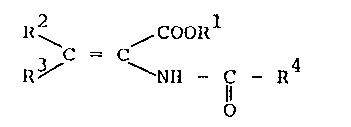Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1~77988
The present invention ~elates to a p~ocess for
producillg N-acyl.-2,3-dehydro-amino carboxylic esters having the
ge~leral formula
1~ ,coo~l
R~' ~ Nll - C R4 (I),
o
.
wherein Rl represents a straight-chain or branched (Cl-C4)
alky]. radi.cal, a phenyl radical or a benzyl radical, ~2 re-
preserll:s hydrogen or a methyl radical, R3 represents hydrogen, a
stra;.ghl:.-chai.n or branched (Cl-C16)alkyl radical, a (C3-C~)
cycloalkyl radi.cal, a (Cl-C6)alkoxy radi.cal, a phenoxy radical,
a (C1-C~)alkyl mercapto radi.cal, a uhenyl mercapto radical or a
methoxy-carbonyl-methyl mercapto radical and R represents a
str:aight-chaitl or branched (C1-C16)alkyl radical OJ: (C2-C16)
alkenyl .radical, either unsubst:ituted by halogen, a methoxy
group 01. by an acet:yl-thio group, an unsubstituted (C3-C8)
cycloalkyl ra~ical whi.ch may be mono- or poly-substituted by
alogen or a methyl group, an unsubstituted phenyl radical which
may be mono- or poly-substituted by halogen, a nitro group, a
(Cl-C4)alkyl or alkoxy group or by a trifluoromethyl group, an
unsubstituted cinnamyl radical, which may be mono- or poly-
substituted by halogen, an unsubstituted heteroaryl radical
which may be mono- or poly-substi.tuted by halogen, a (Cl-C4)
alkyl or alkoxy group or by a trifluoromethyl group, an aryl or
heteroaryl methyl radi.cal or an unsubstituted phenoxy methyl
radi.cal or phenyl-ethyl radical which may be mono- or poly-
30 substituted by halogen, a nitro group, a (Cl-C4)alkyl group or
by a trifluoromethyl groyp~ or a 2-(2',2'-dichloroethenyl)-
3,3-di.methyl cyclopropyl radical.
. --
-- 1 --
7~388
The N-acyl-2,3-dehydro-amino carboxylic esters havirlg
the gel~eral formula (I), as such or Oll saponification of the
es~:er ~urlction, are effective inhibitors for the dipeptidase
[E.C.3.4.13.11~ and thus valuable substances for maintajning the
activity of ~ -lactam antibiotics, particularly those of the
thienamycin group. They can also be used as intermediate pro-
ducts for the production of the corresponding optically active
2-acyl-amino carboxylic estexs by asymmetric cataly-tic hydro-
genation of the prochiral C-C double bonds. When required, the
2-acyl-amino carboxylic esters can then be saponified to the
corresporldirlg optically active 2-acyl-amino carboxylic esters.
'rhe production of N-acetyl-2,3-dehydro-amino carboxy-
lic ester by reactirlg the corresponding 2-azido-carboxylic
esters wi~h a mixture of acetic acid anhydride and acetic acid
in the L~resence of rhenium (VII) sulphide is disclosed in German
Patent No. 3 140 227. However, this process does not permit the
~oduction of the N-acetyl compounds.
In the process according to the present invention a 2-
azido-carboxylic ester having the general formula
~2
Cll - ~CH - CoO~l (II),
N3
wherein Rl, R2 and R3 have the Meanings specified hereinbefore,
are reacted at a téhperature of between 0 and 150C in the pre-
sence of a perrhena~e with a carboxylic acid halide having the
general formula
3 o 1~ 4 - C` - H~ I
o ~ III ),
1'~7~79~38
wherei~ 4 llas the meanings specified hereinbefore and the ~lal
rep~esents chlorine or brornine.
ln this manner all the N-acyl-2,3-dehydro-amino
carboxylic estes can be readily produced in a single-stage
process with good yields.
The perrhenate is preferably used in an amount of
between 0.005 and 10 mole percent, particularly between 0.1 and
3 mole percent, relative to the amount of 2-azido-carboxylic
ester having the general formula (II). Suitable perrhenates are
for example, sodium, potassium or ammoniurn perrhenate or sub-
stituted ammonium perrhenates such as tetraethyl, tetrabutyl,
tetrabenzyl or tricapryl-methyl ammoniurn perrhenate.
Under certain conditions it is advantageous to carry
out the reac-tion of the 2-azido-carboxylic ester having the
general formula (lI) with the carboxylic acid halide having the
general formula (III) in the additional presence of an acid
amide, a lactam or of a substituted urea. These substances
serving as activators are suitably used in at least an equimolar
amount relative to the carboxylic acid halide having the general
formula (III). Suitable acid amides are, for exmaple, dimethyl
formamide, dimethyl acetamide or hexamethyl phosphoric triamide.
N-methyl pyrrolidone is preferred as loctam. Tetramethyl urea
and N,N'-dimethyl-propylene urea are particularly sutiabe as
substituted ureas.
The 2-azido-carboxylic esters having the general for-
mula (II) and serving as starting materials can be produced
according to the process described in Chem. Ber. 117, page 1497
to 1512 (1984) by reacting the corresponding 2-chloro-2-bromo
carboxylic esters with an aqueous sodium azide solution in the
30 presence of a phase transfer catalyst. Examples of the 2-azido-
carboxylic esters having the general formula (II) which can be
reacted by means of the process according to the present inven-
1~77988
tiOIl are the esters of 2-azido-propionic acid, -butyric acid,
-3-methyl-butyric acid, -3-phenyl-propionic acid, -pentanoi.c
acid, -4-methyl-pentanoic acid, -3-cyclopentyl-propionic acid,
-3-cyclohexyl-propionic acid, -hexanoic acid, -heptanoic acid,
-octanoic acid, -norlanoic acid, -decanoic acid, -hendecanoic
acid, -dodecanic acid, tridecanoic, -tetradecanoic acid,
-pentadecanoic acid, -hexadecanoic acid, -heptadecanoic acid,
-octadecanoic acid, -nonadecanoic acid, -3-methyl-mercapto
propionic acid, -3-methoxy-carbonyl-methyl-mercapto pro-
10 pionic acid, -3-pherlyl-mercapto propionic acid or 3-phenoxy
propionic acld.
The carboxylic acid halides having the general formula
(J.II) can be produced by means of generally known processes from
the basic carboxylic acids with chlorinating agents such as
thionyl chloride, phosphorus trichlorie or phosgene or bromina-
tion agents such as a mixture of red phosphorus and bromine or
phosphorus tribromide. The acid chlorides are preferably
applied as carboxylic acid halides having the general formula
,,
(III). The carboxylic acid halides are suitably used in an
20 amount of 1.0 to 1.5 moles, perferably in an amount of 1.0 to
1.1 moles per mole of applied 2-azido-carboxylic acid having the
general formula (II).
The process according to the present invention is
preferably carri.ed out at a temperature of between 20 and 80C.
The reacti'on is carried out with advantage in an inert
solvent. Suitable solvents are, for example, carboxylic alkyl
estes, such as acetic ethyl ester, acetic propyl ester, acetic
isopropyl ester, acetic butyl ester, propionic ethyl esters;
: acetonitrile, nitro-methane, tetrahydrofuran, dioxane or
30 d:imethoxy ethane. ~lowever, an excess of the acid amides,
~ lactams or substi-tuted u~eas mentioned above can also be applied
: instead of an additional solvent.
- 4 -
779t~8
To avoid losses in yield it can be expedient to carry
out the reaction in the presence of a small amount of an
inhibitor for radical polymerizations, for exa~ple, hydroquinone
or hydroquinone monomethyl ether. Suitable amount for the
application of these inhibitors are between 0.001 and 10 percent
by weight, particularly between 0.1 and 3 percent by weight,
relative to the 2-azido-carboxylic ester having the general
formula (II).
'~, ' ' i
In the case of 2-azido-carboxylic esters that are par-
ticularly sensitive to acids it can be advantageous to carry out
the reaction with the carboxylic acid halides having the general
formula (III) in the additional presence of an acid acceptor,
which is suitably applied in an amount equivalent to the
carboxylic acid halide. Suitable acid acceptors are particu-
larly the acetates or oxalates of sodium or potassium.
For example, the process according to the present in-
vention can be so carried out that the 2-azido-carboxylic acid
having the general formula (II) in an inert solvent is put into
a reactor and that the perrhenate and, when required, the acti-
vator and/or polymerization inhibitor are added to the solution,
whexeupon the carboxylic acid halide having the general formula
(III) is added. In the case of small mixtures the carboxylic
acid halide can be added all at once but in the case of large
mixtures it is more expedient to add, it slowly, for example,
within 1 to 3 hour$.~ The carboxylic acid halide can be added in
the pure form or as à solution in the inert solvent used. The
rate of addition is suitably so selected that the generation of
nitrogen can be kept properly under control.
., ,
When adding the carboxylic acid halide at room tem-
perature the reaction temperature can be kept below approxi-
mately 25C by water cool~ing. When operating without external
cooling the reaction will sooner or later become exothermic.
~: -- 5 --
" 1'~7'7~8
'l'he reaction temperature can then reacll the boiling temperature
of t~le ;l~ert solvent -temporarily. In any case the reaction
mixl:ure is stirred until the generation of gas is terlllirlated.
When required, the end of the reaction can also be determined
IR-spectroscopically by the disappearance of the azide bands~
On terminating the reaction the solvent and, when
required, the activator are removed, suitably under reduced
pressure, for exa~iple, with a rotary evaporator. The residue is
taken up in a readily volatile solvent, for example, acetic
ethyl ester, acetic isopropyl ester or diethyl ether, shaken out
with an aqueous sodium hydrogen carbonate solution and then
rewashed with water. 'rhe organic phase dried over sodium sul-
phate or magnesium sulphate is then again concentrated by eva-
poratioll under reduced pressure, once more in a rotary evapora-
tor. In many cases the remaining residue can be crystallized by
addi.ng n-pentane. The crystals are then filtered with suction
and dried under reduced pressure at a temperature of maximally
.~ SOC.
When the residue is a non-crystallizing oil a further
purification by distillation in high vacuum, for example, in a
bulb tube, can be attempted. In many cases it can also be
advantageous to purify the residue by chromatography on a silica
gel column. E'or this purpose a mixture of acetic ethyl ester
and a low-boiling petroleum ether in a ratio by volume of
between 1:1 and l:9,lcan be used as eluate. On evaporating the
eluate and removing the last solvent residue in high vacuum the
analytically puxe N-acyl-2,3-dehydro-amino carboxylic esters are
obtained.
I'he present invention will be explained in greater
30 detail by the l~xamples hereafter.
Fxample 1
13.7 mg (0.05 mmole) of sodium perrhenate, 715.7 mg (5
1~775~8
mmoles) of 2-azido~propanoic ethyl ester and 383.8 ~ng (5.25
nlmoles) of dimethyl formamide were dissolved in 5 ml of
acetonitrile. 592.9 mg (5.25 mmoles) of chloro acetyl chloride
were then added at room temperature while stirring vigorously.
- The reaction proceeded while homogeneously generating nitrogen
within 5 to 6 hours. When the generation of gas was terminated
the acetonitrile was removed under reduced pressure. The resi-
due was taken up in ~cet:ic ethyl ester, spaken out with 10.5 ml
of a 0.5 molar sodium-hydrogen-carbonate solution. The separa-
ted organic phase was once more washed with water and dried over
magnesium sulphate. On removing the drying agent the concentra-
ted organic phase was chromatographed over sil~ca gel with a
mixture of low-boiling petroleum ether alld acetic ethyl ester in
the ratio by volume of 9:1 as the eluate.
On evaporating the eluate there remained 581.5 mg of
analytically pure 2-chloro-methyl-carboxamido propenoic ethyl
ester (i.e., 60.7% of the theoretical,yield)
H ~ ~NH-C-CII Cl
llloClN03(191~614)
~C %~ %N %Cl
- computed: 43.88 , 5.26 7.31 18.50
obtained: ' 43.99 5.40 7.02 18.46
melting point: 63C
NMn~CDC13):
= 8,9 (s, lH) NH;
6,64 (s, lH) H-CI3=;
6~0 (d, lH) H-CH=;
.
- 7 -
~77988
4~37 (q, 21~) OC~2-:
(s, 2~ C~I2-Cl:
1~38 ppm (t, 3H)-CH3.
Il~ (KBr)~NIl = 3282 cm
~CO = 1716 cm
1679 c~~ .
Example 2
13.4 mg (0!.05 mmole) of ammonium perrhenate 715.7 mg
(5 mmoles) of 2-azido-butanoic methyl ester and 383.8 mg (5.25
mmoles) of dimethyl formamide were dissolved in 5 ml of
acetonitrile, whereupon 592.9 mg (5.25 mmoles) of chloroacetyl
chloride weré added at room temperature while stirring vig-
orously. The reaction mixture was stirred for 9 hours at room
temperature, followed by further treatment analogously to that
of Example 1. 845.6 mg (88.3% of the theoretical yield) of
~ analytically pure 2-chloro-methyl-carboxamido-2-butenoic methyl
- ester were obtained.
-
H3C~ ~ Nll-C-CH Cl
It~ ~CO2C~l3
C7llloclNo3(l9l~6lq)
%C %1l ~ %N ~Cl
; computed:43.88 5.26 7.31 18.50
obtained:43.75 5.23 7.19 18.37
melting point: 58.5 to 59C
.~
-- 8 --
1'~77~
1 Il-NMI~ ( CDCl 3 ):
'~ = B,05 ~s, 1~) NH
6,95 ~q, l~ C~C~3)=;
4,19 (s, 2~) C~2Cl;
3,80 ~s, 3H) OCH3;
l,8 ppm ~d, 311) CH3-CH=.
IR (KBr)~'N11 = 3260 cm
- ~CO = 1727 cm
' 1676 cm
F ample 3
134 mg (0.5 mmole) of ammonium perrhenate, 9.25 (g (50
mmoles) of 2-azido-butano.ic-n-butyl ester and 3.85 (52.6 mrnoles)
of dimethyl formamide were dissolved in 50 ml of acetonitrile,
whexeupon 5.93 g (52.5 mmoles) of chloro acetyl chloride were
added at room temperature while stirring vigorously. The
mixture reacted exothermically within 30 minutes.
When the generation of gas was terminated the aceto-
nitrile was removed under reduced pressure. The residue wastaken up in acetic ethyl ester, shaken out with lO0 ml of a 0.5
molar sodium-hydrogen-carbonate solution; the separated organic
phase was once more washed with water and dried over sodium sul-
phate. On removing the drying agent the concentrated organic
phase was crystalliz,ed with n-pentane, filtered with suction and
dried.
9.8 g (83.9~ of the theoretical yield) of analytically
pure 2-chloro-methyl-carboxamido-2-butenoic-n-butyl ester were
obtained.
_ g
~ 77~
C~13C~ c_ocll2c1~2cll3
,IIN-~-cll2 Cl
o
CloHl6No3cl(233~69)
,~
. , %C ~H %N %Cl
computed: 51.40 6.90 5.9915.17
obtained: 51.36 7.01 5.9815.20
mel-ting point: 53.5C
:
Il-NMI~ (CDC13/TMS): .
= 7,85 (s broad~ lH) NH;
6~8 (q, lll) -CH=;
4,15 (t, 2H) -C~OCH2-;
4,1 (s, 2H) -CH2dl;
1,8 ~d, 311) Cl13-CH=;
. 20 U~6 - 2~0 ppm (m, 7H) -CH2-CH2-CH3.
Example 4
134 mg (0.5 mmole) of ammonium perrhenate, 9.25 g (50
mmoles) of 2-azido-butanoic-n-butyl este~ and 3.85 g (52.6
mmoles) of dimethyl;formamide were dissolved in 50 ml of aceto-
nitrile, whereupon 7.75 g (52.5 mmoles) of dichloro acetyl
: chloride were added at room temperature while stirring vig-
, ,
orously. The exothermic reaction started immediately and was
terminated within 20 minutes. The further treatment was like
that i.n Example 3.
