Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- l -
This invention relates to the promotion of
plant growth, and more specifically, to a method of
promQting the photosynthetic action and root-forming
action of a plant and promoting its growth.
One method of increasing the ability of a plant
to produce a substance is to increase its photosynthetic
ability. Some attempts have been made to promote the
photosynthesis of plants by selecting substrates or
employing chemical means. For example, choline salts are
known to have a plant growth promoting effect. U. S.
Patent 4,309,205 discloses a method of increasing the
quantity and quality of flowers and fruits of a plant
growing in soil which comprises applying to a mature
plant during its reproductive stage a flower ox fruit
quantity and quality improving effective amount of at
least one non-toxic salt of choline in an aqueous medium.
U. S. Patent 4,488,901 discloses a method of increasing
the cold resistance of a plant, which comprises treating
a cultivated plant before a temperature drop with an
2s~ aqueous solution of at least one compound of the formula
HO~CH2tnNE12 wh~rein n is an integer of 2 to 5
or its N,N,N-trimethyl-quaternary ammonium salt thereof~
The present inventors have long worked in
search of a substance capable o effectively promoting
the photosynthetic and root-forming action of plants by
chemical treatment, and have now found that a certain
aminoe~hanol derivative has a cytokinin action and the
ability to promote significantly the photosynthetic,
growing and root-forming actions of plants.
According to this invention, there is provided
a method of promoting the growth of a plant, which com-
prises applying an effective amount of at least one
active compound selected from the group consisting of
agriculturally acceptable salts of compounds represented
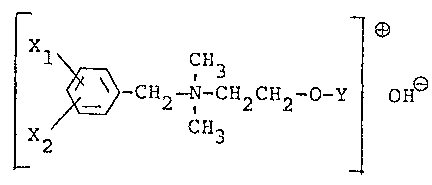
by the formula
,~-r~S
.,
.
.: :
~t'~9~76~3
~ ~ ' 3 ~ (I)
wherein Xl represents a hydrogen or chlorine
atom, or a methyl, trifluoromethyl, ni ro,
methoxy or t-butyl group, and X2 represents
a hydrogen atom, or both Xl and X2 represent
a chlorine atom or a methyl group; and Y re-
presents a hydrogen atom, or a C2 6 alkyl-
carbonyl, benzoyl, N-phenylcarbamoyl, N-3,4-
` dichlorophenylcarbamoyl, chloropropylcarbonyl,
methoxycarbonyl, carbamoyl or methacryloyl group,
to the stalks, leaves, roots or seeds of the plant or to
soil.
- In formula (I), the alkyl moiety of the C2_6
alkylcarbonyl group represented by Y may be linear or :branched, and specific examples of the C2_6 alkylcarbonyl
group include acetyl, propionyl, n-butyryl, isobutyrylp
n-valeryl, caproyl and capryloyl groupsO
In formula (I~, the moiety ~ - is preferably
one in which both Xl and X2 are hydrogen atoms, or
one in which Xl is a 2-methyl or 3-methyl group, and
X2 is a hydrogen atomO
The agriculturally acceptable salts of the
compounds of formula (I) include, for example, hydrohalo~
genates such as hydrochlorides and hydrobromides, in-
organic acid salts such as phosphates, nitrates, sulfatesand carbonates, and organic acid salts such as acetates,
citra~es, lactates and L~+~-tartrates~ Of these, the
hydrochlorides and hydrobro~ides are preferred.
The agriculturally acceptable salt of the
, ' .
~'
. , - . '
--
.'7~3'~3
compounds of formula (I) can be produced, for example,
by (a) reacting an N,N-dimethyl ethanolamine compound
represented by the formula
CH3\
N-CH2CH2-o-y (II)
CH3
wherein Y is as defined hereinabove,
with a halide represented by the formula
Xl
~2 -CH2-Hal (III)
wherein Hal represents a halogen atom, and X
and X2 are as defined above,
to obtain a compound represented by the following formula
_ ' _ '0 1,
Xl ~ CH3 Hal~ ~IV)
_X2 CH3 .
wherein Xl and X2 are as defined above, or
~b) reacting a compound of formula tIV) in which Y is a
hydrogen atom with a C2_6 alkylcarbonyl halide, C2_6
alkanoic acid anhydride, benzoyl halide, N-pbenylcarbamoyl
halide, N-3,4-dichlorophenylcarbamoyl halide, chloropro-
pylcarbonyl halide or methoxycarbonyl halide, carbamoyl
halide or methacryloyl halide to form a compound of
formula ~I) wherein Y is as defined but other than a
hydrogen atom.
