aryl sulfonyloxy.
Alternatively the synthesis can be performed
in a stepwise fashion
R3 Rl Rl ~3
H N-CH-COOH ~ X-C - COR --~RCO - C - NH-CH - COOH
2 R2 R
IV XI X
R4 R5 o Rl R3 O R4 R5
f HN - C - COR6 ~ R-C-C -NH-CH-C-N -C -CGR6
R7 ~2 R7
VI
.
X = Cl , Br, I , alkylsulfonyloxy or arylsulfonyloxy.
.
..
:.
.
,- :. .
~2~7~35;~
- 8 - 162 Y
The aminoacid IV is alkylated by the Q-halo-
acid (ester or amide) or ~-sulfonyloxy acid (ester or
amide) XI under basic conditions to yield compounds X.
This is condensed by standard methods as indicated under
Route 1 with the aminoacid ~ester or amide) Vl to afford
I. _
Reductive cleavage of a benzyl ester I (where
R6 is benzyloxy and R is alkoxy) will yiel~ compounds
of Formula I wherein R is alkoxy and R6 is hydroxy, and
where R6 is alkoxy and R is benzyloxy, will yield com-
pounds of ~ormula I wherein R is hydroxy and R6 is alkoxy.
Route 2
RlR3 O R4RS
R-C - C -NH2 + X-CH-C-N-C -COR
R2 R7
VI I XI I
R R O R R O
R-C-C-NH-CH-C-N-C -CRV
.. . .
o R2 R7
X = Cl, Br, I, alkyl sulfonyloxy or aryl sulfonyloxy~
The aminoacid or derivative VII is alkylated
with the appropriately substituted c-haloacetyl or a~
sulfonyloxy acetyl aminoacid XII under basic conditions
in water or other solvent to obtain compounds of Formula I.
Alternatively, the synthesis can be performed
in a step-wise fashion by condensing an aminoacid
ester VII with a substituted
-- .
. . ~ . . ..
,
.
. . . . .
~ . .
- 9
16~71Y
Rl R3 Rl R3
RCO -C-NH2 + X-CH-COOH ~ RCO-C-NH-CH-COOH
R2 ~2
VII XIII X
.
R4R5
X ~HN-c~-coR6 _
Rl '
VI
Rl R30 R R O
R-c-c-NH-c-c-N-c --CR6
,. . . .
O R R
I
a-haloacetic acid ox q-sulfonyloxy acetic acid (XIII) to
yield the intermediate X. By known methods described
under Route 1, X can be coupled with an aminoacid VI or
derivative to give I.
As desired, protecting groups may be removed
by ~nown methods.
The starting materials which are required for the
above processes herein described are known in the
literature or can be made by known methods from known
; 10 ~ starting materials.
: In products of general Formula I, the carbon atoms
to which Rl, R3 and ~ are attached may be asymmetric. The
compounds accordingly exist in disastereoisomeric forms or
in mixtures thereof. The a~ove described syntheses can
;
.
' ~ . ' . . - . ........... ' ~'
. :
.
.
.: ~ :,
i3~
16271
utilize racemates, enantiomers or diastereomers as
starting materials. When diastereomeric products result
from the synthetic procedures, the diastereomeric products
can be separated by conventional chromatographic or frac-
5 tional crystallization methods. In general, the aminoacidpart-structures, i.e.,
O Rl ~3 R
n ~ ~ 1 7 n
R-C-C-NH- , ~NH-CHCO -- and -N-C -C-
R ! R7
of Formula (I) are preferred in the S-configuration.
The compounds of this invention form salts with
various inorganic and organic acids and bases which are
10 also within the scope of the invention. Such salts
include ammonium salts, alkali metal salts like sodium
and potassium salts (which are preferred), alkaline earth
metal salts like the calcium and magnesium salts, salts
with or~anic bases e.g., dicyclohexylamine salts, N-methyl-
15 D-glucamine, sal~s with amino acids like arginine, lysine
and the like. Also salts with organic and inorganic acids
may be p~-epared, e.g., HCl, HBr, H2SO4, H3PO4, methane-
sulfonic, toluensulfonic, maleic, fumaric, camphorsulfonic.