11.2 g (83.5~ of the theoretical yield) of analyti-
-- 10 -- ~ I
.,
, ~ . . ~ .
cally pure 2-dichloro-met:llyl-carboxamido-2-butenois n-butyl
ester were obtained.
C1-13CII=~-C-OC~2c~l2c~2c~l3
~N-C-CIIC12
O
, CloH15N03C12t268,14)
%C %H %N ~Cl
computed: 44.79 5.64 5.22 26.44
obtained: 44.75 5.81 5.20 25.16
meltirlg point: 58.5C
~CDCl3/TMS)
= 7,85 (s b~d, lH) Nll;
6,BS (q, 1ll) -CH=;
5,95 (s, lli) -CIIC12;
4,1 (t, 21~) -COOCl~2-;
1,7S (~, 3~1) C~13-CI~=;
0~6 - l~B5 ppm ~m, 7H) -CH2-CH2-Cll3.
Example 5
134 mg (0.5 mmole) of ammonium perrhenate, 9.25 g (50
mmoles) of 2-azido-butanoic-n-butyl ester, 50 mg (0.45 mmoles)
of hydroquinone and 3.85 g (52.6 mmoles) of dimethyl formamide
were dissolved in 50 ml of acetonitrile. At room temperature
14.4 g (52.3 mmoles) of hexadecanoic acid chloride were then
30 added while stirring vigorously. Within 1.5 hours the mixture
reacted exothermically. ~'he crude product precipitated on
cooling. It was filtered with suction and washed with water
-- 11 --
'1,'~7'7_3~8
uni.tl free from chloride.
1~.5 g (98.5~ of the theretical yield) of analyti-
cal.ly pure 2-(pentadecyl-carbonyl-amino)-2-bu-tenoic-n-butyl
ester were obtained.
CH3 C~=f-~-oc~2c~2c~2c~3
HN-~~ -CH2 - ~ C1~2 ) 1 3C~
CZ~45NO3(395,63)
%C %H %N
computecl: 72.86 11.46 3.54
o~tai.rled: 72.86 11.76 3.18
melting point: 66 ko 69C
lH-NMII (CDC13/TMS):
~ = 6,9 (s broad, lH) Nl3;
6,75 (q, lH) -CH=;
4,1 (t, 2l1) -COOCH2;
2,25 (t, 2H) -COCH2-;
1,75 (d, 311) CH3-CH=;
0 55 - 1,G5 ppm (m~ 36H) -(CH2,)l3c~3; (CH2)3 3
. , ' .
_xample 6
134 mg (0.5 mmole) of ammonium perrhenate, 7.85 g (50
30 mmoles) of 2-azido-pentanoic methyl ester, 50 mg (0.45 mmoles)
of hydroquniorle and 3.85 ~ (52.6 mmoles) of dimethyl formamide
were dissolved .in 50 ml of acetoni.tri.le, whereupon 10.6 g (52.3
- 12 -
~ '~779~38
mmoles) of 10-hendecenoic acld chloride were added ~t room tem-
peratllre while stirrillg vigorously. The mixture reacted exo-
therlllically within 30 minutes and was then further processed
analogously to Example 3.
The crude product thus obtained was distilled in high
vacuum (0.06 mbar) at an instrumellt temperature of 200 to 250C
in the bulb tube.
11.5 g (77!.8~ of the theoretical yield) of analyti-
cally pure 2-(decen-9-yl-carbonyl-amino)-2-pentanoic methyl
ester were obtained.
CH C~ CH=C-C-OCH3
HN-lcl-cH2-~cH2)7cH CH2
~C ~H ~N
computed: 69.129.89 4.74
20 obtained: 68.9110.16 4.36
melting point: 34 to 35C
-N~ (DMso/TMs~:
= 9,1 ~s, 1~1) NH;
6,3 (t, lH~) -CH=C0;
5.4 - 6~05 ~m, lH) -CH2-CH=CH2;
4,6 - 5~2 ~m, 2H) -CH=CH2;
3.6 (s, 3~1) -COOC~3;
1~65 - 2~5 (m, 6H) CH3CH2; -COCH2; -CH2=CH;
0~7 - 1,75 ppm (m, 1511) CH3; -(CH2)6.
b
\
~;~77988
Exan~le 7
146.6 rng (0.05 mmole) of potassium perrhenate, 7.85 g
(50 mmoles) of 2-aæido-pentarIoic methyl ester and S0 mg (0.45
mmoles) of hydroquinone were put into a reactor at 80C wi.th 25
ml of acetic ethyl ester. 5.93g (52.5 mmoles) of chloro acetyl
chloride, di.ssolved in 25 ml of acetic ethyl ester, were then
added dropwise within 30 minutes while stirring vigorously. The
reacti.on mixture was kept at 80C for a total of 2.5 hours while
sti.rri.ng vigorously, whereupon it was further processed as in
Example 3.
7.0 g t68.1% of the theoretical yield~ of analyti-
cally pure 2-chloro-methyl-carboxamido-2-pentenoic methyl ester
wre obtaiI-led.
O
C113C112CII=C~-C-OC113
o
C~ 2Cl(205,64)
..
%C %II %N ~Cl
computed: 46.72 5.84 6.81 17.24
obtained: 46.89 5.81 6.80 17.31
melting ponint: 62C
; II-N~IR (CDC13/T~IS):
= 7~85 (s broad, llI) NII;
6~75 tt, lH) -CH=;
4,15 (s, 2H) -CH2Cl;
3,~ (s, 3~1) COOC113;
l~7 - 2~65 (m~ 2H) -CIi2-;
1,U5 ppm (t, 313) CI13-.
- 14 -
1~77988
}`xample 8
. . ~
138.5 mg (0.5 mmole) of sodium perrhenate, 7.85 g (50
mmoles) of 2-azido-pentanoic methyl ester and 50 mg (0.45
mmoles) of hydxoqujnone were put into a reacto~ with 25 ml of
acetic ethyl ester at 80C. Within 30 minutes 7.75 g (52.5
mmoles) of dichloro acetyl chloride, dissolved in 25 ml of
acetic el:hyl ester, were then added dropwise while stirring
vigorously. The re~ction mixture was kept at 80C for a total
of 3 hours while stirring vigorously and then further processed
as in Example 3.
7.5 g (62.5~ of the theoretical yield) of analytically
pure 2-dichloro-methyl-carboxamido-2-pentelloic me-thyl ester were
obtained
CH3cH2c~ c-c OC1~3
HN-ICl-CIIC12
o
C81111N03C12(24'87)
~C ~H %N %Cl
computed: 40.02 4.62 5.8329.53
obtained: 40.22 4.62 5.8229.65
melting point: 85C
-NM~ (cDcl3/TMs):
= 7.8 (s broad, lH) Nll;
68 (t, lll) -CH=;
6,05 (s, 111) CHC12;
3~5 (s, 311) COOC~13;
1,75 -~2.6 (m, 2H) -CH2-;
1~1 ppm (t, 311) CH3-.
~7~7~88
Example 9
134 mg (0.5 mmole) of ammonium perrhenate, 9.26 g (50
mmoles) o~ 2-azi.do-pentanoic isopropyl ester and 50 mg (0.45
mmoles) of hydroquinone were put into a reactor with 25 ml of
acetic ethyl ester at 80C. Withi.n 45 minutes 5.93 g (52.5
mmoles) of chloro acetyl chloride, dissolved in 25 ml of acetic
ethyl ester, were then added dropwise while stirring vigorously.
The reaction mixture was kept at 80C~or a total of 4 hours
while stirring vigorously, whereupon it was further processed as
in ~xample 3.
7.3 g (62.5 ~ of the theoretical yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-2-pentanoic isopropyl
ester were o~tained.
Cfl3C112CII-f-C8-OCI~lC113
tHN-~ -C112Cl
O
No3cl~233,69s)
,,
~C ~H %N %Cl
computed: 51.39 6.90 5.99 15.17
obtained: 51.87 6.68 5.94 15.24
melting t~oint: 96C
: ,
= 7,85 (.s broad, 1ll) Nll;
6,65 (t, 11l) -Cll=;
4~7 - 5,65 (m, lH) - COOCII;
4,15 (s, lH) -CIIC12;
1,65 - 2~65 (m, 213) -CH2-;
1~3 (d, 6H) C-(CI13)2;
1,05 ppm ~, 311) Cl13-.
- 16 -
;
~L~717~8~
~xa~ le 10
134 mg (0.5 mmole) of ammonium perrhenate, 9.26 g (50
mmoles) of 2-azi.do-pentanoic isopropyl ester and 50 mg (0.45
mmoles) of hydroquinolle wele put into a reactor with 25 ml of
acetic ethyl ester at 80C. Within 70 minutes 7.75 g (52.5
mmoles) of dichloro acetyl chloride, dissolved in 25 ml of
ace1-.ic ethyl ester, were then added dropwise whi.le stirring
vigorously. The reaction mixture was kept at 80C for a total
oE 5 hours while stirring vigorously and then further processed
as in r,xample 3.
10.6 g (79% of the theoretical yield) of analytically
pure 2-clichloro-methyl-carboxamido-2-penta}loic isopropyl ester
were obtai.ned.
Cll3CI12CII=IC-C-O ~ 3
I~N-C-CflCl
1~ 15 3cl2(268~l4)
%C ~ N %Cl
computed: 44.79 5.63 5.22 26.44
obtained: 45.25 5.50 5.20 26.38
melting point: 110Ç
Il-NMR ~CDC13/T~IS):
= 7,85 (s broad~ lH) Nll;
6,7 (t, 1ll) -Cl3=:
6~0 (s, 113) CHC12;
4~75 - 5~4 (m, 1ll) COOC~I;
1,8 - ~2,5.(lll, 211) -Cll2-;
1,3 (d, 611) -C-(CII ) ;
1~05 ~t, 3l3) Cl13-.
- 17 -
~77~t~8
E:xaml>le 11
134 mg (0.5 mmole) of ammmonium perrhenate, 7.85 g (50
mlnoles) of 2-azido-pentanoic methyl ester and 50 mg (0.45 mmole)
of hyclroquinorle were put into a reactor with 25 ml of aceti.c
ethyl este.r at 80C. Within 30 minutes 9.3 g (52.6 mmoles) of
difluoro benzoyl chloride, dissolved in 25 ml of acetic ethyl
ester, were then added dropwise. The reaction mixture was kept
at 80C for a total!of 10 hours while stirring vigorously,
whereuE)on it was further processed as in Example 3.
9.75 g ~72.4~ of the theoretical y.ield) of
arlalytically pure 2-[2',6'-difluoro-phenyl)-carbonyl-amino]-
2-pen~arloic methyl ester were obtained
O
CH3cfl2cl~-c-c OC
HN-C~
F
Cl 2111 33NF2 ~ 269, ~s
%C %H %N
computed: 57.19 4.86 5.20
obtained: 58.12 4.58 5.13
melting l~oint: 138.5C
,
Il-N~ (CDC13/l'MS):
= 6~4 - 7~85 (m, 5H) aromat.-CH; -Cll= Nll;
3,8 (s, 3~1) COOCI13;
1,85 - 2,65 ~m, 2H) -C112-;
1,1 ppm ~t, 3H) C1l3-.
-`18 -
`` 1~77~8~
I~'Yan1P I e 12
146.6 mg (0.5 mmole) of po~assium perrhenate, 9.26 g
(5~ mmoles) of 2-azido-pentanoic isopropyl ester and 50 mg (0.45
mmoles) of hydroquinone were put into a reactor with 25 ml of
acetic ethyl ester at 80C. Within 30 minutes 9.3 g (52.6
mmoles) of 2,6-difluoro benzoyl chloride, dissolved i.n 25 ml of
acetic ester, were then added dropwise. The reaction mixture
was kept, at 80C for a total of 10 hours while stirring vigor-
ously, whereupon it was further processed as in Example 3.
10.9 g (73.3% of the theoretical yield) of analyti-
cally pure 2-[(2'6'-difluoro-phenyl)-carbonyl-amino]-2-pentanoic
isopropyl ester were obtained.
: Cll3CI~2CII=C-C-OC~ 3
¦ F CH3
0,~
F
%C %H %N
co~nput:ed: 60.60 5.76 4.71
obt;ained: 61.14 5.50 4.66
meltirlg ~oint: 91C
H-NMI~ (CDC13/TMS):
= 6.45 - 7.9 (m, 5il)aromatic'~CH; -CH=; NH;
4~7 - 5,4 (m, 113) COOCH:
1~75 - 2,65 (m~ 2H) -CH2-;
1,3 (d, 611) C-(CI13)2;
1~1 pplll (t, 3y) C1i3.
~.f~ 88
Example_13
536 mg (19.9 mmoles) of ammollium perrhenat:e, 31.4 g
(0.2 Inmole) of 2-azido-pentanoic methyl ester and 15.4 g (0.21
mmole) of dimethyl formamide were dissolved in 150 ml of
acetonitrile, ~hereupon 25.3 g (0.21 mmole) of 3-methyl-
butanoic acid chloride were added at room temperature while
stirring vigorously. The mixture reacted exothermically with-
in 45 mirlutes, whereupon it was further processed as in Example
3.
30.2 g (70.8~ of the theoretical yield) of analyti-
cally pure 2-[(2'-methyl-propyl)-carbonyl-amino]-2-pentanoic
methyl ester wexe obtained.
Il
Cli3C112Cll= ~C-c-ocll3~cH3
HN- -CH CH
2 ~C113
C111119N03t213'28)
%C %1l ~N
comuuted: 61.95 8.98 6.57
obtained: 61.84 9.01 6.51
melting pOillt: 45 to 47C
H-NMR ~C~C13/TMS):
= 7,05 (s broad, .lH) Nll;
6,65 (t, 1ll1 -Cl~=;
3,75 (s, 3il) -COOCH3;
1,7 - 2,6 (m, 5H) -C112-C=; ~-CH2-CII-;
1~15 (s, 3~1) Cll3-C112;
1,0 ppm (d, 611) C~cll3.
- 20 -
~ ~77~
xample 14
. .=
134 mg (0.5 mmole) of ammonium perrh~nate, 7.85 g (50
mmoles) of 2-azido-pentanoic methyl ester, 3.85 g (52.6 mmoles)
of dimethyl formamide and S0 mg (0.45 mmole) of hydroquinone
were dissolved in 50 ml of acetonitrile, whereupon 8.5 g (52.3
mmoles) of 2-ethyl hexanoyl chloride were added at room tempera-
ture while stirring vigorously. The reaction mixture was
sti~red for 120 hours, the exothermic reaction reaching a maxi-
mal ternperature of 80C.
The further processing was analogous to that of
Example 3. 'rhe crude product thus obtained was distilled in
high vacuum (0.06 mbar) at an instrument tempe,rature of 180 to
210C in the bulb tube.
7.0 g (54.8% of the theoretical yield) of 2-[(1'-
ethyle~rle-pentyl)-carborlyl-amino]-2-pentenoic methyl ester were
obta:ined .
' C113Cfl2C~ C-C-ocH3
3N-~ cEl-cll2cl32cH2c~l3
O C132C113
10 25 03~255,36)
~ %C %H %N
computed: 65.85 9.87 5.48
obtained: ~ 65.11 10.31 5.52
i-NMI~ (C~C13/~'MS):
~= 6,85 (s brad' lli) NH;
6~55 (t, 1ll) -C~3=;
~,7 (s, 3ll) -COOCI~3;
0~6 - 2~45 ppm (m~ 2011) Cll3C112-C=; -Clll-(CII2)3-C1l3.
