Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
.27~7l~
CATALYTIC TWO-STAGE COAL HYDROGENATION
AND HYDROCONVERSION PROCESS
B~CKGROUND OF THE INVENTION
This invention pertains to an improved catalytic
two-stage coal hydrogenation and hydroconversion process to
produce increased yields of low-boiling hydrocarbon distillate
liquid products. It pertains particularly to such a process in
which the coal feed is rapidly heated and catalytically hydro-
genated in a first reaction zone containing an ebullated
catalyst bed, and then further hydrogenated and hydrocracked in
a close-coupled second catalytic reaction zone at slightly
higher temperature conditions to produce increased yields of
desirable low-boiling hydrocarbon liquid products while minimizing
hydrocarbon gas yields and catalyst deactivation.
In the H-Coal single stage coal liquefaction process,
a particulate coal feed is usually slurried in a coal-derived
recycle oil and the coal-oil slurry is preheated to a temperature
near the reaction temperature and then fed with hydrogen into a
catalytic ebullated bed reactor, which operates at relatively
high temperatures. In the reactor, a major portion of the coal
is liquefied to produce hydrocarbon gas and distillate liquid
fractions, but an undesirably large fraction of the coal lique-
faction product is residual oil containing preasphaltenes andasphaltene compounds. The preasphaltenes are highly unstable
species at elevated temperatures, and can decompose thermally
in the presence of hydrogen to form asphaltenes while releasing
gaseous hydrocarbons and water, but they can also rearrange,
aromatize, and even condense to form char. In the reactor, the
asphaltenes break down further to heavy and light distillates,
naphtha and gaseous hydrocarbons.
In order to achieve satisfactory hydrocarbon liquid
products in single-stage catalytic reaction processes, the
reactor must be operated at a relatively high temperature which
usually produces retrograde materials and places a limit on the
distillate liquid yields which can be achieved. Conventional
single-stage catalytic processes for coal liquefaction and hydro-
genation are generally disclosed in U.S. Patent Nos. 3,519,555
a ~
~ ~'76S~8
-- 2
and 3,791,959. In attempts to overcome the deficiencies of
single-stage catalytic processes for coal liquefaction and
hydrogenation, various two-stage catalytic processes have been
proposed, including processes having a thermal first stage
reactor as well as catalytic-catalytic processes utilizing low
first stage temperatures of only 600-700F. Examples of such
coal hydrogenation processes using two stages of catalytic
reaction are disclosed by U.S. Patent Nos. 3,679,573; 3,700,584;
4,111,788; 4,350,582; 4,354,920; and 4,358,359.
Although these processes using two stages of coal
hydrogenation have generally provided some improvements over a
single stage coal liquefaction process, such processes usually
produce low quality liquid solvent materials in the reactor
and do not provide for the desired hydrogenation and high
conversion of the coal feed to produce high yields of desirable
low-boiling hydrocarbon liquid products with minimal yields of
hydrocarbon gas and heavy residuum fractions. Such improved
results have now been achieved by the present two-stage
catalytic coal hydrogenation and hydroconversion process.
SUMMARY OF THE INVENTION
The present invention provides an improved process
for direct two-stage catalytic hydrogenation, liquefaction and
hydroconversion of coal to ~roduce significantly increased yields
of desirable low-boiling hydrocarbon distillate liquid products
with minimal yields of hydrocarbon gas and high-boiling resid
fractions. In the process, a particulate coal such as bituminous,
sub-bituminous or lignite and a process-derived recycled hydro-
carbon liquid solvent material are mixed together and theresulting flowable coal-oil slurry is hydroqenated and liquefied
using two staged direct-coupled ebullated bed catalytic reactors
connected in series.
The coal-oil slurry is fed into the first stage
back-mixed catalytic reaction zone which is maintained at
Si 78
-- 3 --
selected moderate temperature and pressure conditions and in
the presence of a particulate hydrogenation catalyst which
promotes controlled rate liquefaction of the coal, while
simultaneously hydrogenating the recycle solvent oils at
conditions which favour hydrogenation reactions at temperatures
less than about 800F. The first stage reaction zone contains
an ebullated bed of a particulate hydrogenation catalyst to
hydrogenate the aromatic rings in the particulate coal, recycle
solvent and dissolved coal molecules and produce the desired
low-boiling hydrocarbon liquid and gaseous materials.