The non-toxic phvsiologically acceptable salts are pre-
20 ferred, although other salts are also useful, e.g., inisolating or purifying the product.
The salts may be formed by conventional means,
as by reac~ing the free acid or free base forms of the
product with one or more equivalents of the appropriate
25 base or acid in a solvent or medium in which the salt is
insoluble, or in a solvent such as water which is then
removed _ vacuo or by freeze-drying or by exchanging the
cations of an existing salt for another cation on a
suitable ion exchange resin.
-': . ' ' .
- ,' ~ ~. - : - -~ :
~2~
16271Y
The compounds of this invention inhibit angio-
tensin converting enzyme and thus block conversion of the
decapeptide angiotensin I to angiotensin II. Angiotensin
II is a ~otent pressor substance. Thus blood-pressure
lowering can result from inhibition of its biosynthesis
especially in animals and humans whose hypertension is
angiotensin II related. Furthermore, converting enzyme
degrades ~he vasodepressor substance, bradykinin. There-
fore, inhibitors of angiotensin converting enzy~e may lower
blood-pressure also by potentiation of bradykinin. Al-
thou~h the relative importance of these and other possible
mechanisms remains to be established, inhibitors of angio-
tensin converting enzyme are effective antihypertensive
agents in a variety of animal models and are useful
clinically, for example, in many human patients with r no-
vascular, malignant and essential hypertension. See, for
example, D. W. Cushman et al., Biochemistry 16, 5484 (1977~.
The evaluation of converting enzyme inhibitoxs
is guided by in vitro enzyme inhibition assays. For
example, a useful method is that of Y. Piquilloud,
A. Reinharz and M. ~oth, Biochem. Biophys. Acta, 206, 136
(1970) in which the hydrolysis of carbobenzyloxyphenyl-
alanyl~istidinylleucine is measured. In vivo evaluations
may be made, for example, in normotensive rats challenged
with angiotensin I by the technique of J. R. Weeks and
J. A. Jones, Proc. Soc. Exp. Biol. Med., 104, 646 ~1~60)
or in a high renin rat model such as that of S. Koletsky
et al., Proc. Soc. Exp. Biol. Med., 125, 96 (1967).
Thus, the compounds of this invention are useful
as antihypertensives in treating hypertensive mammals,
including humans and they can be utilized to achieve the
reduction of blood pressure by formulating in compositions
such as tablets, capsules or elixirs for oral administra-
tion or in ~terile solutions or suspensions for parenteral
administration. The compounds of this in~ention can be
: , : . ................... . : . . , -
:, :
. ~ ' '- ,
: . . :,
~27~35~)
12 -
16271Y
administered to patients tanimals and human) in need of
such treatme-t in a dosage range of 5 to S00 mg per
patient generally given several times, thus giving a total
daily dose of from 5 to 2000 mg per day. The dose will
S vary depending on severity of disease, weight of patient
and other factors which a person skilled in the art will
recognize.
Also the compounds of this invention may be
given in combination with other diuretics or antihyper-
tensives. Typically these are combinations whoseindi~idual per day dosages range from one-fifth of the
minimally reco~mended clinical dosages to the maximum
reco~mended levels for the entities when they are given
singly. To illustrate these combinations, one of the anti-
lS hypertensives of this invention effective clinically inthe range 15-200 milligrams per day can be effectively
combined at levels ranging from 3-200 milligrams per day
with the following antihypertensives and diuretics in
dose ranges per day as indicated:
hydrochlorothiazide (15-200 mg), chlorothiazide (125-
2000 mg), ethacrynic acid (15-200 mgJ, amiloride ~5-20 mg),
furosemide (5-80 ma), propanolol (20-4~0 mg), timolol
~5-50 mg.) and metnyldopa (65-2000 mg). In addition, the
triple drug combinations of hydrochlorothiazide (15-200 mg)
plus amiloride (5-20 mg) plus converting enzyme inhibitor
of this invention (3-200 mg) or hydrochlorothiazide (15-
200 mg) plus timolol (5-50 mg) plus the converting
enzyme inhibitor of this invention (3-200 mg) are effect-
ive combinations to control blood pressure in hypertensive
patients. The above dose ranges will be adjusted on a unit
basis as necessary to permit divided daily dosage.