C'12CI~3
- 21 -
1~77~t~B
llxample 15
__ ___.__
134 mg (0.5 mmole) of ammonium perrhenate, 7.85 g (50
mmoles) of 2-az:ido-pentanoic methyl ester, 3.85 g (52.6 mmoles)
of dimethyl formamide and 50 mg (0.45 mmole) of hydro~luinone wre
dissolved in 50 ml of acetonitrile, whereupon 7.8 g (52.5
mmoles) of 2-methyl-2-ethyl-butanoic acid chloride were added at
room temperature while stirring vigorously. The reaction mix-
ture was stirred foi 120 hours, the exot4ermic reaction reaching
a temperature of 80C. The further treatment was analogous to
that of Exar.lple 1. The oily crude product thus obtained was
distilled in high vacuum (0.06 mbar) at an instrument tem-
perature of 100 to 150C in the bulb tube.
6.4 g (53% of the theoretical yield) of 2-[(1'-
methyl-l'-ethyl-propyl)-carbonyl-amino]-2-pentanoic methyl ester
were obtained.
C133C132cl3=f- -OCll3
CH2CH3
I HN_C_C_CH2CH3
O C13
3 '
C131123NO3(241~33)
%C 9~H 96N
comput:ed: 64.709.,61 5.80
obtaitled: ' 63.7010.16 6.25
-N~I~ (cDCl3/TMs):
= 7,15 (s broac~, lH) Nll;
6~65 (t, lH) -CH=;
3,75 (s, 311) -COOC133;
0,65 - 2,35 ppm (~, 1813) C113C132-C=; -C-(CH2C133)2.
C113
.~
77~38
ExamL)le 16
134 mg (0.5 mmole) of ammonium perrhenate, 7.85 g (50
mmoles) of 2-azido-pen-tanoic methyl ester, 50 mg (0.45 mmole) of
hydroquinone and 3.85 g (52.6 mmoles) of dimethyl formamide were
dissolved in 50 ml of acetic ethyl ester. 8.5 g (52.9 mmoles)
of thiophene-2-acetic acid chloride were then added dropwise at
roo~n temperature while stirring vigorously. Within 30 minu-tes
the mixture reacted!exothermically.
When the generation of gas was terminated the reaction
mix-ture was diluted with 50 ml of acetic ethyl ester, shaken out
with a 0.5 ~lolar sodium hydrogen carbona-te solution and the
organic pllase was once more washed with water and dried over
sodium sulE~hate. On removing the drying agent the concentrated
organic phase was crystallized with n-pentane.
12.1 g (95.5% of the theoretical yield) of analyti-
cally pure 2-(thenyl-carbonyl-amino)-2-pentanoic methyl es~er
were obtained.
~, O
Cl33c~l2c~3=f-c-ocl33
13N-7-C13--
C12l~l5NO3S(253,26)
~C %1l %N %Cl
computed: 56.91 5.97 5.53 12.63
obtained: 56.80 5.86 5.66 12.63
meltirlg point: 67 to 70C
- 23 -
~'~77~8
Il-NI~ (CDC13/TMS):
J` = G,B5 - 7,5 ~m~ 411) Nll; 1~;
G,65 (t, 111) Cll=;
3~9 (s, 211) COCE12;
3,75 (s, 31~) COOCH3;
1,65 - 2~55 (m, 2~ C112-;
1~05 ppm (t, 31~) CH3-.
!
Examp e 17
134 mg (0.5 mmoles) of ammonium perrhenate, 7.85 g (50
mmoles) of 2-azido-pentanoic methyl ester, 50 mg (0.45 mmole) of
hydro~uinone and 3.85 g (52.6 mmoles) of dirnethyl formamide were
reacted Wit}l 9.5 g (52.6 mmoles) of 3-acetyl-thio-2-methyl-pro-
piol~ic acicl chloricle analogously to Example 16.
On further E~rocessing as in Example 16 10.9 g (79.8%
of the theoretical yield) of analytically pure 2-[(1'-methyl-
2' acetyl-thio-ethyl)-carboxamido]-2-pentanoic methyl ester were
obtained .
o
C113C112C~ C-C-OC113
IIN-C-CII-CH2-S-C-CII
O cll3 O
( 12lll9N04S(273,29)
%C I g61~ ~6N 96Cl
.,' ';
computed: 52.74 7.01 5.12 11.71
obtained: 52.46 7.01 5.22 11.56
rnelting pOillt: 56 to 58C
-- 24 --
~'~775~
111-NM1~ ( CDC1 3/~rMs ~:
= 7,55 (s broad, 1~l) Nll;
6,65 (~, lli) Cl~=;
3,8 (s, 3~) COOC1~3,
3,1 (t, 21~) S-CII2~;
2,4 ~ ?-9 (m, lH) COCH;
2~35 (s, 3HJ COCH3,
1,8 - 2~4 (m, 2~3) ~CH2-;
1,25 (d, 3H) CH-CH3;
1,05 ppm (t, 3H) Cll3-CI12.
~xamp e 18
134 mg (0.5 mmole) of ammonium perrhenate, 9.25 g (50
mmoles) of 2-azido-pentanoic isopropyl ester, 3.85 g (52.6
mmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hydro~uinone were dissolved in 50 ml of acetonitrile, whereupon
12.35 g (52.5 mmoles) of 2,6-dichloro cinnamic acid chloride are
added at roorn temperature while stirring vigorously. The
reaction mixture was stirred for 16 hours while the product
precipitated. The residue was separated, washed with water
until free from chloride and dried.
12.5 g (70.2% of the theoretical yield) of analyti-
cally pure 2-[2'-(2,6-dichloro-phenyl)-vinyl-carbonyl-amino]-
2-pentanoic isopropyl ester were obtained.
: '' O
' CH3cl~2cl~=c-e'
H1-lCI-CH-CH
Cl
30C17~19NO3C12(356,27)
\
- 25 -
~C %1l ~N%Cl
computed: 57.31 5.37 3.53 19.90
obtained: 57.31 5.76 4.06 18.45
melt:ing point: 166C
', 1
Il-NMI~ ~CDC13/TMS):
,. ~
~ = 7,75 (d, lH) -COCH=;
6,B5 _! 7,5 (m, 4~) NH; ,aromatic CH;
6~65 (d, 113) -C=CH-;
6,55 (t, 1~l) C~12-C~I=;
4,65 - 5,3 (m, lH) COOCH-;
1,85 - 2,5 (m, 2H) -CH2-;
1,25 (d, 611) C-(CI13)2;
1,05 ppm (t, 311) CH3-.
E, ml~le__9
27.3 mg (0.1 mmole) of sodium perrhenate, 1.71 g (10
mmoles) of 2-azido-hexanoic methyl es-ter and 767.6 mg tlO.5
nmloles) of dimethyl formamide were dissolved in 1 ml of acetoni-
trile, whereupon 1.19 g (10.5 mmoles) of chloro acetyl chloride
were added at room temperature while stirring vigorously. The
mixture reacted exothermically within 30 minutes and was then
further processed as in Example 3.
1.8109 y (82.4~ of theoretical yield) of analyti-
cally ~ure 2-chloro-methyl carboxamido-2-hexenoic methyl ester
were obtained.
'' ' O
3 2C~l2 ~C
, 11 . C02C113
:
C9~ clNo3(2l9~66B)
- 26 -
77988
%C ~H %N %Cl
cornpu~ed: 49.21 6.42 6.38 16.14
obtained: 49.21 6.33 6.41 16.15
melting point: 63.5 to 64C
.~ lH-NMR (CDC13):
= 8,0 (s, lH) NH;
6,86 (t, lH)-CH2-CH=;
4~19 (s, 2H) C112Cl;
3,81 (s, 3H) OCH3;
2,18 ~q, 2H)-C1l2-CH=;
1,8 - 1,28 (m, 211) CH3-CH2-;
0~95 ppm (t, 3H) CH3-CH2.
ln(KBr) ~NII = 3255 cm 1,
~CO = 1722 cm 1,
1664 cm 1.
- Example 20
_.
134 Ing (0.5 mmole) of ammonium perrhenate, 9.25 g (50
mmoles) of 2-azido-hexanoic ethyl ester, 3. 85 g (52.6 mmoles) of
dirnethyl formantide and 50 mg (0.45 mmole) of hydroquinone were
dissolved in 50 rnl of acetic isopropyl ester, whereupon 5.95 g
(52. 6 mmoles) of chloro acetyl chloride were added at room
temperature while stirring vigorously. The mixtures reacted
exothermically withln 40 minutes. The mixture was further pro-
cessed as in Example 16.
8.7 9 (74.,4% of the theoretical yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-2-hexenoic ethyl ester
were obtainecl.
" C11 3C11 2C~1 2C~=f -l-oc~l 2cll 3
HN-I~-c1~2cl
Clolll6No3cl(233~69)
- 27 -
.
~'~775~
%C ~1l %N ~Cl
comput:ed: 51.40 6.90 6.0 15.17
obtained: 51.56 6.83 6.06 15.23
meltillg point: 49 to 49.5C
N~ (cDcl3/TMs)
= 7,9 (s ~roaa, lH) NH;
6,75 (t, lH) -CH=;
4,2 (q, 211) -CH2Cl;
1.8 - 2~4 (m~ 2EI) -C112-CH=;
1~2 - 1.8 ~m, 2H) CH3-CH2-;
1,3 (t, 311) COOC~12Ci~3;
0~9 ppm (t, 3H) CH3-CH2.
Exam~le 21
a) 134 mg (O.S mmole) of a~mnonium perrhenate, 9.25 g
(50 mmoles) of 2-azido-hexanoic ethyl ester, 5.2 g (52.5 mmoles)
of N-methyl pyrrolidone and 50 mg (0.45 mmole) of hydroquinone
were put into a reactor with 50 ml of acetonitrile, whereupon
7.75 g (52.5 mmoles) of dichloro acetyl chloride were added at
room temperature while stirring vigorously. The mixture reacted
exothermically within 20 minutes. It was further processed as
in Examule 3.
~ 10.1 g (75.3% of the theoretical yield) of analyti-
- cally pure 2-dichlor4-methyl-carboxamido-2-hexenoic ethyl ester
were obtained.
b) 134 mg (0.5 mmole) of ammonium perrhenate, 9.25 g
(50 mmoles) of 2-azido-hexanoic ethyl ester, 6.1 g (52.5 mmoles)
of tetramethyl urea and 50 mg of hydroquinone were reacted with
7.75 g (52.5 mmoles) of dichloro acetyl chloride analogously to
a).
9.4 g (70.1% of the theoretical yield) of analyti-
- 28 -
,_ '1,~7798~ '
cally r)ure 2-dichloro-methyl-carboxamido-2-hexenoic ethyl ester
were obtained.
c) 134 mg (0.5 mmole) of ammonium perrhenate, 9.25 g
(50 Inllloles) of 2-azido-hexanoic ethyl ester, 9.4 g (52.5 mmoles)
of N,N,N',N', N', N" of hexamethyl phosphoric acid triamide and
50 mg (0.45 mmole) of hydroquinone were reacted with 7.75 g
(52.5 mmoles) of dichloro acetyl chloride analogously to a).
7.3 g (54.!5% of the theoretical yield) of analyti-
cally pure 2-dichloro-methyl-carboxamido-2-hexenoic ethyl ester
10 were obtained.
CH3cl~2cl~2cll=c~ -C-OCH2ClH3
HN-C~CHC12
O
clol~l5No3cl2(268l14)
96C 96EI 96N ~6Cl
computed: 44.79 5.64 5.2226.44
obtained: 44.61 5.30 5.2026.31
melting point: 75C
H-NMII (CDC13/TMS)
= 7~9~ (s b~ad~ lH) NH;
6,8 ~t, lHJ -CH=;
6,05 (s, 11~) -C~C12;
4~25 (q, 2H) COOC112-;
1,9 - 2,4 (m, 211) -C112-CII=;
1,2 - 1,85 (m~ 211) C113-C112-;
1,35 (t, 31~) -CO0C132CE~3;
0~95 ppm~(t, 3H) CH.~-CH2.
-- 29 --
~ 77~8f~
Example 22
134 mg (0.5 mmole) of ammonium perrhenate, 9.95 g
(49.9 mllloles) o~ 2-azido-octanoic methyl ester, 3.85 g (52.5
mmoles) of dimethyl formamide and 50 mg (0.45 mmole) of hydro-
qui.none were dissolved in 50 ml of acetonitrile, whereupon 5.95
g (52.6 mmoles) of chloro acetyl chloride are added at room
temperature while stirri.ng vigorously. The mi.xture ,reacted
exothermically wlth~n 25 minut,es. It'was further processed as
in F,xample 3.
10.8 g (87.3% of the theoretical yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-2-octenoic m~thyl ester
we~e obt:ained.
C113C~12C112C~12C~2C~ C-C-OC~13
N-lC-Cll2C
C~ 8No3cl(247~72)
%C ~}l %N %Cl
computed: 53.33 7.32 5.65 14.31
obtai.ned: 53.29 7.41 5.52 14.27
melting point: 52.5C
Il-NMI~ ( CDCl 3/TMS):
= 7,85 (s bLoad, lll~ NH;
6~8 (t, 1ll) -Cll=;
4~2 (s, 2~) -c~2Cl;
3~85 (s, 31~) -COOCI-~3;
2~2 (dt, 2ll) -Cl12CII=;
0 65 - 1~85 ppm (m, 911) CH3CII2C1l2C 2
- 30 -
~2~77988
Example 23
246 mg (0.5 mmole) of tetra-n-butyl ammonium
perrhenate, 9.95 g (49.9 mmoles) of 2-azido-octanoic methyl
ester, 3.85 g (52.6 mmoles) of dimethyl formamide and 50 mg
(0.45 mmole) of hydroquinone were dissolved in 50 ml of
acetonitrile, whereupoll 7.75 g (52.5 mmoles) of dichloro acetyl
chloride were added at room ~erat ~ while stirring vigor-
ously. The mixtuL~e!immediately reacted exothermically. The
further treatment was like that in Example 3.
10.4 g (73.85~ of the theoretical yield) of analyti-
cally pure 2-dichloro-rnethyl-carboxamido-2-octenoic methyl ester
were obtained.
Cl13C~2C~l2cll2cll2cll=f-c-ocil3
o
, .
Cll11l7NO3Cl2(282,17)
%C ~ Cl
computed: 46.826.07 4.9625.15
obtained: 46.886.22 4.8724.63
meltitlg point: 57C
, , , i ,
lll-NMR (CVC13/TMS):
.
= 7,8 (s broad,, lH) NH;
6~75 (t, lH) -CH=;
5,95 (s, 1ll! -C~IC12;
3,8 (s, 3H) -COOCH3;
2,15 (dt, 2H)~ -Cll2-CII;
0,55 - 1,75 ppm (m~ 91l) CH3C1l2CH2CH2~-
- 31 -
1~7988
Exalr~le 24
. .
246 mg (0.5 mmole) of tetra-n-butyl ammonium
perrhen~te, 11.35 g (49.9 mmole) of 2-azido-octanoic isopropyl
ester, 3.85 g (52.6 mmoles) of dimethyl formamide and 50 mg
(0.45 mmoles) of hydroquinone were dissolved in 50 ml of acetic
ethyl estex, whereupon 5.95 g (52.6 mmoles) of chloro acetyl
chloride were added at room temperature while stirring
vigorously. The mixture reacted exothermically within 1 hour.
It was further treated as in Example 16. 10.5 g (76.3% of the
. 10 theoretical yield) o~ analytically pure 2-chloro-methyl-car-
.(: boxamido-2-octenoic isopropyl ester were obtained.
C~3CH2CI~2CI~2C~I2C~1 f C ~ C113
IIN-ICl-Cl~2C
, O
3l~22NO2C1(275,78)
i~:
;~ %C %H %N ~Cl
computed: 56.628.04 5.079 12.85
obtained: 56.558.32 5.00 12.83
- mel~.ing point: 55C
~'
Il-N~ ( CDCl 3/TMS):
= 7~85 (s b~oad; lH) NH;
: 6,7 (t, 113) -Cll=;
5~35 - 4,7 (m, lH) -COOCII-;
4,1 (s, 2~l) -C1~2Cl;
2,1S (dt, 211) -CH2-CH=;
~,.: 30 1~35 (d, 6H) -CH-(CH3)2;
,,, 0,5 - 1,8 ppm (m, 913) CH3CH2CH2C~12--
.. ...