The catalyst used in each stage reactor should be
selected from the group consisting of oxides or other compounds
of cobalt, iron, molybdenum, nickel, tin, tungsten and mixtures
thereof, and other hydrocarbon hydrogenation catalyst metal
oxides known in the art, deposited on a porous base or support
material selected from the group consisting of alumina, magnesia,
silica, titania, and similar materials. Useful catalyst particle
sizes can range from about 0.030 to 0.125 inch effective
diameter.
The first stage reactor is maintained at conditions
of 650-800F temperature, 1000-4000 psig hydrogen partial
pressure, and at 10-60 lb coal/hr/ft3 reactor feed rate or
space velocity to produce a high quality hydrocarbon solvent
materialr while achieving at least about 50 W ~ conversion of
the coal to tetrahydrofuran (THF) soluble materials. At such
mild reaction conditions, hydrocracking, condensation and
polymerization reactions along with formation of hydrocarbon
gases are all advantageously minimized. Preferred first-stage
reaction conditions are 700-7gO~ temperature; 1500-3500 psig
hydrogen partial pressure and a coal space velocity of 15-50 lb
coal/hr/ft3 reactor, with the preferred conditions being
specific to the type of coal being processed.
From the first stage reaction zone, the total effluent
material, containing hydrocarbon gases and liquid fractions, is
passed with additional hydrogen directly to the second stage
back-mixed catalytic reaction zone where the material is further
7~
-- 4
hydrogenated and hydrocracked at a temperature at least about
25F higher than for the first stage reaction zone. Both
stage reaction zones are upflow, well mi~ed ebullated bed
catalytic reactors. For the second stage reactor, operating
conditions are maintained at higher severity conditions which
promote more complete thermal conversion of the coal to liquids,
hydroconversion of primary liquids to distillate products, and
product quality improvement via heteroatoms removal at tempera-
ture greater than 800F, and with similar hydrogen pressure
and a hydroconversion catalyst such as cobalt-moly on alumina
support. The desired second stage reaction conditions are
750-875F temperature, 1000-4000 psig hydrogen partial pressure
and coal space velocity of 10-60 lb coal/hr/ft3 reactor volume
to achieve at least about 90 W % conversion of the remaining
reactive coal along with the asphaltene and preasphaltene
compounds to lower boiling hydrocarbon materials, and the
heteroatoms are further reduced to provide THF soluble product
materials. Preferred second stage reaction conditions are 800
860F temperature, 1500-3500 p~ig hydrogen partial pressure,
and coal space velocity of 15-50 lb coal/hr/ft3 reactor volume~
This two-stage catalytic coal liquefaction process
provides high selectivity to low-boiling hydrocarbon liquid
products and desired low yields of Cl-C3 hydrocarbon gases and
residuum materials, together with minimal deactivation of the
catalyst as measured by residuum conversion, which provides
for extended activity and useful life of the catalyst. Overall,
the present two-stage catalytic process produces higher yields
of distillate and lower molecular weight products which are
considerably more paraffinic an~ "~etroleum-like" in terms of
their chemical structure, than are produced by other single or
two-stage direct coal liquefaction processes. It has been
determined that the Watson characterization factor for the
hydrocarbon liquid products in relation to their mean average
boiling point from the present catalytic two-stage process are
intermediate those products produced by the H-Coal~ single-stage
catalvtic process and by petroleum catalytic hydroconversion
processes.