~lso, the dose will vary depending on the severity of the
disease, weight of patient and other factors which a person
skilled in the art will recognize.
Typically the combinations shown above are
formulated into pharmaceutical compositions as discussed
below.
: ~
.: ' ~ .:~ . , . . -
.: : . :
~:7~3~i~
- 13 - 16~7~Y
~ bout 10 to 500 mg. of a compound or mixture of
compounds of ~ormula I or a physiologically acceptable salt
is compounded with a physiologically acceptable vehicle,
carrier, excipient, binder, preservative, stabilizer,
flavor, etc., in a unit dosage form as called for by
accepted pharmaceutical practice, The amount of active
substance in these compositions or preparations is such
that a suitable dosage in the range indicated is obtained.
Illustrative of the adjuvants which may be
incorporated in tablets, capsules and the like are
the following: a binder such as gum,tragacanth, acacia,
corn starch or gelatin; an excipient such as microcrystal-
line cellulose; a disintegrating agent such as corn starch,
pregelatinized starch, alqinic acid and the like7 a lubri-
cant such as magnesium stearate; a sweetening a~ent suchas sucrose, lactose or saccharin; a flavoring agent such as
peppermint, oil of wintergreen or cherry. When the dosage
unit form is a capsule, it may contain in addition to mate-
rials of the above type, a liquid carrier such as fatty oil.
~o Various other materials may be present as coatings or to
otherwise modify the physical form of the dosage unit.
~or instance, tablets may be coated with shellac, sugar or
both. A syrup or elixir may contain the active com-
pound, sucrose as a sweetening agent, methyl and propyl
parabens as preservatives, a dye and a flavoring such
as cherry or orange flavor.
Sterile compositions for injection can be
formulated according ~o conventional pharmaceutical
practice by dissolving or suspending the ac~ive sub-
stance in a vehicle such as water for injection, anaturally occurring vegetable oil like sesame oil,
coconut oil, peanut oil, cottonseed oil, etc. or a
- ' - ' , ' ' ' '' ~ ':
. . . - . ~ . . .
.. . . . .
ii35~
- 14 - lfi~71Y
synthetic fatty vehicle like ethyl oleate or the like.
Buffers, preservatives, antioxidants and the like can be
incorporated as required.
Th~ following examples are illustrative of the
invention and constitute especially preferrea embodi-
ments~ The preferred diastereomers of these examplas
axe isolated by column chromatography or fractional
crystallization.
EXAMPLE 1
A. N-(l-Carbox~-3-phenylpro~yl)-L-lysyl-L-proline
A solution of the sodium salt of 2-oxo-4-
phenylbutyric acid and N-t-Boc-L-lysyl-L-proline is
adjusted to pH 7 with caustic and treated with sodium
cyanoborohydride at room temperature for several days.
Essentially all of the t-Boc protecting group
is cleaved when the product is absorbed on strong acid
ion exchange resin. The crude N-(l-carboxy-3-phenyl-
propyl)-L-lysyl-L-proline is eluted from the resin with
10% ammonia, freeze dried, and purified by gel fil-
tration chromatography (LH-20). A minute peak for t-Boc
protons in the Nmr spectrum disappears when the product
is treated with ethyl acetate that is 4 N in hydrogen
chloride gas. ~he nmr spectrum of th~ resulting HCl
salt of the product is consistent with structure. The
mass spectrum shows a molecular ion at 693 m/e for the
tetrasilylated species. Chromatography on XAD-2 resin
using 3.5% acetcnitrile in 0.1 molar ammonium hydroxide
affords N-~-(l(S)-carboxy-3-phenylpropyl)-L~lysyl-L-
proline.