. ~,
- 32 -
:
t77988
L~`xamE~le 25
_ ___,_ __ _
246 nlg (0.5 mmole) of tetra-n-butyl-ammonium per,r-
llenate, 11.35 g (49.9 mmoles) of 2-azido-octanoi,c i.soproE~yl
ester, 3.85 g (52.6 mmoles) of di.methyl formamide and 50 mg
(0.45 mmolé) of hydroquinone were dissolved in 50 ml of ace-
toni-t~ile, whereupon 7.75 g (52.5 mmoles) of dichloro acetyl
chloride were added at room temperature while stir.ring vig-
orously. The reaction mixture was stirred for 18 hours a-t room
ternperal..ure and was then further treated as in Example 3.
12.3 g (79.3~ of the theoretical yield) of analy-
tically pure 2-dichloro-methyl-carboxamido-2-octanoic iso-
propyl ester we.re obtained.
O
3 2 2 2 2CEI C C OC~
I C113
IIN-C-CIIC 1
' Cl3ll2lNo3cl2(3lo~22)
.
%C ~H %N ~Cl
cornpuled: 50.33 6.82 4.51 22.85
obtained: 50.11 6.96 4.50 22.77
meltirlg point: 88C
-NMI~ (cDcl3/TMs):
= 7,9,5 (,S br3ad, 11l) NH;
6~8 (t, 1ll) -Cll=;
6,05 (s, 1ll) -CIIC12;
5,4 - 4,7 (m, 1ll) -COOCII-;
2,15 (dt, 2H) -Cll2-CH=;
1,35 (d, 611) -Cll-(CH3)2;
0 6 - 1~8 ppm (m, 9ll) Cll3CII2C 2 2
.
," - 33 -
77988
xaml~le 26
134 mg (0.5 mmole) of ammonium perrhenate, 9.85 g
(49.9 mmole) of 2-azido-3-cycloperltyl propionic met~lyl ester,
3.85 g (52.6 mmoles) of dimethyl formamide and 50 mg (0.45
mmole) of hydroquinolle were dissolved in 50 ml of acetonitrile,
whereupon 59.3 g (52.5 mmoles) of chloro acetyl chloride were
added at room temperature while stirring vigorously. The re-
action mixture was !stirred for 13 hours at room temperature and
then fuxther treated as in Example 3.
6.9 g (56.3% of t:he theoretical yield of analytically
pure 2-chloro-methyl-carboxamido-3-cyclopentyl propenoic methyl
ester were obtained.
~ CII=C- -OCE~
HN-f-CH Cl
o
C~ l6No3cl~245~7l)
~C %H ~N ~Cl
computed: 53.77 6.56 5.70 14.43
o~tained: 53.61 6.63 5.29 14.78
melting ~oint: 65.5C
lH-NMR ~CDC13/l'MS):
., I
~= 7,8 ~,sb ~ d, lH) Nll;
~ 6,7 ~d, lH) -CH=;
4,15 Is, 211) -CH2Cl;
~: 3,8 (s, 3H) COOCH3;
2,15 - 3,1 (s breit, 113) C,CH;
1,0 - 2.2 ppm ~m, 8ll) H ~ il
- 34 -
1'~77988
~_amL~le 27
134 mg (0.5 mmole) of ammonium perrhenat:e, 9.85 g
(4~.~ mmole) of 2-azi.do-3-cyclopentyl-propionic metllyl ester,
3.85 g (52.6 mmoles) of di.methyl formamide and 50 mg of (0.45
mmole) of hdyr~quinone were dissolved in 50 ml of acetonitri.le,
whereupon 7.75 y (52.5 mmoles) of dichloro acetyl chloride were
added at room temperature while stirring viyorously. The re-
action was stirred.for 15 hours a~ room cemperature and then
further processed as in Example 3.
10.9 g (77.9% of the theoretical yield) of analyti-
10 cally pure 2-dichlor-metllyl-3-cyclopentyl-propenoj.c methyl ester
we~e obta.ined.
/~ U
~CEE= ~C-C-OCH3
EIN-C-CHCl z
~ O
Clllll5No3cl2l28o~l5)
~C %H %N %Cl
20 computed: 47.16 5.40 5.00 25.31
obtained: 47.06 5.32 4.98 25.00
melting point: 85 t.o 86C
NMI~ (CDC13/TMS):
= 7,75 (s broad~ lH) Nll;
6,75 (~d, lH) -CH=;
6,0 (s, lH) -CHC12;
3, 8 ( s, 311 ) COOCIE3;
2,25 - 3,0 (s breit, 1ll) CC,CH;
0,9 - 2,2 ppm (m, 8H) EI~EI -
-- 3s --
Hixalnt~le 28 ~ ~77988
134 mg (0.5 mmole) of ammonium per,rhenate, 11.25 g
(4~.9 mMoles) of 2-a~ido-3-cyclopentyl propionic isopropyl
ester, 3.85 g (52.6 mmoles) of di.methyl formamide and 5U mg
(0.45 mmole) of hydroquinone were dissolved in 50 ml of
acetonitri.le, whereupon 5.93 g (52.5 mmoles) of chloro acetyl
chloride were added at room temperature while stirring vig-
orously. The Mixture reacted exother~ically within 30 minutes.
The mixture was fur~her processed as in Example 3.
11.2 g (81.8% of the theoretical yield) of analytically pure
2-chloro-methyl-carboxamido-3-cyclopentyl propenoic isopropyl
ester were obtained.
O- Cll=f-C-OCII '3
HN- lC~ -C112C
O
13~l2oNo3cl~273~76)
~C %H %N %Cl
com~uted: 57.04 7.36 5.12 12.95
obtained: 57.04 7.71 5.15 13.01
~:: melting point: 72C
tl-NMn '(CDC13/~'MS):
= 7,75 ~s broad, 111) NH;
' 6,65 (d, 111) -CH=;
4,7 - 5,35 (m, 111) COOCH;
: 4.15 (s, 211) -CH2Cl; -C
2.25 - 3.0 (s.breit, lH) c`CII;
- 2,15 (m, 811)11t_t~H
1~
1,30 ppm (b, 6tl) COOC'cl'l3.
-- 36 --
7'7988
xam~le_29
134 mg (0.5 mmole) of ammonium perrhenate, 11.25 g
(49.9 mmoles) of 2-azido-3-cyclopentyl propionic iso~ropyl
ester, 3.85 g (52.g mmoles) of dimethyl formamide and 50 mg
(0.45 mmole) of hydroquinone were dissolved in 50 ml of
acetonitrile, whereupoll 7.75 g (52.5 mmoles) of dichloro acetyl
chloride were added at room temperature while stirring vigor-
ously. The mixture!reacted exo~hermically within 45 minutes.
The mixture was further processed as in Example 3.
8.7 g (56.6~ of the theoretical yield) of analyti-
cally l~ure 2-dichloro-methyl carboxatnido-3-cyclopentyl-propenoic
isopropyl ester were obtained.
O- CH=T-C--C"
HN-~-cllcl ?
: o
C131ll9NO3C12(308,205)
%C %H %N ~Cl
comput.ed: 50.66 6.21 4.54 23.00
s obtai.ned: 50.82 6.26 4.63 22.79
melting point: 99C
, 1
Il-N~ (CDC13/l'~lS):
.
J= 7,85 (s brad , lH) Nll;
6~75 (d, lH) -Cll=;
6.05 (s, 1l~) CIIC12;
4,7 - 5,4 ~m, lH) COOCII;
2,25 - 2~95 (s breit; 1ll) cC`CH;
2~2 (n~, 8l~)~H ~ 1l;
1,35 ppm (d, 6H) COOC~cll3.
- 37 -
, :: ::
.
~ ~7~7988
Lxa~ le 30
134 mg (0.5 mmole) of ammoni.um perrhella~e, 10.55 g (50
mmoles) of 2-azido-3-cyclohexyl-propionic me-thyl ester, 3.85 g
(52.6 mmoles) of di.methyl formamide and 50 mg (0.45 mmole) of
hydroclulnone were dissolved in 50 ml of acetic-n-pxopyl ester,
whereupon 5.93 g (52.5 mmoles) of chlo~o acetyl chloride were
added at room temperature while stirring vigorously. After one
hour ~he reactio~ miY~ture was maximally exothermical and was
then further processed as in Example 16.
10 10.3 g (79.3~ of the theoretical yield) of analyti-
cally pure 2-chloro-methyl carboxamido-3-cyclopentyl propenoic
methyl ester were obtained.
o
-C-OC~3
~N-ICI-CH2C
O
C12~N03Cl(259~73)
%C %11 %N %Cl
comEJuted: 55.49 6.995.39 13.65
obtained: 55.39 7.284.75 13.83
meltillg pOillt: 117 to 118C
; '
Il-NM~ (~DC13/T~ISJ:
= 7,7 (s broad,~ 1 H) N)3;
6.6 (d, lH) -CH=;
4,15 (s, 211) -CH2Cl;
3,85 (s, 311) COOCH3;
0,75 - 2,6 ppm (m, 1113) ~ .
- 3~ -
7798~
I~'xaml~le 31
134 mg (0.5 mmoles) of ammollium perrhellate, 10.55 g
(50 mmoles) of 2-azido-3-cyclohexyl propionic methyl ester, 3.85
g (52.6 rnmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hydro(luinone were dissolved in 50 ml of acetonitrile, whereupon
7.75 g (52.5 mmoles) of dichloro acetyl chloride wer~ added at
room -cemperature while stirring vigorously. The reaction
mixture was stirred!for 16 hours at ~oom temperature and was
then further processed as in Example 16.
11.7 g ( 79.5% of the theore-tical yield) of analyti-
cally pure 2-dichloro-methyl-carboxamido-3-cyclohexyl propenoic
methyl ester were obtained.
o
acll=~c-c_Oc~l3
~IN-C-CIIC 1
12l3l7No3cl2~294ll8J
%C ~H %N %Cl
computed: 48.99 5.82 4.76 24.10
obtalrled: 48.95 5.91 4.70 24.06
melting point: 98C
1ll-N~ (CDC13/T~S):
= 7,65 (s bL~ad~ 111) N13;
6,65 (d, 111) -Cll=;
5,95 (s, 111) -C}12Cl;
3,8 (s, 311) COOC113;
1,9 - 2,65 (s breit, 111)C`C-H;
0"7 - 2rQ pplll ~m, 1013)~3337~11 .
'79~8
_Y~ mple 32
13.7 mg (0.05 mMole) of sodium perrhenate, 715.7 my (S
mmoles) of 2-azido-hutanoic rnethyl ester and 383.8 Ing (5.25
mmoles) of dimethyl formamide were dissolved in 5 ml of
acetoIIitrile, whereupon 1.195 g (5.25 mmoles) of 2-(2',2'-
dichloro-etheIlyl)-3,3-dimethyl-cyclopxopane carboxylic acid
chloride were added at room temperature while stirring vigor-
ously. The reaction mix-ture was stir~ed for 30 hours at room
temperat:ure, whereupon it was further treated as in Example l.
1.31 g (85.6~6 of the theoretical yield) of analyti-
cally pure 2-[2'-(2,2-dichloro-ethenyl)-3',3'-dimettlyl]-cyclo-
p~oL)a~ -carboxamido-2-hexelloic methyl ester were obtained.
H /CO2CfI3
113C NII-ICl ~CII=CC12
I~3C CIJ3
C13II17C12NO3~306,189)
%C %H 96~1 %Cl
compuled: 51.00 5.60 4.57 23.16
obtained: 50.79 5.66 4.29 23.11
meltiIly point: 109 to 109.5C
Il - NMII (CDC13)
~= 7,32 (s, lII) Nll; IR ~rNII = 3320 cm,
6,8 (q, lII) li-C(CII3)=; ~rcO = 1720 cm,
6,42 (d, lII) II-C=CCL2; 1658 cm
3,77 (s, 311) OC113;
1,76 (~3, 3II)IJ3C-C~I=;
1,28 (s, 311) ~ \\7
1,25 ppln (5~ 3~)J~t3c~cIl3
~'~77~
I`xa~ )le 33
_ _ _ _ _ _
13.7 mg (0.05 nImole) of so~i.um perrhenate, 856 mg (5
mI;loles) of 2-azido-hexanoic methyl ester and 383.8 I~Iy (5.25
mmoles) of ~i.methyl formamide were dissolved in O.S ~ll of
acei-oIli.tri]e whereupon 1.1945 g (5.25 mmoles) of 2-(2'-2'-
dichl.oro-ethenyl)-3~3'-dimethyl-cyclopropane carboxylic acid
chloride we.re added at room 'ernpe-rature whi.le sti.rring vigor-
ously, The reaction mixture was kept for 15 hours at room
ternL~erature and then further treated as in Example 1.
1.40 g (83.8% of the theoretical yield) of analyti-
cally pure 2-[2'-(2,2-dichloxo-ethenyl)--3'-3'-dimethyl]-
cycloproparle-carbox~mido-2-hexelloic methyl ester were ob-tained.
Il ~ C02C113
C=C 11 1]
CII3CI12C1l2 NII-C ~ ~ CI3=CC12
O ~'~
~13C Cil3.
lslJ2lcl2No3~334~243)
%C %~I %N %Cl
comL~uted: 53.90 6.33 4.19 21.21
obtained: 53.62 6.51 3.98 2~.. 44
melt~ g L~oint: 105 to 106C
I3-N~ CDC13)
= 6,97 (s, 113) NII; IR ~NI3 = 3300 cm
G,73 (t, 113) -CII2-CII=; ~CO = 1715 cm 1,
6,43 (d, lII) I3-C=CC12; 1655 cm
3~8 (s, 3II) OCII3;
2 38 - 0,77 (m, 9I3) CII3CII2CI32;
1~3 (s, 311)
1~27 ppn~ (s~ 3II)J ~13C/~C~33
l~7~7~8~
I:xaml~le 34
13.7 Ing (0.05 mmole) of sodium perrhenate, 1.0261 y (S
n:moles) of 2-azido-3--phellyl propanoic rnethyl ester and 383.8 mg
(5.25 mnloles) of di.nlethyl formamide were dissolved in 5 ml of
acel:onitrile, ~hereupon 592.9 rng (5.25 mmoles) oE chloro acetyl
chlorLdewere added while stirring vigorously. The reactiorl
mixture was stirred for 7 hours at room temperature and then
fur:ther processed as in rxarnple 1.
1.1657 g (91.9% of -the theoretical yield) of analyti-
cally I~ure 2-chloro-methyl carboxamido-3-phenyl propenoic methyl
esle~ ~/ere obtained.
6115~ N~l_C_
~C=C
Il 2CH3
No3(253~6g5)
~C %H ~N %Cl
compu~ed: 56.82 4 77 5.52 13.98
obtai.ncd: 56.90 4.83 5.38 13.93
mel~:ing point: 113.5 to 114C
-N~ (C~C13)
(m, 511) aromat~C -Cll Nll ~ 3235 cm
4,09 (s, 2H) Cll2Cl; CO 1617 cm 1
3,~2 ~plll (s, 311) Cll3.
- 4~ -
77~
k'xample~ 35
_ _ _ _
13.7 mg (().05 mmole) of sodium perrhenat~, 1.()261 mg
(S mllloles) ol 2-azjdo-3-pherlyl pro~anoic rme~h~l estel- and 383.8
ng (5.25 mrnoles) of dimethyl formamide were dissolved in 5 ml of
acel:onitrile, whereupon 1.1945 g (5.25 mmoles) of 2-(2',2'-di-
chloro-ethenyl)-3,3-dimethyl cyclopropane carboxylic acid
chloride were added at room temperature while stirring vigor-
ously. The reaction~mixture was stirred for 15 hours at roorm
~empcrature and then further processed as in Example 1. 1.7136
g (93.1 ~ of the theoretical yield) o~ analytically pure 2-[2'-
(2,2-dichloro-ethellyl)-3',3'-dimethyl]-cycloproparle-carboxamido-
3-phenyl propenoic methyl ester were obtained.