~2~ o'~
The present two-stage direct coal liquefaction process
advantageously provides a significant improvement over the
single-stage H-Coal~ coal liquefaction process, by providing
an integrated recycle solvent hydrogenation step upstream of
the conventional catalytic ebullated bed reactor~ The reaction
conditions are selected to provide controlled hydrogenation and
conversion of the coal to liquid products (as defined by
solubility in quinoline, tetrahydrofuran, or other similar
solvent), while simultaneously hydrogenating the recycle and
coal-derived product oils. Because the coal feed is dissolved
in a high quality hydrocarbon solvent in the low temperature
first-stage reactor, the potential for retrogressive (coke
forming) reactions is significantly reduced and solvent quality,
hydrogen utilization and heteroatom removal are appreciably
improved, which increases potential conversion of the coal while
extending the catalyst life. The high quality effluent slurry
material from the first stage reactor is fed to the close-
coupled second stage catalytic reactor operated at somewhat higher
temperatures to achieve increased coal conversion to mainly
distillate liquid products. The process thermal efficiency is
advantageously improved over other two-stage coal liquefaction
proeesses. Also, because of the high percentage conversion of
coal to low-boiling hydrocarbon distillate liquids which is
achieved, higher boiling residuum fractions can be recycled to
the first stage reactor. Thus, the present process advantageously
achieves higher yields of distillate and lower molecular weight
hydrocarbon products and less heteroatoms with lower energy input
than for single stage catalytic processes, and also for other
thermal and thermal/catalytic two-stage coal liquefaction
processes.
BRIEF DESCRIPTION OF DRAWINGS
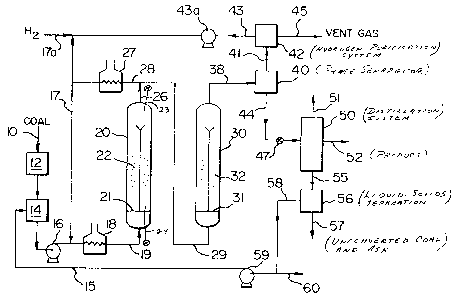
Fig. 1 is a schematic flow diagram of a two-stage
catalytic eoal hydrogenation and liquefaction process in
aceordance with the invention.
~: 7'~ 7~
-- 6 --
Fiy. 2 is a graph showing the effect of first stage
reactor temperatures on the yield of C4-975F hydrocarbon product
liquid.
DESCRIPTION OF THE INVENTION
In the present invention, improved hydrogenation and
liquefaction of coal is achieved by a two-stage catalytic
process using two well-mixed ebullated bed catalytic reactors
direct-connected in series. As is shown in Fig. 1, a coal such
as bituminous, sub-bituminous or lignite is provided at 10 and
passed through a coal preparation unit 12, where the coal is
ground to a desired particle size range such as 50-375 mesh
(U.S. Sieve Series) and dried to a desired moisture content such as
3-10 W % moisture. The particulate coal is then slurried in
tank 14 with sufEicient process-derived recycle solvent liquid
15 having a normal boiling temperature above about 550F to
provide a flowable slurry. The weight ratio of solvent oil/coal
is usually 1.4 5.0, with 1.5-3.0 being preferred. The coal/oil
slurry is pressurized at pump 16, mixed with recycled hydrogen
at 17, preheated at heater 18 to 600-650F temperature and is
then fed into the lower end of first stage back-mixed catalytic
ebullated bed reactor 20. Fresh make-up high-purity hydrogen
is provided as needed at 17a.
The coal-oil slurry and hydrogen streams enter reactor
20 containing an ebullated catalyst bed 22, passing uniformly
upwardly through flow distributor 21 at flow rate and at
temperature and pressure conditions to accomplish the desired
hydrogenation reactions. The operation of the ebullated bed
catalytic reactor including internal recycle of reactor liquid
upwardly through the expanded catalyst bed at a recycle ratio
exceeding about 2:1 is generally well known and is described
by U.S. Patent No. 4,437,973, Huibers et al., issued March 20,
1984. The first stage reactor 20 preferably contains a
particulate hydrogenation catalyst such as cobalt molybdate,
nickel molybdate, or nickel tungsten on an alumina or silica
~ 276~f~3
support material. In addition, fresh particulate hydrogenation
catalyst may be added to reactor 20 at connection 23 in the
ratio of about 0.1 to 2.0 pounds of catalyst per ton of coal
processed. Spent catalyst may be removed at connection 24
to maintain the desired catalytic activity within the reactor.
Operating conditions in the first stage reactor are
maintained at a moderate temperature range of 650-800F, 1000-
4000 psig hydrogen partial pressure, and coal feed rate or
space velocity of 10-60 lb coal/hr/ft3 reactor volume which is
equivalent to about 22-132 lb/hr/ft3 catalyst settled volume
in the reactor. The preferred reaction conditions of 700-790F
temperature, 1500-3500 psig hydrogen partial pressure and
15-50 lb coal/hr/ft3 reactor volume, or about 33-110 lb/hr/ft3
catalyst settled volume in the reactor, will be specific to
the particular coal beinq processed, because different coals
convert to liquids under thermal conditions at different rates.