Some embodiments of their production are shown
below.
. - .- -- - :
.. .
. . '
- . - . .
- . :
. -- . . -- .
.
~,7~ti)s`t~
.
PRODUCTION EXAMPLE 1
Under ice cooling, 4.83 g ~30 millimoles~ of
2-chlorobenzyl chloride was added to 2.67 g (30 milli-
moles) of dimethylethanolamine dissolved in 10 ml of
ether. The mixture was left to stand at room temperature
for 2 days. The crystals that precipitated were sepa-
rated by filtration, washed with ether, and dried under
reduced pressure to give N-2-chlorobenzyl-N,N-dimethyl-2-
hydroxyethanol ammonium chloride (compound No. 4 in Table
1 given hereinbelow) represented by the following formula.
CH2-N-CH~CU2-OH; Cl~
Yield~ 48 %
PRODUCTION EXAMPLE 2
In 20 ml o~ isopropyl ether were dissolved
4.47 g (30 millimoles) of 2-dimethylaminoethyl butyrate
CH3\
( N-CH2CH2-Q-COCH2CH2CH3) and 3.8 g t30 millimoles) of
CH3
benzyl chloride~ and the solution was left to stand a
room temperature Eor 4 days. The solvent was then evapo-
rated~ The crystals that precipitated were separated by
suction filtration, washed ully with isopropyl ether,
and dried under reduced pressure to give N-benzyl-N-(2-
butyryloxyethyl)-N,N-dimethylammonium chloride (compound
No. 29 in Table 1 given hereinbelow) represented by the
following formula.
_ _ ,~
CH3
~ CH2-N-c~2cH2-o-coc~2cH2cH3 Cl~
CH3
Yield: 29 %
,
_ 5 _
In the same way as in Production Examples 1 and
2, the salts of compounds of formula ~I) shown in Table 1
can be obtained. Table 1 also describes the compounds
obtained in Production Examples 1 and 2.
.
- : . . .
.
:- .
': : ' ' - . ' ' -
,
- : . :
r7~
-- 6
. ._ _ _____ - -T. I . . __.. __ _ _ _ ____ . ___
~ ::~ ~ :~ ~
~ ~ _ U~ _ ~ _ ~
5 r-l q 11 5 11 m
~ ~ ~ `
J~ ~ oi_,~ ~ JJ ~ ~ ~n
t~ ~ ~ X-- l _ ~ _ ~_ _ l _ _, o
~ ~ ~ ~ ~D C~l ~ ~ O 1~ O G~
~ ~ ~ ~ ~ ~ ~ ~r ~ I` U~ U7 ~ .
~ r~ 3~ 1~ ~ ~ 1~ ~ ~r ~ ~ :c
~! ~c n m ~ m m ~ c m :~
~ O !n J- U~ U~ ~ ~ U~
_, _ _ _ _ _ _ _ _,
N ~ ~ ~ ~ ~ ~ ~ r~
. __ _ _ _ ... __ ___ . _._ .
o ul C~ ~ ~ ~> o a~ ~
,~r ,~ ~1 _1~ o~o ~OG~
o`~ 0~ o`~ ~ o~ ~ ~ , ..
~ ~ ~D 1` In O U~ O 1~ U~ U~ ~ U7 i`
1~ u ~ ~ 00 ~ ~o ~r o ~ c~ c:~ !`U~ ;~ r-1 ~--1 r-~ ~ l r.~ r
~j _ tn~ ~ ~ ` 1-~ C`i U`) In C:
C~ N 0~ N ~ C`J O N Cl~ t~
-l - -~ - - -------------- - - -- --
q~ .~ r~ ~ er
~ E~ l l l l l ,~
- ~ -- --- - - - -~ -
~ ~ ~t ~ ~
U~ t~ H C_~ C.~ ~) H
. ,,___ ._. . __ ... ~
~ 5: ~ m ~ ~: rr~
N . ___ .. __ . ~ - . __ - __
x m ~: ~c 5: 3:: tl:
.. - . . . - .