~C02C1~3
C=C 11 11
C61~5 \NII-C~CII=CC12
113C C113
~:l8lll9cl2No3(368~26)
~C ~l %N %Cl
comput:ed: 58.71 5.20 3.8019.25
obtain~d: 58.61 5.12 3.8419.27
melting point: 127 to 128C
Il-NMI~ ( CI)Cl 3 ~:
~= 7,65 - 7,15 (m, Sll) aromabC-C~l=; lR ~NII = 3270 e~
6,35 (d, 111) -Cll=CC12;~CO = 1720 cm ,
3,85 (s, 311) OC113;1635 em 1.
1,25 (s, 3H)
1,23 pum (s, 311~ ~
1~3 ~113
- 43 -
~ t7'7988
I~'xan~)le 3 fi
27.3 mg (O.l mmole) of sodi.um ~er~henate al1d 1.57 g
(10 mrnoles) o:E 2-azido-3-methyl-butanoic methyl estex we~e put
i l~tO a reactor with 5 ml of acetic ethyl ester at 80C, where-
UpOIl ~ g (10.5 mmoles) of chloro acetyl chloride, dissolved
in 5 Inl of acetic ethyl ester, were added dropwise within 20
m.inu~es while stirring vigorously. 'rhe reaction mi.xture was
then ]ce1~t at 80C for 3 hours while stirring vigorously ar1d then
further processed as in Example l.
l.97 g (95.8~ of the theoretical yield) of analyti-
cally ~Jure 2-chl.oro-methyl-carboxamido-3-methyl-2-butenoic
metllyl esl~e~ we~e obtained.
3\C C/ 2 3
C133/ NH-Ii_CH2C
C~ 2ClN03 (205,641)
ZO %C %~1 %~ %Cl
com)uted: 46.73 5.88 6.81 17.24
obtained: 46.76 S.79 6.75 l7.34
mell:.ing pOillt: 106C
IJ-NMI~ ( CDCl ~
cS = 7,83 (s, 111) N13; I~ `rN}1 = 3275 cm
4,17 (s, 211) C132Cl; ~CO = 1719 cm,
3,8 (s, 311) OC113; 1660 cm
2,22 (d, 313) 113C~_ ;
`N
1,89 ~pln ~s, 311)~113c~ `N.
-- 4* --
7'7'~
I'.XCIIII~ 37
13~ g (~.5 mmole) of am~loni,um perrhe~ e, ~.s5 g (49.9
nlmol(-~s) (~ a%:i~lo-3-mel~ y] butarl~ic e~:tlyl es~,er, 4.6 g (52.8
Mmoles) of butanoic ethyl esteI, 416 g (52.8 ~moles) of diemthyl
acetam:ide and 5~ Illg ~ O . 45 mmoles~ of hydroquinone were put into
a react:oi- with 50 ml of acetonitrile, whereupon 5.95 g (52.6
Mmoles) of chloro acetyl chloride were added at rool~ ter,peratue
while stir ing vigorously. The mixture reacted exothermically
witllin 15 minutes.
The further treatment was analogous to that of Example
3.
9.35 g (85.3% of the theoretical yie]d) of analyti-
cally pure 2-chloro-Methyl-carboxamido-3-methyl-2-butenoic ethyl
ester were obtained.
C113 q
' ~c=c-c-oc~l2cl~3
Cll ~IN-C-CI~ 2C 1
Cglll~NO3Cl(219~67)
%C%1l ~N %Cl
computed: 49.21 6.42 6.38 16.14
obl:ained: 49.11 6.52 6.31 16.29
melting pOillt: 117CC
lI-NMIi(CDC13 /TMS ):
~= 7,7 (s kroad, lH) N)l;
4,15 (q, 211) COOCI12-;
4,1 (s, Zll) -C112Cl;
2,2 Is, 3Ji) Cl~3-C=;
1,~5 (s, 311) C113-C=;
1,25 U~ (t, 311) COOCII2CI13.
1,~77988
~ rll~l~ 3~
____ _ ._
134 Ing (O.S mmole) of ammollium perrhenate, ~.55 g
(4~.'3 Inl;loles) of 2-a~ido-3-methyl hulanoic etllyl ester, 3.~5 g
(52.6 r,lrlloles) of dimethyl formamide and 50 mg (0.45 mlnoles) of
hydroquinol-le we~e E)ut into a reactor with 50 ml of acetonitrile,
whereu~on 7.75 g (52.5 mmoles) of dichloro acetyl chloride were
added at ~oorn temperature. The mixture reacted exothermically
witll:irl 40 minutes. ,It was further processed as in Example 3.
9.8 g (77.3~ of ~he theoretical yield) of analyti-
cally pul-e 2-dichloro-methyl-carhoxamido-3-methyl-2-butenoic
ethyl est:er were obtained.
Cl3 O
3\ \ 11
~C= IC-C-OC112C113
C113 llN-l CEIC 2
13 3 2~ 54,11)
~C ~l-l %N ~Cl
col~lputed: 42.54 5.16 5.51 27.90
ohtailled: 42.20 5.19 5.44 27.58
meltirlg point: 145C
1ll-N~]I~ (CDC13/'l'MS)
~= 7~8 (s broad, 111) Nll;
S,95 (s, 1l~) CIIC12;
4,2 (q, 211) COOC~l2;
2,25 (s, 3~ ) C113-C=;
1,9 (s, 311) C113-C=;
1~25 ppm (t, 3~1) COOC1l2Cll3.
- 4~ -
as
~a~ple 3~
_ __.__
:l34 ~g (0.5 mmole) of amlnorlium perrhellat~, 8.55 g
(49.'~ lnoles) of 2-azi,do-3-methyl-butarloic ethyl e~ter, 3.85 g
(52.6 mmoles) of dlmethyl formamide and 50 mg ~0.45 mmoles) of
hydroquinone were put into a reactor with 50 ml of acetonitrile,
whereuE>on 6.45 g (52.4 mmoles) of acetyl bromide were added at
xoom terll~erature while stirring vigorously. The reaction
mi,xture was stirred,for 3 hours while'a maximal reaction tem-
perature of 35C was reached. The reaction mixture was further
processed as in Example 3.
4.8 g (51.9~ of the theoretical yield) of analyti,
cally ~ure N-acetyl-2,3-dehydro-valine ethyl ester were
obl:ainecl.
!
C~13 1l
~ C=~-C-OC~12C~13
C~l3 1IN-IC-C~13
~9lll5NO3(l~5~22)
,' 20
%C ~H %N
comput:ed: 58.36 8.16 7.56
obt,ained, 57.71 7.88 7.52
melting point: 77C
NMI~ (CDC13/TMS):
~= 7,9 (s broad, lH) NH;
4,15 (q, 211) -COOC132-;
2,1 ~s, 311) COC~13;
2,05 (s, 3~1) C~13-C=;
1~ (s, 311)~C113-C=;
1,2 pplll (t, 3~1) -COOC112-C~13.
- 47 -
7~7<~8
l~Xc~ f.~ 40
_. _ _ __
134 In~] (0.5 mmole) of amlllo~ m perrhenate, ~.55 g
(49.9 mmoles) of 2-azido-3-me-thyl-hutanoic ethyl ester, 3.85 g
(r,2.6 nl~lo:Les) of dimethyl formamide and 50 mg (0.45 mmole) of
hydro(lu:inolle were put illtO a reac-tor with 50 ml with acetoni-
tri]e, whereupon 5.7 g (52.5 mmoles) of methoxy acetic acid
chloxjde ~ere added a~ room tem~era-tu.re while stirri.rl~ vigorox-
ously. 'rhe reacti.on mixture was stirred for 70 hours at room
temperatul-e an~ then further processed as in ~.xample 3. The
crude u~oduce thus obtained was distilled in vacuo (0.4 mbar).
6.0 g (55.8% of the theoretical yield) of analy-~ically
pure N-methoxy-acetyl-2,3-dehydro-valine ethyl ester were
vb~a:illcd .
C1~3~ ~C
C=C - -OCH 2CH 3
CH 3 IIN - ICl -CH 2OCH 3
- 20 C101117N4 ( 215 ~ 25 )
%C ~H ~N
compute~: 55.80 7.96 6.51
obtailled: 55.87 8.60 6.64
meltin~ point: 112 to 114C
1 Il-N~ ( CDCl 3/l'MS ):
J = 7,65 (s broad~ 111) NH;
4, 2 ( q, 211 ) -COOC112-;
3,95 (s, 211) C0-C~12-0;
3,45 (s, 311) -OC~13;
2,15 (s, 311) Cll3~C=;
1,~5 ~s, 311) C113~C=;
1, 25 ppm ( t, 311 ) -COOC112-C113 .
-- 4~ --
7~7988
Example 41
__ __
134 mg (0.5 mmole) o ammonium perrhellate, 8.55 g
(4~.9 l~moles) of 2-azido-3-methyl-butalloic methyl ester, 3.85 g
(52.6 mmoles) of di.methyl formamide and 50 mg (0.45 mmole) of
hydroc~ui.none were put into a reac-tor with 50 ml of acetonitrile,
whereupoll 6.35 g (52.6 mmoles) of 3-methyl-butanoic acid
chlori.de wexe added at room temperature while stirri.ng vigor-
ously. ~rhe reaction mixture was stirred for 9 hours at room
temperature and then furthex processed as in Example 3.
6.5 g (57.3~ of the theoretical yield) of analytically
pUI e 2-[2'-methyl-propyl)-carbonyl-amillo]-3-methyl-2-butenoic
ehl:yl ester were obtained.
Cll3 O
~C=j-C-oc~l2clJ3
3 ,CI~
IIN-~C-CII2CII 3
C~13
12 21N3(227,30)
~C ~H ~N
computed: 63.41 9.31 6.16
obta.ined: 64.00 10.18 6.25
melt:ing pOillt: 123 to 125C
; 30
~ 77
NI`11~ (CDC13/1MS)
= G,8 (s broad, 111) NH;
4,2 (q, 211) -C00C112-;
1,7 - 2~4 (m, 311) C0C!12-C13;
2,2 (s, 311) C113-C=;
1,9 (s, 3}1) C113-C=;
1,3 (t, 311) C113-C~I2;
1, 05 ppm ( d, 611) -C_ccl33.
r.xam~le 42
134 mg (0.5 mmole) of ammonium perrhellai:e, 8.55 g
(49.9 mliloles) of 2-a~ido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 I,lmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hydro~luillorle were put into a reactor with 50 rml of acetoni.trile,
whe1eupon 7.4 g (5~.5 mmoles) of 4-chloro-blltanoic acid chloride
were added at room temperatur-e while stirri.ng vigorously. The
reacti.otl mixture was sitrred fo~ 10 hours at roorn temperature
ancl then furthe processe~ as in rJxample 3.
~.4 g (67.9% of the theoretical yield) of analyti-
ca].].y pure 2-[(3'-chloro-pr.opyl)-carbonyl-amino]-3-1llethyl-2-
bulerloi.c e-thyl es1:er were obtained.
C113~ 0
C=;C-c_0cH CH
113 I~N -BC- CHzcll2cl~ Cl
N3 C 1 ( 2 4 7 ~ 7
--5C)--
~,7t7~S~
%C %1-1 ~N %CI
c(~r,~l-ul~ 53. 33 7 . 32 5. 65 14 . 31
o~ 5 3 . 3 6 7 . 6 4S . ~ 7 1 4 . 5 0
m~ g l-oin~ 0C
N~lII (CDC13/TI`IS):
= 7,1 (s broad~ ) Nll;
4,15 (q, 211) -COOCH2-;
3,6 (t, 2~1) -C~12Cl;
1,75 - 2,7 (1ll, 413) -COC132C132-;
2,15 (s, 311) C113-C=;
1,~5 (s, 3~1) C113-C=;
1,25 pL)m (t, 3f3) -COOC132-C113.
I~,xampJ e 4 3
134 ~Ig (O.S mmole) of aMmonium perrhenate, 8.55 g
(49.9 nlmoles) of 2-azido-3-methyl-bu'canoic ethyl ester and 3.85
g (52.6 mmoles) of dimethyl formamide were ~issc-lved in 50 ml of
acetic ethyl estex, whereuporl 9.2 g (52.5 mmoles) of 2-chloro-
20 benzyol chlor:ide were added at room tempexature while stirringvigorously. The reaction mixture was stirred for 14 houxs at
XOOIII temperature and then further processed as in Example 16.
7.1 g (50.5% of the theoretical yield) of analtyi-
cally pure 2-[(2'-chloro-phenyl)-carbonyl-amino]-3-methyl-
2-butenoic ethyl ester were obtained.
CIJ3 o
,, 3~=C-C-OC112C113
CI ~
Cl
14 16 3 ~281,74)
7~7~8~
%C ~1l %N %Cl
computed: 59.68 5.72 4.97 12.58
obtained: 58.72 5.96 4.91 11.64
melting point: 86 to 89C
ll-N~I~ (CDCl 3/T~15 J:
~= 7~0 - 7,9 ~ , 5H) NH aromatic-CH;
4,2 (q, 213) COOC112;
2,2 (s, 311) CH3-C=;
1,95 (s, 3H) CH3-C=;
1125 ppJIl (t, 3H) COOC112CH3. -
E,xample 44
-
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-bu-tanoic e-thyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquinone weLe added at room temperature while stirring
vigorously. Tile reaction mixture was stirred for 14 hours at
room temperature and then further processed as in F.xample 16.
10.2 g (72.5% the theoretical yield) of analytically
pure 2-[(3'-chloro-phenyl)carbonyl-amino]-3-methyl-2-butenoic
ethyl ester were obtained.
CH3 ~ ~o
C=C-C-OCH Cll
~IN - iC~ ~
Cl
14 lG 03cl(28l~74)
- 52 -
77~
~C %EI ~N %Cl
computetl: 59.68 5.72 4.97 12.58
obtained: 59.52 5.78 4.89 12.32.
Illeltirlg pOillt: 120 to 122C
-N~ CDC 13/1~MS)
= 7,0 - 8,1 ~m, 511) Nll; arom~tic-CH-
4~15 ~q, 2H) coocl~2;
2,15 (s, 311) C113-C=;
1,~5 ~s, 31i) C113-C=;
,2~ ppm ~t, 3ll) COOCI12CII3.
E~xampl 4_
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hydroquinone were dissolved in 50 ml of acetic ethyl ester,
whereu~on 9.2 g (52.5 mmoles) of 4-chloro-benzoyl chloride were
added at room teml~erature while stirring vigorously. The reac-
tion mixture was stirred for 14 hours at room temperature. The
~recipitated crude product was filtered with suction and washed
unitl free from chloride.
11.1 g (78.9~ of the theoretical yield) of analyti-
cally pure 2-[(4'-chloro-phenyl)-carbonyl-amino]-3-methyl-2-
butenoic ethyl ester were obtained.
Cll3 O
,~ ,C=C-~'-OC~12C113
C 3 ¦ ~ Cl
O
191116N3Ci(281,74)
- 1~7~988
%C %H ~N ~Cl
computed: 59.68 5.72 4.97 12.58
obtairled: 58.97 5.55 4.09 14.08
melting point: 124 to 124.5C
ll-N~ (C~C13/l'MS):
~= 7,1 - 8,25 (m, 511) N13; aroln~tic-CH;
4,2 (q, 2l1) ~OOCI~2;
2~2 (s, 313) Cl13-C=;
1,9 (s, 311) C~33-C=;
1,25 ~plll (t, 311) COOC1l2C1l3.
Exalllple 46
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquinorle were dissolved in 50 ml of acetic ethyl ester,
whereuporl 8.35 g (52.6 mmoles) of 2-fluoro-benzoyl chloride were
added at room temperaturé while stirring vigorously. The
reaction mixture was stirred for 14 hours at room temperature
and then further processed as in Example 16.