The optimal first stage reaction conditions will allow maximum
utilization of hydrogen shuttling solvent compounds, such as
pyrene/hydropyrenes, known to be present in coal-derived
recycled oils, since catalytic rehydrogenation of donor species
occurs simultaneously with solvent-to-coal hydrogen transfer.
Coal-derived oils are also exposed to an efficient catalytic
hydrogenation atmosphere immediately upon their formation,
reducing the tendency for regressive repolymerization reactions
which lead to poor quality hydrocarbon liquid products. First
stage reactor thermal severity has been found to be quite
important, as too hig~ a severity leads to a coal conversion
rate which is too rapid for the catalytic hydrogenation reactions
to keep pace, as well as poorer hydrogenation equilibrium for
the solvent compounds. Too low a thermal severity in the
first stage, while still providing an efficient atmosphere
for solvent hydrogenation, does not provide sufficient coal
conversion to provide a substantial process improvement.
In the first stage reactor, the objective is to
hydrogenate the aromatic rings in molecules of the feed coal,
7~7~3
-- 8 --
recycle solvent and dissolved coal so as to produce in situ
a high quality hydrogen donor solvent liquid in the presence
of hydrogen and the hydrogenation catalyst. At the moderate
catalytic reaction conditions used, heteroatoms are removed,
retrogressive or coke forming reactions are essentially
eliminated, and hydrocarbon gas formations are effectively
minimized. Because of the reaction conditions used, i.e.,
relatively low temperature first stage, the catalyst promotes
coal hydrogenation and minimizes polymerization and cracking
reactions. Also because of these improved conditions in the
first stage reactor, less coke is deposited on the catalyst
at the milder reaction conditions used, and the deposited
coke also has a desirably higher hydrogen/carbon ratio than
for prior processes, which minimizes catalyst deactivation
and appreciably prolongs the effective life of the catalyst.
From the first stage reactor 20, the total effluent
material at 26 is mixed with additional preheated hydrogen at
27 and flows directly to the lower end of close-coupled second
stage catalytic reactor 30. This reactor 30 which operates
similarly to reactor 20 contains flow distributor grid 31 and
catalyst bed 32, and is operated at a temperature at least about
25F higher than for the first stage reactor, and usually in
the temperature range of 750-875F, but at temperatures lower
than conventionally used for single-stage catalytic coal
liquefaction processes. The higher temperature used in
reactor 30 may be accomplished by utilization of the preheated
hydrogen stream 28 as well as the second stage reactor heat
of reaction. The second stage reactor pressure is slightly
lower than for the first stage reactor to permit forward
flow of the coal slurry material without any need for pumping,
and additional ~akeup hydrogen is added at 29 to the second
stage reactor as needed. A particulate catalyst similar to
that used in the irst stage reactor is utilized in bed 32
for the second stage reactor.
~ ~'7~7~
g
In the second stage reactor 30, the reaction
conditions are selected to provide a more complete catalytic
conversion of the unconverted coal to liquids, utilizing the-
high quality solvent liquid produced in the first stage
reactor. I'he remaining reactive coal as well as preasphaltenes
and asphaltenes are converted to distillate liquid products along with additional
heteroatoms removal. Substantial secondary conversion of coal
derived liquids to distillate products, and product upgrading
by heteroatoms removal, is also accomplished in the second
stage reactor. The reaction conditions are selected to minimize
gas formation or dehydrogenation of the first stage liquid
effluent materials. Useful reactor conditions are 750-875F
temperature, 1000-4000 psig hydrogen partial pressure, and
coal space velocity of 10-60 lb coal/hr/ft3 reactor volume.
Preferred reaction conditions will depend on the particular
type coal being processed, and are usually 800-860F temperature,
1500-3500 psig hydrogen partial pressure and 15-50 lb coal/hr/ft3
reactor space velocity.