B. N-a-(l(S)-Car~oxy-3-phenylpropyl)-L-lysyl-L-proline
A solution of the sodium salt of 2-oxo-4-
phenylbutyric acid and N-t-Boc-L-lysyl-L-proline is
adjusted to pH 7 with caustic and treated with sodium
cyanoborohydride at room temperature for several days.
: . . - . ~, '
- ' ~ ., ' .
: - . . .
- . .
;~' ;''''. '' ' '' . ' ' ~ :
~7~
- 15 - 16271Y
The product is absorbed on strong acid ion
exchange resin, and eluted with 2% pyridine in water.
Product-rich cuts are stripped to a glass and treatPd
with 4 N HCl in ethyl acetate to remove the t-Boc pro-
tecting group. The resulting hydrochloride salt isconverted to the free base by absorbing on strong acid
ion exchange resin and eluting with 2~ pyridine in
water. Freeze drying of product-rich cuts affords N-a-
(l-carboxy-3-phenylpropyl)-L-lysyl-L-proline as a white
fluffy solid. The nmr spectrum is consistent with
structure. The mass spectrum shows a molecular ion at
549 for the disilylated species. Chromatography affords
the desired isomer.
EXAMPLE 2
N-~-(l(S)-Carboxy-3-~henylE~opyl)-L-lys~ -proline
N-a- ( l-Carboxy-3-phenylpropyl)-L-lysyl-L-
proline, a mixture of diastereomers prepared as de-
scribed in Exæmple lB is purified by gel filtration
chromatography in methanol (LH-20). The XAD-2 column
prepared as described in Example 1 is equilibxated at
53C with 0.1 M NH40H containing 4% acetonitrile. The
isomer mixture from above (250 mg) is dissolved in 10 ml
of the same solvent and added to the column. When the
col~unn is eluted with this solvent, the first isomer
emerges in the volume range 320-360 ml of eluate. The
second isomer emerges in the range 450-540 ml of eluate.
Intermediate fractions contain a mixture of isomers.
When fractions containing the first isomer are freeze-
dried, 72 mg of fluffy white solid is obtained. This is
the more active isomer and is the S,S,S confi~uration by
analogy to the more active isomer of N-~ (1-carboxy-3-
phenylpropyl)-~-alanyl-L-proline which was established
by X-ray analysis to have the S,S,S configuration. By
thin layer chromatography on silica gel in 1~
ethylacetate/n-butanol/water/acetic acid, this solid is
- . . ................ .
. .
-
, ' - : . . .
ii35~3
- 16 - 16271Y
a singl~ spot having an Rf value of 0.43. The 300 M~z
nmr spectrum shows a triplet for the methine proton y to
tha phenyl substituent at 3.40 ppm. When the fractions
containing the second isomer are freeze-dried, 72 mg of
white fluffy solid is obtained. This solid by thin
layer chromatography is a single spot of Rf value 0.39.
The 300 MHz nmr spectrum shows the triplet for the
methine proton y to the phenyl substituent at 3.61 ppm.
EXAMPLE_3
Benzyl N-(l(S)-carboxy-3-phenylpropyl) L-alanyl-L-
prolinate
Thionyl chloride ~13.1 ml) was added to 150 ml
of benzyl alrohol keeping the temperature below 0
(exothermic reaction). N-(l(S)-carboxy-3-phenylpropyl)-
L-alanyl-L-proline (15 g) was added portionwise to the
cold solution. The cooling bath was removed and the
mixture was stirred at room temperature overnight.
After heating to 45 under vacuum to remove dissolved
gases the reaction was diluted with 500 ml e~her and
washed with 10 x 100 ml of water. A solid appeared in
the organic layer which was filtered and dried to yield
4.9 g of crude monobenzyl ester.
The combined aqueous extracts neutralized with
NaHCO3 gave a second crop weighing 1.4 g. The two crops
were combined and a portion (1.0 g~ was recrystallized
from ethanol-water to yield 0~95 g of pure monobenzyl
ester, m.p. 120-125. Mass spectral analysis indicated
the benzyl ester was attached to the proline ring.
This is a divisional of Canadian Patent
Application S.N. 341,340 filed on December 6, 1979,
~ , ,
.
- - . .
.
,
.