9.0 g (68~ of the theoretical yield) of analytically
pure 2-[(2'-fluoro-phenyl)-carbonyl-amino]-3-methyl-2-butenoic
ethyl ester were obtained.
CH3 o
~C=C-C-OCH CH
IIN-C~'~
o F
(~1411lGNo3F(265~285)
- 54 -
,7~a
%C %H %N
com~uted: 63.38 6.08 5.28
obtaine(l: 63.06 6.04 5.23
melting point: 82.5C
ll-N~ (CDC13/TMS):
~ = 6~5 - 7,65 tlll, 511) Nl~; a~omatic-CH;
4,2 ~q, 21~) -COOC132-;
2~25 ~s, 311) C133-C=;
lt9 (S, 31~) C1~3-C=;
1~35 ppm (t, 31i) -COOC112C1~3.
I ample 47
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49.9 mmole) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
~52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquirlone were dissolved in 50 ml of acetonitrile, whereupon
8.35 g (52.6 mmoles) of 4-fluoro-benzoyl chloride were added at
room temperature while stirring vigorously. The reaction
mixture was stirred at room temperature for 14 hours and then
further processed as in Example 3.
9.3 g (70.2~ of the theoretical yield) of analytically
pure 2-[(4'-fluoro-phenyl)-carbonyl-amino]-3-methyl-2-butenoic
ethyl ester were obtained.
C~i3 ~ o
C=C-C-OCIl Cll
HN-ICl ~ F
Cl4lll6No3Ft265~28s)
- 55 -
~ ~77~
Q~iC 2iH %N
compuled: 63.38 6.08 5.28
obtained: 62.94 6.15 5.11
melting point: 130 to 132C.
Nl`lR (cDcl3/TMs): H
~= 7~6 - 8,2 (m, 311) NH;~
6,7 - 7,3 (m, 211) C~
4~2 (q, 211) -COOC~2-:
2,15 (s, 311) C~13-C=;
1,85 (s, 311) -C~13-C=;
1,25 ppm ~t, 3~3) ~cOocll2-c~l3.
Example 48
134 mg (0.5 mmole) of a~mnonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of di.methyl formamide and 50 mg (0.45 mmole) of
hydroquinone were dissolved in 50 ml of acetonitrile, whereupon
11.0 g (52.5 mmoles) of 2,4-dichloro-benzyl chloride were added
at roorn temperature while stirring vigorously. The reaction
mixture was stirred at room temperature for 14 hours and then
further processed as in Exarnple 3.
9.3 g (58.996 of the theoretical yield) of analytically
pure 2-~(2',4'-dichloro-phenyl)-carbonyl-amino]-3-methyl-2-
butenoic ethyl ester were obtained.
Cil O
3\~'=C-~'-OCI~ Cll
3 I
C141115N02C12(316,18)
-- 56 --
........ . . . ..
.: . .. .: .
7~7~388
~c ~H ~,N ~Cl
comE)ute~: 53.18 4.78 4.43 22.43
obta~ cd: 52.22 4.80 4.41 20.73
melting point: 128.5 to 130C
N~ (cDCl3/l'Ms):
= 7,1 - 7,7 (m, 411) NH; aromatic-CH;
4,2 (q, 2fl) -COOCH2-;
2,2 (s, 311) C113-C=;
1,95 (s, 311) Cli3-C=;
1,3 p~lll (t, 31~ COOCII2-C~33.
l~xample 4_
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquinone were dissolved in 50 ml of acetonitrile, whereupon
11.0 g (52.5 mmoles) of 3,4-dichloro-berlzyl chloride were added
at room temperature while stirring vigorously. The reaction
mixture was stirred for 15 hours at room temperature and the
further processed as in Examule 3.
10.5 g (6.5% of the theroetlcal yield) of analytically
pure 2-[3',4'-dichloro-phenyl)-carbonyl-amirlo]-3-methyl-2-
butenoic ethyl ester were obtained.
C~13 ~ 1l
~C=C-C-OC132C1~3
~ C ~ Cl
C141115N03C12(316~18)
- 57 -
~ ~7988
%c ~H ~N %Cl
computed: 53.18 4.78 4.43 22.43
obtained: 53.40 4.86 4.36 22.11
melting point: 115 to 120C
~ '
Il-N;I~ (CDC13/TMS):
2 ~ 8,2 (m;, 4~3) Nll; aromatic-CH;
~,2 (q, 211) - COOC112-;
2,15 (s, 3~1) Cf33-C=;
1~9 (s, 313) C113-C=;
1~25 ppm (t, 311) -COOC112C}13.
~xanlple 50
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
~49.9 mMole~) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mllloles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquinorle were put into a reac~or with 50 ml of acetonitrile,
whereupon 11.0 g ~52.5 mmoles) of 3.5-dichloro-benzoyl chloride
wexe added at room ternperature while stirring vigorously. The
reactiorl mixture was stirred for 15 hours at room temperature.
The precipi.tated crude product was fi.ltered with suction and
wa~hed w.ith water until free from chloride.
12.3 g (77.9% oP the theoretical yield) of 2-[(3',
5'-dichloro-phenyl)-carbonyl-amino]-3-methyl-2-butenoic ethyl
ester were obtained.
_ .,
3 ~ C C U-OCfl Cf3
3 3 ill-C
Cl4lll5No3cl2(3l6~l8)
- 58 -
%C %H %N ~Cl
computed: 53.18 4.78 4.43 22.43
obtailled: 50.44 4.88 3.91 21.12
melting point: 171C
ll-N~1~ (CDC13/TMS):
~= 7,15 - 7,85 (m, 411) Nll; aromati.c-CH;
4,2 (q, 211) - COOC1~2-:
2,2 (s, 311) C113-C=;
1,9 ~s, 311) C113-C=;
1,25 ppm ~t, 311) -COOC112-C113.
Example 51
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
(49 9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 9
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hydroqui.none were put into a reactor with 50 ml of acetonitrile,
whereupoll 12.6 g (52.6 mmoles) of 2,6-dichloro-phenoxy acetyl
chloride were added at room temperature while stirring vigor-
ously. The reaction mixture was stirred for 14 hours at room
temperature. The precipitated crude product was filtered with
SUCtiOtl and washed with water until free from chloride. 14.4 g
(87.4~ of the theoretical yield) of analytically pure 2-
. [(2',6'-dichloro-phenoxy)-acetyl-amino]-3-methyl-2-butenoic
ethyl ester were obtained.
3 ~ ~
Cll ~ C l-C-c~J2c~l3
IIN-C-CII -O ~ Cl
~ Cl
C15lll7NO3cl2(3~o~2lJ
- 59 -
779~8
%c %H %N %Cl
comput:ed: 54.56 5.19 4.24 21.47
obtained: 50.24 5.12 4.12 20.30
melting point: 124 to 125C
ll-NI~ CL)C13/'l'MS):
Ct = 7,95 (s broad~ 1ll) NH;
6,65 - 7,5 (m~ 211) aromat'~-n Cll;
4,6 (s, 211) COCII2-O-;
4,2 ~q, 2119 COOC112;
2,2 (s, 311) C~12-C=;
1,9 (s, 311) CH3-C=;
1,25 pplll (t, 3ll) COOCII2C~13.
13xample 52
134 mg (O.S mmole) of ammonium perrhenate, 8.55 g
(49.9 mmoles) of 2-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmole) of
hdyroquinone were put into a react:or with 50 ml of acetonitrile,
whereupon 11.3 g (52.5 mmoles) of 4.5-dichloro-thiophene-2-
carboxylic acid chloride were added at room temperature. The
reaction mixture was stirred at room temperature for 15 hours
while a maximal exothermical reaction temperature of 45C was
attained. The reaction mixture was then further treated as in
Example 3.
11.6 g (72.196 of the theoretical yield) of analyti-
cally pure 2-[2'-(4,5-dichloro-thienyl-carbonyl-amino]-3-
methyl-butenoic ethyl ester were obtained.
-- 60 --
. .
~'~77988
Cll o
= ~ -C-oC112C1l3
13N-C ~ 1
O
12 12 3 2 (32Z,21)
~C ~H %N ~S ~Cl
computed: 44.74 4.07 4.35 9.49 22.00
10 obtained: 43.16 4.20 3.60 9.49 21.65
melting pOillt: 9S to 98C
ll-N~IJ~ (CDC13/~MS)
~= 8,45 (s, 1ll) N~]
7,55 (s, 1l~) ll ~ Cl
4,2 (q, 211) -COOCH2-;
2,15 (s, 3~3) Cli3-C=;
1,9 (s, 311) Cl33-C=;
20 l,3 pplll (t, 31~) -COOCll2-Cl~3.
~xample 53
134 mg (0.5 mmole) of ammonium perrhenate, 8.55 g
~49.9 mmoles ) of 2~-azido-3-methyl-butanoic ethyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (G.45 mmoles) of
hydroquinone were put illtO a reactor with 50 ml of acetoni-
trile, whereupon 7.1 g (52.7 mmoles) of 2,2-dimethyl butanoic
acld chloride were added at room temperature while stirring
vigorously. The reaction mixture was stirred for 35 hours at
room temperature and theA further processed as in Example 3. In
- 61 -
7988
order to purify the crude product, it was distilled in vacuo
(U.53 mbar) at llO to 150C in the bulb tube. The oil thus
obtained was crystallized w:ith n-pentane.
8.8 g (73.1% of the theoretical yield) of analytically
pure 2-[(l',l'-dimethyl propyl)-carbonyl-amino]-3-methyl-2-
butenoic methyl ester were obtained.
C113 ~ 0
~C=C-C-OC}1 CH
CI33 l ICl33
IIN-~C~ - I -C112C~33
o c~l3
12II23N3~291~33)
~C ~H %N
computed: 64.70 9.61 5.80
obtained; 64.8l 9.89 5.67
melting point: lO0 to 105C
1H-NMI~ ~CDC13/T~Is):
~= 6,85 (s hroa~ 111) Nll;
9,15 (q, lfl) -COOCII2:
2,15 (s, 311) Cl33-C=;
1,~ (s, 31I) Cl~3-C=;
0,65 - 1,85 ppIl~ (m, 1411) C-(CH3-C1~2C133; 0-C112CII3.
Example 54
13.7 mg (0.05 rnmole) of sodium perrhenate, 926 mg (5
mmoles) of 2-azido-3-methyl-butnaoic isopropyl ester and 383.8
mg (525 nm~oles) of dimethyl formamide were dissolved in 2.5 ml
of acetonitrile, whereup~n 529 mg (5.25 mmoles) of chloro acetyl
chloride were added at room temperature while stirring vigor-
- 62 -
779f~
ously. The reaction mixture was stirred for 7 hours at room
tempera~ure and then further pl:ocessed as in Example 1.
1.0657 g (81.2% of the theoreti.cal yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-3-methyl-2-butenoic iso-
propyl ester were obtained.
Cl13 ~ / CO2C~I~C113)2
~ C=C~
C~13 N~
C'1UlllGC'1N03(233,695)
%C %H %N %Cl
computed: 51.40 6.90 5.99 15.17
obtained: 51.41 6.97 5.77 15.16
melting point: 126C
IIJ-NI~ CDC13):
1~' = 7,9 (s, 111) Nll; I~ ~NII = 3265 Clll
5,13 ( septet , 111 ) O-CII (C113)2 ~tco 17
~,15 ~S, 211) C112Cl: 1663 clll 1.
2,2 (s, 31l) 1 3C-C=C~N:
(S~ 311~lll3c~ N
1,2g ppm (d, 611)-CH~C113)2.
Example 55
13.7 mg (0.05 nmloles) of sodium perrhenate, 1.17 g (5
mmoles) of 2-azido-3-methyl-butanoic benzyl ester and 383.8 mg
(5.25 mrnoles) of dimeth~l formamide were dissolved in 1.5 ml of
acetonitrile, whereupon 592.9 mg (5.25 mmoles) of chloro acetyl
-- 63 --
77988
chloride were added at room temperature while stirring vigo~-
ously. '1'he reactio11 mixtu~e was stirred for 15 hours at room
temp~rature and then further processed as in Example l.
l.32 g (97.7 % of the theoretical yield) of analy-
tically pure 2-chloro-methyl-carboxamido-3-methyl-2-butneoic
benzyl ester were obtained.
C~13 ~ ~ CO2Cl12 C6~15
~C=C
C133~ N~ ~
14 16 3(281,739)
%C %H ~N ~Cl
computed:59.68 5.72 4.97 12.58
obtained:59.75 5.64 4.77 12.60
melting point: 122 to 122.5C
Il-N~ ( C~Cl3):
~= 7~76 (s, l11) N11;I1~ ~N11 = 3255 c1n
7~4 ~s, 5ll)aJ~o~a~ic-~cHc; ~C0 = 1705 Clll
5,25 (s, 211) -O-C~12-;1660 c
4,l3 (s, 2H) -C112Cl;
2,24 (d, 311) 3 C=C~N;
1,9 ppln (s, 311) 113C' `N
- 64 -
r;t7988
Example 56
13.7 mg (0.05 rnmole) of sodiwn perrhenate, 1.0961 g (5
mmoles) of 2-azido-3-methyl-butanoic phenyl ester and 383.8 mg
(5.25 mmoles) o~ dimethyl formamide were dissolved in 1.5 ml of
acetonitrile, whereupon 592.9 mg (5.25 mmoles) of chloro acetyl
chloride were added at room temperature while stirring vigor-
ously. The reaction mixture was stirred for 15 hours at room
temperature and then further processed as in Example 1.
1.24 g (92.6~ of the theoretical yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-3-methyl butenoic phenyl
ester were obtained.
Cll3 ~ ~ Co2-c6l~5
Cll-- -C~
3 N~ -CII2Cl
Cl3lll4clNo3(267~7l2)
~C ~H %N %C1
cornputed: 58.32 5.27 5.23 13.24
obtained: 58.34 5.20 4.96 13.42
melting poi-lt: 126C
-N~ ( CDC13)
;,1 (m, 511) aromab'~ v'NIl _ 1724 ~
2,29 (d, 311)113C'C=~N;
1,98 pplll (s, 3l~ C~=~ -
\
- 65 -
77~&~
Ex~mple 57
27.3 mg (0.1 mmole) of sodiudm perrhenate and 1.57 g
(10 mmoles) of 2-azido-3-methyl-butanoic methyl ester were put
into a reactor with S ml of acetic ethyl ester at 80C, where-
upon 1.55 g (10.5 mmoles) of dichloro acetyl chloride were added
dropwise within 45 minutes while stirring vigorously. The reac-
tion mixture was kept at 80C for a total of 3 hours while stir-
ring vigorously and thereafter it was further processed as in
13xample 1.
2.27 g (94.7% of the theoretical yield) of analyti-
cally puxe 2-dichloro-methyl-carboxamido-3-methyl-2-butenoic
methyl ester were obtained.
cll3~ ~C02C~13
~C=C\
C~13~ , 2
O , ;
C~Hllcl2No3(24o~o86)
%C%H ~N %Cl
computed: 40.04 4.64 5.83 29.53
obtained: 39.93 4.51 5.78 29.72
melting pOillt: 118C
~ .
Il-NMI~ ( CDCl 3 ):
~= 7,75 (i5, 111) Nll;IR ~NII = 3265 c~
6~05 (s, 1ll) CIIC12; ~CO = 1722 clll 1,
3,79 (s, 3IJ) OC~13; ' 1670 c
2,26 (d, 311)~ 3C~=~N;
1~9 pplll (s, 3H)Il C~=~N-
- 66 -
77~388
Example 58
27.3 mg (0.1 mmole) of sodi.um perrhenate and 1.57 g
(10 mmoles) of 2-azido-3-methyl butanoic methyl ester were put
:into a reactor with 5 ml of acetic ethyl ester at 80C, where-
upon 1.48 g (10.5 mmoles) of benzoyl chloride were added drop-
wise within 15 minutes while stirring vigorously. The reaction
mixture was kept at 80C for a total of 5 hours while stirring
vigorously and was then further processed as in Example 1.