It is an important characteristic of this process
that very little change in the hydrocarbon compounds composition
occurs between the first and second stage reactions. It has
been found that the 850~F-distillate liquids contain much
lower levels of condensed aromatics and are significantly
more aliphatic than are products produced from a conventional
single stage catalytic coal hydrogenation process. Recycle
of residual oil greatly enhances hydrogenation and hydro-
conversion of the coal in the first stage reactor.
From the second stage reactor 30, the effluent
material at 38 is passed to a phase separator 40 operating
at near reactor condition.s, wherein a vapour fraction 41 is
separated from a solids-containing liquid slurry fraction at
44. The vapour fraetion 41 is treated at hydrogen purification
section 42, from which hydrogen stream 43 is withdrawn for
recycle by compressor 43a to the reactors 20 and 30. Fresh
make-up hydrogen is added as needed at 17a. A vent gas
,, ,
.,
. .
~.~765i7~
-- 10 --
containing undesired nitroyen and sulfur compounds is removed
from purification section 42 as stream 45.
The slurry liquid fraction 44 is pressure-reduced
at 47 to near atmospheric pressure, such as about 200 psig,
and passed to a distillation system generally shown at 50.
The resulting liquid fractions are recovered by a vapour/liquid
flash in the distillation system 50, including atmospheric
and vacuum distillation steps to produce light distillate
product stream 51 and a heavier higher-boiling distillate
liquid product stream 52. A bottoms stream 55 is passed to
a liquid-solids separation step 56, from which unconverted
coal and ash solids are removed at 57. The liquid stream 58
having reduced concentration oE solids is recycled by pump 59
as slurrying oil 15. If desired, a reduced solids concentration
product liquid stream can be withdrawn at 60.
The recycle slurrying oil stream 58 is prepared
by blending a portion of the atmospheric separator bottoms
liquid slurry (containing 500F+ distillate, residuum,
unreacted coal and ash), the atmospheric fractionation bottoms
material (600F+ distillate), and vacuum gas oil. This
slurrying liquid stream 58 is then recycled back as stream 15
to the mixing tank 14, where it is mixed with the coal feed
to form the flowable slurry feedstream to the first stage
reactor.
The recycle oil preparation in liquid-solids
separation step 56 can be improved by reducing its solids
concentration (a-sh and unconverted coal) by using known solids
removal means in separation step 56, such as by use of hydro-
clones, centrifuges, filters or solvent deashing techniques,
with use of liquid hydroclones usually being preferred.
This invention will be further described and better
understood by reference to the following Examples of com-
parative operations, which Examples should not be construed
as limiting the scope of the invention.
i., `
-- 11. --
EXAMPLE 1
Several runs were made using the present two-stage
catalytic process on Illinois No. 6 coal at the reaction
conditions shown in Table 1, i.e., 750F first stage reactor
temperature and 825F second stage reactor temperature. From
the results provided in Table 1, it is seen that substantially
improved results including increased hydrogen efficiency and
improved distillate liquid yields were achieved, as compared
to results for a single stage cataly-tic coal liquefaction
process operating at substantially the second stage reaction
conditions. It should be noted that the yields of C4-975F
and 390F-975F materials are both significantly greater for
the present two-stage process than for single stage processes.
TABLE 1
CATALYTIC TWO-STAGE PROCESS PERFORMANCE
Feed: Illinois No. 6 Coal - 70 U.S. Mesh size
Catalyst: First Stage - Amocat lC
Second Stage - Amocat lA Single Stage
Average Catalyst Age, Catalytic~2)
Lb Dry Coal/Lb Catalyst 216.3 664.3 Process
OPERATING CONDITIONS
Temperature, F
First Stage 750 750
Second Stage 825 825 850
Pressure, psig 2506 2515 2500
Dry Coal Space Velocity (ea.stage),
Lb Dry/Coal/Hr/Ft3 Catalyst 68 68 68
Total Material
Recovery, (Gross) W %97.6 97.31
NORMALIZED YIELD, W % Dry Coal
Cl-C3 Gas 5.6 5.9 12.1
C4-390F Liquid -17.9 16.21 21.2
390-500F Liquid 48.6 13.9 12.2¦45.2
500-650F Liquid _16.8 16.8J
650-850F Liquid -11.7}15 7 ~14.6
850-975F Liquid 62.1 4-0 64.0 3.7
390-975F Liquid -46.4 -49.4 32.0
C4-975F Liquid 64.3 65.6 53.2
975F+ Material 4.9 4.6
, .. ... - .~ .