2.0877 g (89.5% of the theoretical yield) of analy-
tically pure 2-phenyl-carboxamido-3-methyl-2-butenoic methyl
ester were obtained.
C113~ ~C z 3 ,.
CH3 NH-~C-C6115
C13lllSNO3(233~267)
%C %H %N
computed: 66.94 6.48 6.00
obtained: 66.96 6.59 6.01
melting point: 132C
'
NMn ~CDC13):
~ = 7,91 - 7,29 (m, SH) aroma~iC~cH=; IR ~NI~ = 3280 cm 1,
: 3,72 ~s, 3H) OCH3;~CO = 1725 cm 1,
2,18 (d, 3ll) 3~=~ ;lG43 cm 1.
l~B9 ppln (s, 31l)Cll3~ ~N-
`
- 67 -
77988
Example 59
27.3 mg (O.l mmole) of sodium perrhenate and 1.57 g
(10 mlnoles) of 2-azido-3-methyl-butenoic methyl ester were put
into a reactor with 5 ml of acetic ethyl ester at 80C, where-
upon l.lO g (lO.5 mmoles) of cyclop~opane carboxylic acid
chlori,de, dissolved in 5 ml of acetic ethyl ester, were added
dropwise within 30 minutes while stirring vigorously. The
reaction mixture was kept at 80C for a total of 4 hours while
stirring vigorously and was then further processed as in Example
10 1.
l.74 g (88.3% of the theoretical yield) of analyti-
cally pure 2-cycloyropyl-carboxamido-3-methyl-2-butenoic methyl
e6t e:~ we~e obtaisled.
cl,3 ~C2C113
~C=C~
cll3 Nll~ / H
O ~
C~5N03 ( 19 7 ~ 2 3 4 )
%C %H %N
computed: 60.90 7.67 7.lO
obtained: 60.65 7.53 7.05
melting point: 150C
Il-NMI~ ~ CVCl 3):
~= 7,13 (s, 111) Nll; I~ ~N~ = 3270 cm
3,74 (s, 311) OC~13; ~CO = 1719 c~
2,11 ~s, 3ll)C113~ ; , 1644 cm 1.
~N
1,84 (s, 31l)c~ N;
1~6~ - 0,6 pplll (1ll; 5ll) ~ ll
H
- 68 -
~ ~7~88
Ixample 60
27.3 mg (0.1 mmole) of sodium perrhenate, 1.57 g (10
mmoles) of 2-azido-3-methyl-butanoic methyl ester were put into
a reactor with 5 ml fo acetic ethyl ester at 80C, whereupon
1.25 g (10.5 mmoles) of l-methyl-cyclopropane carboxylic acid
chloride, dissolved in 5 ml of acetic ethyl ester, were added
dropwise within 30 minutes while stirring vigorously. The
reaction mixture was kept at 80C for a total of 4 hours while
stirring vigorously and was then further processed as in ~xample
10 1.
19.5 g (92.4% of the theoretical yield) of analyti-
cally pure 2-[(1'-methyl-cyclopropyl)-carboxamido]-3-methyl-2-
butenoic methyl ester were obtained.
3~ C C ~ 2 3
C~ 7N03(211~261)
%C %H %N
computed: 62.54 8.11 6.63
obtained: 62.34 7.97 6.71
melting pOillt: 73.5C
ll-NMI~ ( C~C13):
~= 6,97 (s, 1ll) NH ll~ ~NII = 3325 c~
3,77 (s, 311) 0C113; ~CO = 1717 cm 1,
2,16 (s, 3ll)Cll3 ; 1645 cln
1,B4 (s~ 311)Cl~ N ;
1,43 (s, 311) C113; ~l
2,0 - 0,5 ppm (m, 41l)
- 69 -
~ '~77988
Example 61
27.3 mg (O.l mmole) of sodium perrhenate and 1.57 g
(lO mmoles) of 2-azido-3-methyl-butanoic methyl ester were put
into a reactor with 5 ml of acetic ethyl ester at 80C, where-
upon 1.97 g (lO.S mmoles) of 2,2-dichloro-l-methyl-cyclopropane
carboxylic acid chloride, dissolved in S ml of acetic ethyl
ester, were added dropwise within 30 minutes while stirring
vigorously. The reaction mixture was kept at 80 C for a total
of 4 hours while stirring vigorously and was then further
processed as in Exa~ple l.
2.61 g (93.l~ of the theoretical yield) of analyti-
cally pure 2-[(2',2'-dichloro-l'-methyl)-cyclopropyl-carbox-
amido]-3-methyl-2-butenoic methyl ester were obtained.
Il 11
ll lS 2 O3(280,151)
~C %~1 ~N ~Cl
computed: 47.16 5.40 5.00 25.31
obtained: 47.05 5.45 4.88 25.35
melting point: 104C
Il-NMI~ ( CDCl 3 ):
d = 7,31 (s, 1~1) N~l; IR rN11 = 3280 cm
3,71 (s, 311) OCH3; ~CO = 1721 cm 1,
2,25 (d, l11) V~11 ; 1650 cm
2,18 (s, 311) C113~= ;
1~86 ~5~ 3H) C113- ~';
1,66 (s, 311) C~13;
1,39 ppm (d, lH)
-- 70 --
77988
Exam_ e 62
27.3 m~ (0.1 mmole) of sodium perrhena-te and 1.57 g
(10 mmoles) of 2-azido-3-methyl-butanoic methyl ester were put
into a reactor with 5 ml of acetic ethyl ester at 80C, where-
upon 2.39 g (10.5 mmoles) of 2-(2',2'-dichloro-ethenyl)-3,3-
dimethyl-cyclopropane carboxylic acid chloride, dissolved in 5
ml of acetic ethyl ester, were added dropwise within 30 minutes
while stirring vigorously. The reaction mixture was kept at
80C for a tol:al of 4 hours while stirring vigorusly and then
further processed as in Example 1.
3.08 g (96.3% of the theoretical yield of analyti-
cally pure 2-[2'-(2,2-dichloro-ethenyl)-3',3'-dimethyl]-
cyclopropyl-carboxamido-3-methyl-2-butenoic methyl ester were
obtailled.
C~l33~=c~C2C~13 1~
C~J3 ~NH-C Ik,l Cll=CC12
1~3C C1~3
C~ 9cl 2No3 ~ 3 2o ~ 2 l 6 )
%C %H %N %Cl
computed: 52.51 5.98 4.37 22.14
obtained: 52.29 6.13 4.25 22.40
melting point: 151.5 to 152.5C
ll-NMI~ (CDC13):
~= G,69 ~s, 11l) Nll; Il~ ~)'Nll = 3345 cm 1,
6,46 (d, lH) -Cll=CC12; ~rcO = 17()0 Cln
3,76 (s, 3ll) OC1l3; , 1665 cm
2,16 (s, ill) ~13C`=~N;
1,85 ~s, 3l~) H3~ ~N;
1,29 (s, 3l~) l ~
1,27 ppm ~s, 3H~ l13C CH3
-- 71 --
~77~88
Exam~le 63
-
245 mg (0.5 mmole) of tetra-n-butyl ammonium
perr11et1ate, 7.85 g (50 mmole) of 2-a~ido-3-methyl-butanoic
methyl ester, 5.2 g (52.5 mmoles) of N-methyl pyrrolidine and 50
mg (0.45 mmole) of hydroquinone were dissolved in 50 ml of
acetonitrile, whereupon 9.75 g (52.5 mmoles) of 4-nitro-benzoyl
chlor,i~e were added at room temperature while stirring vigor-
ously. The reaction mixture was stirred for 14 hours at room
temperature and then further treated as in Example 3.
8.9 g (64.0~ of the theoretical yield) of analytically
pure 2-[(4'-nitrophenyl)-carbonyl-amino]-3-methyl-2-butenoic
methyl ester were obtained.
C~1
c=c -1 -OCI~
C113
o
~ 2
13 l4N2os(27B~27)
%C %H %N
computed: 56.ll 5.07 lO.07
obtai,ned: 55.84 5.13 9.21
melting point: 122C
-NM~ ~CDC13/T~1S):
= 8,9 - 7,6 (m, SH) N11; ar~mat:iC Cll=,
3,75 (s, 311) COOC113;
2,15 (s, 31~) C113-;
1,9 pp111 ~s, 3,11) C113-.
Ixample 64
246 mg (0.5 mmole) of tetra-n-butyl ammonium perr-
- 72 -
~;:7798~3 `
henate, 7.95 g (50 mmoles) of 2-azido-3-methyl-butanoic methyl
ester, 5.2 g (52.5 mmoles) of N-methyl pyrrolidone and 50 mg
(0.45 mmoles) of hydroquinone were dissolved in 50 ml of ace-
tonitrile, whe~eupon 11.05 g (52.5 mmoles) of 2,6-dichloro-
isonicotinic acid chloride were added at room temperature while
stirring vigorously. The mixture reacted exothermically within
15 minutes while the crude product precipitated. The crude
product was filtered with suction and washed with water until it
was free from chloride.
8.9 g (58.7% of the theoretical yield) of analytically
pure 2-(2',6'-dichloro-isonicotinoyl)-amino]-3-methyl-2-butenoic
ethyl ester were obtained.
3~C=C-I-OCf~
c~3~ l Cl
o~Cl
Cl2lll2N2o3cl2~3o3~l5 t
%C %H %N %Cl
computed: 47.54 4.00 9.24 23.40
obtained: 47.50 3.84 8.39 22.69
melti.ng point: 186 to 187C
,
ll-NMI~ (DMSO-J6/TMS):
~= 10,1 ~s broad, 111) NH;
7,95 (s, 211) aro~a~:iC CH
3,6 Is, 311) -COOC113;
2,1 Is, 311) C113-;
l~n5 pp~ 8, 31~) C113-.
-- 73 --
1'~77988
_~ample 65
24G mg (0.5 mmole) of tetra-n-bu-tyl arnmonium perrhen-
ate, 9.25 (50 mmoles) of 2-azido-butanoic-n-butyl ester, 3.85 g
(52.6 mmoles) of dimethyl formamide and 50 mg (0.45 mmoles) of
hydroquinone were dissolved in 50 ml of acetonitrile, whereupon
ll.05 g (52.5 mmoles) of 2,6-dichloro-isonicotinic acid chloride
were added at room temperature while stirring vigorously. The
mixture reacted exothermically within 15 minutes. It was
furlher processed as in Example 3.
ll.8 g (71.25% of the theoretical yield) oE analyti-
cally pure 2-[(2',6'-dichloro-isonicotinoyl-alnir1o]-2-butenoic-
~-bulyl ester were obtained.
o
C1~3-C11=C-C-OC11 (Cll J C
Cl
Il ~Cl
Cl4lll6N2o3cl2(33l~2o)
%C ~H %N %Cl
computed: 50.77 4.87 8.46 21.40
obtained: 50.82 4.65 8.08 20.0l
melt.ing point: 123 to 124C
l1-NMI~ (CDC13/T~lS):
~= 8,05 (s broad- ll1) Nl~;
7,65 (s, 21i) aromatic C~l;
G,9 (q, lll) -Cll=;
~,lS (t, 211) -COOC112-;
1,75 (d, 311) C113-;
U 55 - 2~0 pp~ 711) -C1l2cll2 C 3
- 74 -
~ ~ ~7~988
Example 66
27~3 mg (0.1 mmole) of sodium perrhenat:e and 2.05 g
(10 mmoles) of 2-azido-3-phenyl-p~opanoic methyl ester were put
into a reactor with 5 ml of acetic ethyl ester at 80C, where-
upon 1.95 g (10.5 mmoles) of 2-bromo-butanoic acid chloride,
dissolved in 5 ml of acetic ethyl ester, were added dropwise
withirl 40 rninutes while stirring vigorously.
The recation mixture was kept at 80C for a total of 3
hours while stirring vigorously and was then further processed
as in E~xample 1.
2.23 g (68.2% of the theoretical yield) of analyti-
cally pure 2-[(2'-bromopropyl)-carboxamido]-3-phenyl propenoic
methyl ester were obtained.
I~C=C~COOCf~3
fl5C6 Nll-nc-cl-l-cll2-cl~3
o Br
Cl 41~1 6BrNo3 ( 326 ~ l 95 )
%C %H %N %Br
computed: 51.55 4.94 4.29 24.50
obtained: 51.49 4.87 4.30 24.27
melting point: 115C
ll-NMI~ ( Cl)C13 ):
d`= 7,80 (s, 1ll) Nfl; lrNll = 3210 cm
7, 6 3 - 7, 25 ( m, 5H ) a~Omal:iC~C~I=; ~CO
4,38 (t, llJ) -CllBr-; . 1661 cm
3,~6 (s, 311) OC~13;
2,4 - 1,95 (m, 2fJ) -C112-C113;
1, 1 1 pplll ( t, 3~1 ) -ClJ2-Cfl3 .
-- 75 --
~"~77988
~xample 67
2.46 mg (0.5 mmole) of tetra-n-butyl ammonium perrhen-
ate, 9.25 g (50 mmoles) of 2-azido-butanoic-n-butyl ester, 5.2 g
(52.5 mmoles) of N-~nethyl pyrrolidone and 50 mg (0.45 mmole) of
hyd~oquinone were dissolved in 50 ml of acetonitrile, whereupon
10.7 g (52.3 mmoles) of l-naphthyl acetyl chloride were added at
room temperature while stirring vigorously. The reaction mix-
ture was stirred for 20 hours at room tempexature and then
further processed as in Example 3.
10.3 g (62.8~ of the theoretical yield) of analyti-
cally pure 2-(1'-naphthyl-acetyl-amino)-2-butenoic-n-butyl ester
were obtained.
~1` ~
~C=~C-C-OcH2c1~2cH2c~3
3 11N~7~C~12
0~
:':
C2oll23NO3(325,41)
%C %H %N
computed: 73.82 7.12 4.30
obtained: 73.10 7.10 4.24
melting point: 101 to 102C
ll-NM~ (CDC13/TMS):
~= 7,1 - 8,2 ~ m, 7H) aromatic-CH;
6,8 (s broad, 111) Nll;
6,6 (q, 111) Cll3-CII=;
4,05 (s, 211) COC112-;
3~95 (t, 211) COOC~12-;
1,65 (d, 311) C113-C=;
0,5 - 1~7 ppl; (m, 711) -CH2CH2cll3-
- 76 -
Example 68 1~77988
2.46 mg (0.5 mmole) of tetra-n-butyl ammonium perrhen-
at~, 50 mg of hydroquinone and 6.85 g (52.5 mmoles) of furan-
2-carboxylic acid chloride were put into a reactor at 80C with
25 ml of acetic ethyl ester saturated with hydrogen chloride,
whereupon 7.85 g ~50 mmoles) of 2-aæido-3-methyl-butanoic methyl
ester, dissolved in 25 ml of acetic ethyl ester, were added
dropwise while stirring vigorously. After a reaction time of 4
hours the generation of gas was terminated. The reaction mix-
ture was then further processed as in Example 16.
7.4 g (66.3% of the theoretical yield) of analytically
pure 2-furyl-carbonyl-ami.no-3-methyl-2-butenoic methyl ester
we~e obtained.
C113 ~
C=C-C-OCII
C113''' Ill-C
11 13N4 ~223,23)
%C ~H %N
computed: 59.19 5.87 6.27
obtained: 59.36 5.35 6.29
melt:ing point: 95 to 98C
Il-N~ (CDC13/TMS):
= 7,55 (s broad~ lH) Nll;
7,35 - 7,5 (m, lH)~
7,0 - 7,15 (m, lH)~
6,2 - 6,6 (m, 1~
3,65 (s, 311) COOC113;
2,2 (s, 311) C~3-C=;
1,9 ppm (s, 3~1~ C113-C=.