: ,.
S~7~
- 12 -
TABLE 1 (Cont'd)
Unconverted Coal
Ash 11 11.1
H2O ~ 9.2 9.0
CO+CO2 13.51 0.46 0.3 14.1
NH3 1.2S 1.1
2 _2.6 2.7
Total (100 + H2Reacted) 106.1105.56
PROCESS PERFORMANCE
Coal Conversion, W % M.A.F. 94.3 94.8 94.6
975F+ Conversion, W % M.A.F. 86.9 82.2 72.0
Hydrogen Efficiency 10.5 10.7 9~6
C4-975F, W % M.A.F. 72.3 67.2
Organic Sulfur Removal, W % 98.0 96.6
Nitrogen Removal, W %79.2 66.5
C -975F DISTILLATE Q~ALITY
-4
Gravity, API 25.5 23.7
Sulfur, W % 0.035 0.037
Nitrogen, W % 0.19 0.33
Space velocity expressed in terms of catalyst bed settled
volume, equivalent to 34 lb coal/hr per ft 3 reactor volume.
This indicates that relatively little change occurs
in chemical structure of the compounds in the second stage
reactor compared to ~hose in the first stage, and that
significantly more aliphatic type compounds are produced in
the two-stage catalytic process.
From the improved results achieved by the present
process, it was also unexpectedly found that the 850F minus
distillate fraction contained much lower levels of condensed
aromatics and are significantly more aliphatic than the similar
boiling fractions from a single stage catalytic coal lique-
faction p~ocess, as is shown in Table 2, showing the proton
distribution of the 850F minus distillate liquid.
'~'
78
- 13 -
TABLE 2
PROTON DISTRIBUTION OF 850F-DISTILLATES
Single Stage Two-Stage
H-Coal~ Process Catalytic Process
First Second
Stage Stage
Aromatics
Condensed 24.8 7.4 8.1
Uncondensed 7.0 7.2 7.6
Totals 31.8 14.6 15.7
Alpha Aliphatics
Alkyl 11.8 10.8 10.1
Cyclic 18.2 15.5 14.3
Beta Aliphatics
Alkyl 16.6 24.4 25.0
Cyclic 13.5 21.1 20.1
Gamma Aliphatics8.0 13.6 14.8
Totals 68.1 85.4 84.3
EXAMPLE 2
Additional runs were made for this two-stage
catalytic process on sub-bituminous Wyodak coal. Comparative
results with the Illinois No. 6 bituminous coal runs of
Example 1 are shown in Table 3.
TABLE 3
COMPARATIVE PROCESS PERFORMANCE
-
WYODAK(2)
ILLINOIS NO. 6 WYODAK(13 H-COAL~
TWO STAGE TWO STAGE SINGLE STAGE
Cl-C3 Gas, W ~ M.A.F. Coal 5-7 7-10 5-13
C4-975F, W ~ M.A.F. Coal 63-68 54-68 47-51
Coal Conversion, W ~ M.A.F.
Coal 94-95 79-92 82-91
Hydrogen Consu~ption 6-7 6-8 5-7
Hydrogen Efficiency 10-11 8-9 7-10
975F+ Conversion 81-87 74-90 69-78
(1) Preliminary Data
(2) Run 227-4 and 177-87, H-Coal~ Single Stage Catalytic Process
It is noted that percent coal conversion and yield
of C4-975F material is somewhat less for Wvodak coal than for
the Illinois No. 6 coal. Results for the present two-stage
'!''`i`~
;7~
- 14 -
catalytic process compared with the H-Coal~ single stage
catalytic process on Wyodak coal are also shown in Table 3.
It is noted that although the percent conversion of the Wyodak
sub-bituminous coal is comparable to that for the single
stage process, the C4-975F yield and the conversion of the
975F+ material are significantly higher than for the single
stage process.
EXAMPLE 3
During two-stage catalytic operations on Wyodak
sub-bituminous coal, the effect of first stage reactor
temperature on hydrogen content of reactor liquids and the
solvent quality and on C4-975F liquid yields were investigated.
The first stage reactor temperature was varied between 650F
and 775F with the second reactor stage temperature maintained
at 810F and at 45 lb/hr/ft3 catalyst space velocity in each
reactor. The results for hydrogen content of the reactor
liquid as indicated by hydrogen/carbon ratio are shown in
Table 4.
TABLE 4
HYDROGEN CONTENT OF REACTOR LIQUIDS - RUN 227-22
FIRST STAGE HYDROGEN TO CARBON RATIO
REACTOR 650-850F LIQUIDS 850F+ LIQUIDS
TEMP. F FIRST STAGE SECOND STAGE FIRST STAGE SECOND STAGE
650 1.35 0.03 1.32 1.09 0.10 0.99
700 1.36 0.01 1.35 1.11 0.04 1.07
750 1.34 0.04 1.30 1.04 0.06 0.98
775 1.29 0.00 1.29 0.97 0.03 0.94
From these data, it is seen that the hydrogen to
carbon ratios are greater for the first than for the second
stage reactors at temperatures up to about 750~F and decline
at 775F first stage temperature. Also, it is pointed out
that these hydrogen/carbon ratios are among the highest
reported in the literature for processing Wyodak coal.
The effect of first stage reactor temperature on
solvent quality is shown in Table 5.
. ,
- 15 -
TABLE 5
SOLVENT QUALITY OF REACTOR PRODUCTS - RUN 227-22
FIRST STAGE
REACTOR COAL CONVERSION, W % M.A.F. COAL~2)
TEMP F FIRST STAGE SECOND STAGE
650 64.5 60.0
700 70.4 60.8
750 64.1 47.9
750 64.6 49.7
750tl) 51.6 54.2
775 42.6 46.7
(1) Wet coal feed producing lower H2 partial pressure
(2) HRI Solvent Quality Test Conditions
Coal - Upper Wyodak
Temperature - 750F
Residence
Time - 30 Minutes
Type Test - Thermal
Solvent - Stage 1 - Filtered Liquid Product
- Stage 2 - Filtered Atmospheric Still
Bottoms
Conversion - As measured by solubility of
microautoclave product in THF
It is noted that the solvent quality is higher in
the first stage reactor up to a first stage reactor temperature
of about 750F.
The effect of first stage reactor temperature on
C4-975F liquid yields is shown in Fig. 2. It is seen that
improved C4-975F yields are obtained for increasing first
stage reaction temperature from 650F up to about 750F, and
that liquid yields are further increased as the second stage
reactor temperature is increased from 810F to 825F. Thus,
the improved solvent liquid quality achieved in the first
and second stage reactors is indicated by the high hydrogen
content of the 650-850F and 850F+ reactor liquid fractions
as shown in Table 4 and the coal conversions obtained based
on the standard test for solvent quality as shown in Table 5.
.~
..,
~ ~7~5i7~
- 16 -
, .~
EXAMPLE 4
The present two-stage catalytic coal liquefaction
process is compared with other two-stage thermal-catalytic
5 coal liquefaction processes, as shown in Table 6.
TABLE 6
COMPARISON WITH THERMAL CATALYTIC PROCESSES
KERR-MCGEE
TWO-STAGE HRI THERMAL- THERMAL-
CATALYTIC C ALYTIC _ATAL~TIC
Coal <--------Wyodak Clovis Point-------->
Cl-C3 Gas, W ~ 8.1 9.9 12.8
C4-850F Liquid, W % 65.5 52.9 52.6
Coal Conversion, W %
M.A.F. Coal89.3 90.2 92.1
Hydrogen Consumption 8.1 6.6 5.2
Hydrogen Efficiency 8.1 8.5 10.1
975F+ Conversion87.4 76.9 78.6
From this comparison, it is seen that the present
catalytic two-stage process provides improved results of
reduced Cl-C3 gas yields, increased C4-850F liquid yields,
and increased conversion of 975F+ fraction material compared
to the other processes.
Although this invention has been described broadly
and in terms of certain preferred embodiments thereof, it will
be understood that modifications and variation of the process
can be made within the spirit and scope of the invention,
which is defined by the following claims.