- 77 -
,77988
l~xample 69
2.46 mg (0.5 mmole) of tetra-n-butyl-ammonium perrhen-
ate, 7.~s g (50 ~moles) of 2-azido-3-methyl-butanoic methyl
ester, 5.2 g (52.5 mmoles) of N-methyl pyrrolidone and 50 mg
(0.45 mmole) of hydroqui~one were dissolved in 50 ml of acetoni-
'crile, whereupon 9.55 g (52.5 mmoles) of trichloro-acetyl
chloride were added at room temperature while stirring vigor-
ously. Within 20 minutes the reaction mixture reacted exother-
mically. It was then further processed as in Example 3.
7.9 g (57.6~ of the theoretical yleld) of analyti-
cally pure 2-trichloromethyl-carboxarnido-3-methyl-2-butenoic
met:hyJ ester were obtained.
3~ c=c_ll-
I ~Cl
IIN-C-IC-Cl
O Cl
8~l1oNo3cl3(274~5o)
~C %H ~N ~Cl
computed: 35.00 3.67 5.10 38.75
obtained: 33.99 3.61 4.83 39.80
ll-NMI~ (C~Cl3/TMS)
~e 7,85 (s broad, ll1) N11;
3,7 (s, 3H) COOCH3;
2,25 (s, 31~) C113-;
l,85 ppm (s, 3H) C113.
~xample 70
2.46 mg (0.5 mmole) of tetra-n-butyl-ammonium perr-
hena1:e, 9.25 g (50 mmoles) of Z-azido-butanoic-n-butyl ester,
5.2 g (52.5 mmoles) of ~-methyl pyrrolidone and 50 mg (0.45
mmole) of hydroquinone were dissolved in 50 ml of acetonitrile,
- 78 -
77g88
whereupon 9.55 g (52.5 mmoles) of trichloro-ace-tyl chloride were
added at room temperature while stirr.ing vigorously. The reac-
tion mi.xture was sitrred for 16 hours at ~oom temperature and
then further processed as in Example 3. The crude product thus
obtai.ned was distilled in high vacuum (0.013 mbar) at an instru-
ment temperature of 150 to 190C in the bulb tube.
9.5 g (62.7% of the theoretical yield) of analytically
pure 2-trichloromethyl-carboxamido-2-butenoic-n-butyl ester were
obtained.
Il~ p
=f-C-OC'l2CH2CH2CH3
HN-~ C-C
O Cl
Clolll4N03C13~302,59)
~C ~H %N ~Cl
computed: 39.69 4.66 4.63 35.15
obtained: 39.11 4.67 4.06 32.77
Il-NM~ (CDC13/T~IS):
~= 8,05 (8 broad~ 111) Nll;
6,9 (q, 11~) -C~l=;
4,15 (t, 211) COOC~12-;
1,75 (d, 313) CH3-C=;
0,65 - 1,75 pptn (m, 7H) -C112C112CH3.
- 79 -
7ssa '
~xample 71
134 mg (0.5 mmole) of aluminum perrhenate, 7.85 g ~50
mmoles) of 2-azido-3-methyl-but.anoic acld methyl ester, 5.2 g
(52.5 mmoles) of N-methyl pyrrolidone and 50 mg (0.45 rnmole) of
hydroquinor~e we~e dissolved in 50 ml of acetic ethyl ester,
whereupon 8.5 g (52 mmoles) of caprylic acid chloride were added
at room temperature while stirring vigorously. The mixture
~eacted exot.hermically within 10 minutes and was then further
processed as in Example 16.
9.5 g (74.4~ of the theoretical yield) of analytically
pure 2-he~tyl-carboxamido-3-1nethyl-2-butenoic methyl ester were
obl:aillcd.
3~ C=c~u-Oc113
C113 1IN-C-~C~l2)6cl~3
Cl~lll5o3N(255l36)
~C ~H %N
computed: 65.85 9.87 5.48
obtained: 65.79 9.92 5.30
melting point: 71C
NMI~ 13/'rMS):
S= 6,6 (s b~ad, 111) NH;
3,65 (s, 311) -COOC113;
1,9 - 2,45 (m, 2H) -COC112-;
2,1 (s, 311) C~13-C=;
! ~ 1,75 (s, 311) C113 C=
1~25 (s, 1011) -(C112)5=;
0,~ pplll (t, 3~1) C112-C113-
- 80 -
7~7988
~xample 72
134 mg (0.5 mmole) of ammonium perrhenate, 7,85 g (50
mmoles) of 2-azido-3-methyl-butanoic methyl ester, 5.2 g (52.5
mmoles) of N-methyl pyrrolidone and 50 mg (0.45 mmoles) of
hydroquinone were put into a reactor with 50 ml of acetic acid
ethyl ester, whereupon 9.0 g (52.7 mmoles) of phenoxy acetic
acid chloride were added at room temperature while stirring vig-
orously. The mixture reacted exothermically within 30 minutes
and was then further processed as in Example 16.
9.0 g (68.4~ of the -theoretical yield) of analytically
pure 2-phenoxy-acety-amino-3-methyl-2-butenoic methyl ester were
obtained.
3~C=
e ~
Cl4lll7o4N(263~3)
%C ~H %N
computed: 63.86 6.51 5.32
obtained: 63.71 6.48 5.21
melting point: 79C
H-N~ cDcl3/TMs):
S= 7,65 (s broad, 111) Nll;
6,65 - 7,55 ~m, 5ll)aroma~ic CH;
4,6 (s, 211) -COC112-;
3,7 (s, 311) COOC~13;
2,25 (s, 311) C113-;
1,~5 p~ (8, 311) C1~3-.
\
- 81 -
~xample 73 ~ ~7~988
246 mg (0.5 mmole) of tetra-n-butyl ammonium
perrhenate, 15.5 g (49.7 mmoles) of Z-azido-hexadecanoic methyl
ester, 5.2 g (52.5 mmoles) of N-methyl py~rolidone and 50 mg
(0.45 mmoles) of hydroquinone were dissolved in 25 ml of acetic
acid ethyl acid and 25 ml of acetonitrile, whereupon 5.9 g (52.3 `
mmoles) of chloro acetyl chlo~ide were added at room temperature
while stirring vigorously. The mixture reacted exothermically
within 30 minutes. It was further processed as in Example 3.
11.5 g (62.3~ of the theo~etical yield) of analyti-
cally pure 2-chloro-methyl-carboxamido-2-hexadecenoic methyl
ester were obtained.
Cl13~Cll2)l2cHFl-c-Ocll3
I~N-~i-
C191134N03Cl(359,94)
%C %H %N %Cl
computed: 63.40 9.52 3.89 9.85
obtained: 63.38 9.51 3.84 9.86
melting point: 74 to 75C
Il-N~ ( CDCI 3/l'MS ):
= 7,75 (s broad, lH) NH;
6,75 It, Il~) -Cl~=;
4,1 (s, 2l1~ -CH~Cl;
3 , 75 (s, 311 ) -COOCt~3;
1,7 - 2,5 (Il~, 2ll) -Cl~2-Cl~=-
1,25 (s, 2211) -~Cl~2)ll-;
3 '
- 82 -
7988
Example 74
246 mg (0.5 mole) of tetra-n-butyl ammonium perrhen-
ate, 9.25 g (49.9 mmoles) of 2-azido-pentanoic acid isopropyl
ester, 3.85 (52.6 mmoles) of dimethyl formamide and 50 ml (0.45
mmole) of hydroquinone were dissolved in 50 ml of acetonitrile,
whereupon 11.8 g (52.5 mmoles) of 2-[4'-(2-methyl-propyl)-
phenyl-propionic acid chloride were added at room temperature
while stirring vigorously. The reaction mixture was then
stirred for 15 hours at room temperature, whereupon it was
further treated as in Example 3.
10.0 g (58.0% of the theoretical yield) of analy-
tically pure 2-~2'-[4-(2'-methyl~propyl)-phenyl]-propionyl-
amido~-2-pentenoic isopropyl ester were obtained.
ClliC112CII~-C-Ocll(c113)2 ~C~13
HN- lC~ - ~CII ~CH2CII
C211131N03~345'48)
%C~H ~N
computed: 73.00 9.04 4.05
obtained: 73.21 9.11 4.21
melting point: 80 to 83C
1 Il-N~ c~cl3/~ ls):
= 6,9 - 7,3 (1~, 411) -Cll= a~omatic; 1 15 ~d, 61~) -COO ~
6,65 ~s broad~ 111) NH; ~C113
6,45 ~t, 111) -C112-CII=;O,g5 ~t, 3~1) C113-C~12;
4,6 - 5,25 (m, 111) COOCII-;~C~l
3,G5 ~q, 111) COCII-;0,85 ppm (d, 6H) -~1
2,4 (d, 211) ~ C112-;
1,G5 - 2,2 ~m, 311) -C~12-CII= -ICII;
1,45 ~, 311) Cl~-C~13;
- 83 -
77988
~xample 75
246 mg (0.5 mmole) of tetra-n-butyl-ammonium perr-
henate, 11.25 g (50 mmoles) of 2-azido-3-cyclopentyl propionic
acid isopropyl ester, 3.85 g (52.6 mmoles) of dimethyl for-
mamide, and 50 mg (0.45 mmole) of hydroquinone were dissolved in
50 ml of acetonitrile, whereupon 9.5 g (52.6 mrnoles) of 3-
acetyl-thio-2-methyl propionic acid chloride were added at room
temperature. The mixture reacted exothermically within 30
minutes and was then further treated as in ~xample 3.
9.6 g (56.2~ of the theoretical yield) of analytically
~ure 2-~(1'-methyl-2'-acetyl-thio)-ethyl-carboxamido]-3-cyclo-
pentyl-propenoic isopropyl ester were obtained.
6 C=IC-g-OC~I 3
IIN-Icl - ~C13-C132-S-c_cH3
O CH3 o
Cl7ll27No4s~341~41)
%C ~H ~N ~S
computed: 59.81 7.97 4.10 9.37
obtained: 59.55 8.22 4.12 9.94
melting point: 63 to 65C
II-N~ CDC13/'~'MS):
= 6,95 (s broad~ lII) NII;
6,6 (d, 1~3) -Cll=;
4,7 - 5,4 ~ In~ ) -COOCIl-;
2,15 - 3,5 (JII, 411) COI 11-;
2,3 (s, 311) COCI33;
l,3 - 2~25 ~m, 913) ~
1,25 ppln (d, 6I3) -C(CI33)2.
- 84 -
7~98~3
Example 76
246 mg (0.5 mmole) of tetra-n-butyl ammonium perr-
heante, 11.25 g (50 mmoles) of 2-azido-3-cyclopentyl-propionic
acid isopropyl ester, 3.85 g (52.6 mmoles) of dimethyl fora-
mide, and 50 mg (0.45 mmole) of hydroquinone were dissolved in
50 ml of acetonitrile, whereupon 8.5 g (52.3 mmoles) of dip-
ropyl acetic acid chloride were added at room temperature while
stirring vigorously. The reaction mixture was stirred for 16
hours at room temperature and then further treated as in Example
3.
9.2 g (56.9% of the theoretical yield) of analytically
pure 2-[(1'-propyl-butyl)-carbonyl-amino]-3-cyclopentyl-
proE~ionic isopropyl ester were obtained.
CII=C-~-OCH 3
IlN-uc-cl~(cll2cll2c1l3)2
O
~19Il33No3(323~48)
%C %H %N
computed: 70.55 10.28 4.33
obtained: 70.02 10.59 4.33
melting point: 148.2C
E~l(cvcl3/T~ls)
~= 6,8 (s broad, lII) NEI;
6,55 (d, lII) -CII=;
4,8 - 5,3 ~ , lII) COOCII-;
0~G5 - 2,8 (m~ 28II) -CII~CII2CII2CII ) ;
1,25 E~ l (d, 61I)~-C(CII3)2.
- 85 -
~;~7~798t3
ExamE~le 77
134 mg (0.5 mmole) of ammonium perrhenate, 7.85 (50
mm~les) of 2-azido pentanoic methyl ester, 3.85 g (52.6 mmoles)
of dimethyl formamide and 50 mg (0.45 mmole) of hydroquinone
were dissolved in 50 ml of acetonitrile, whereupon 8.5 (52.3
mmoles) of dipropyl acetic acid chloride were added at room
temperature while stirring vigorously. The reaction mixture was
stirred for 120 hours and a maximal reaction temperature of 80C
was attained. On further processing the reaction mixture as in
l~xample 3, 7.0 g (54.8 ~ of the theoretical yield) of analyti-
cal ly pure 2-[(1'-propyl-butyl)-carbonyl-amino]-2-perltenoic
methyl ester were obtained:
cll3cll2c~ C-C-Oc113
IIN- ICl -Cll ~ CH2C112CH3 ) 2
C~ 5NO3 ~ 255~36)
%C %H 96N
computed: 65.85 9.87 5.48
obtained: 64.85 10.18 5.26
melting point: 132C
NMI~ (CDCl 3/lrMs ):
~= G,9 ~s broad, lH) NIJ;
; 6,65 (t, 1111 -CH=;
3,75 (6, 311) COOC113;
1,85 - 2,5 ~In~ 211) -C112-CII=;
0,6 - 1~8 ppm (m, 1811) C113-C112; -Cll-(C112C112C 3)2
-86 -
Example 78 ~.27798~3
246 mg (0.5 mmole) of tetra-n-butyl ammonium perr-
herlat:e, 7.85 (50 mmoles) of 2- az1do-3-methyl-butanoic acid
methyl ester, 5.2 g (52.5 mmoles) of N-methyl pyrrolidone and 50
mg (0.45 moles) of hydroquinone were dissolved in 50 ml of
acetonitrile, whereupon 8.75 g (52.5 mmoles) of cinnamic acid
chloride were added at room temperature while stirring vigor-
ously. The reactiorl mixture was then stirred for 14 hours, the
maximal reaction temperature being 40C. The reaction mixture
was then further processed as in Example 3 and 9.3 g ~7.17~6 of
the theoretical yield) of analytically pure 2-[(2'-phenyl-
vinyl)-carbonyl-amino]-3-methyl-2-butenoic methyl ester were
obtained.
3 C=C-C-OCII
cll3 ilN-ICl-Cl~=C
C15ll17N3(259'31)
%C %H %N
computed: 69.48 6.48 5.40
obtairled: 67.44 6.61 5.41
melti.llg point: 138 to 1414C
111-NMI~ ( CDC1 3/TMS ):
c~= 7,1 - 7,6 (m~ 61l)aromati~ CII; Nll;
7,6 ~d, 1ll) -CO-C~I=;
6,5 (d, 1ll) -C=CII-;
3,7 (s, 3~1) COOC1l3:
2,15 (s, 3ll) Cll3-C=;
1,~5 PUIII (s, ~3ll) C113-C=.
-- 87 --
~X77988
Example 79
.
134 mg (0.5 mmole) of ammonium perrhenate, 7.85 g (50
mmoles) of 2-azido-pentanoic acid methyl ester, 50 mg (0.45
mmole) of hdyroquinone and 3.85 g (52.6 mmoles) of dimethyl for-
mamide were dissolved in 50 ml of acetic ethyl ester, whereupon
12.0 g (52.02 mmoles) of 3,4,5-trimethoxy-benzoyl chloride were
added dropwise at room temperature while stirring vigorously.
The reaction mixture was stirred for 240 hours at room tempera-
ture and then further processed as in Example 16.
8.7g g (54.1~ of the theoretical yield) of 2-[(3',
4',5'-trimethoxy-phenyl)-carbonyl-amino]-2-pentenoic methyl
e~ter were obtained.
C113CH2CH=C-C-OCH3
OC~I3
I ~ 3
o OCE13
C16ll21NO6~323,35)
~C ~fl %N
computed: 59.43 6.55 4.33
obtained: 59.21 6.75 3.70
melting point: 137 to 140C
;
1 Il-NMR ( CDC 1 3/TMS ):
~= 7,65 (s bxoad, lH) Nll;
7,05 ~s, 2H)a~o~aticCII=;
6,7 ~t~ 1ll) CII=C;
3,85 ~s, 3ll) COOCII3;
l,B - 2,55 ~m~ 2H) -Cll2-;
1,05 pp~ ~t, 311) C113-.
i
- 88 -