~ ~ ~ ~ ~ y y -~
- -- -- - - ----- ----
~o ~ ~ ~ ~r I
~ - - - - -
~f~ 3 ;iJ~
_ ~ a ~ m ____ m $~
~ ~ ~ m ' ~ L~ ~ ~ o
~_ ~u~ E~ 3 ~ ~ ~itq O
~<1 In~D ~ 1`00 u~ 5 _
~; r~ ~ ~ ~ ~r ~ ~r m _1 ~ ~r
m m m ~ c: m m m m ` c ~q m
~ ~ In ~ ~ u~ ~ ~Q ~ 07 E~ .a
~ D ~ 0 ~ r`.U~ î ~~~
. _ _ . . ___
o ~r _1~ r- _1
,~o ~ ` ~r~
_ ~ 0)o ~ ~0` ~`0`
' . _ _ ~ _ . _ _ ... _ _ _. ...
~ ou~ ~ ~ L~
~ i~ cq m ~ m
__ . . . ... ~ .,
. 33 :~ ~:
. , ~ _
x~ m m m c~) Y
._ _ ,,............... . . _ _
x~ ~ ~ ~ Y Y
.__ ... . __ . .. _ _ _ :
~ r~,, . ... c~ ~o ~
.
.
. : , . .
- . . .
- . . - . - :
': ' -,-
-- 8 --
, _ .u~ _. ~ ~ '-- ~ .
~ 'r 0 ~ tn ~ ~:~
m ~ e ~ ,
êl~ ~ m ~ ~ îq ~ I_~q 8
~~NI~ ~D ~r ~ ~ q
~ ~tq ~ r tn ~ n ~n 5 _ ~__ o
SL?~1 a~ ~ ~ ~ ~ ~D
~q ~ ~ ~ to o ~ ~ ~ ~ ~
_c~ _, _ ~Vl N 1~ ~ N N N
~m ~ ~ ~ ~ m m ~ m ~ m m
~r ~ ~ ~
tQ t~ m tli ~ tO t JJ tn ~ N ~ ~
c~l o a~ o;~ u~ o ~
e R ~ D ,~ D ~ c>
_ _ ~ ~ ~ t~ ~~ d'
. _ .. _ .__ _
Ir) N ~D ~ i O ~1
` ~ ~ O ~ ~ .'
1~ 1~ c~O -t 0~ ~1`
~ ~ ~ co ~r ~ I~ ,1 ~
In ;, ~ _l ~ ~ ~
_ ~ ~-~ ~ ~ ~ .
H ~ I~ O ~I O ~`J ~ ~ 0~
c _~ co ~ _I ~ oo ~ ~_1 1`
, . ~ . _ .__ . _ ._
CO~ .~ C~ ~O
~ O~ ~ l l ~
e ~ ~ ~ r~-
_ _ . __ ~ _ I~ ~.
m lq c~
_ .
. ~ :r ~ .
~ .'
. . _ . . .. _
x~ Y :c ~ m m
~ . .
~ _l ~ ~
x Y ~) ~ m m
. _ ~ .. . _ _
L~ .
o ~ ~ ~r u~
~,x ,~ ,1 _1 ,1 ,~
. , ____ _ ~ .__
3~ 3~3
_ 9 _
_ _ _ . . .. _ _
~ :~ In
~^ ~ ta ~: ~3 C" m ~ ~ ~ c:
m 3~ g
m ~ t~ ~ ~ ~ ~
. ~ _ ~--~t ~0 ~ ~ ~ _ E~ E~ N
N 'I m m ~ N ~ i ~ ~ _ m O
C~ t~ N ~ I~ ~r m~
aJ e 1l ~ m m m e
~, Ul~ ~,0~_~CO
~ N ~ ~ r-J U7 ~ ' ~ ~ ~
cl~m 1l ~ ~ ~ to ~ I~ ~
,
~ u~ Ln o In 1~
ll ~ ~ C`~
~7`'~ O~D~ ~I`CO
-~--- --- ---- - -~ - ---
~ ~ ~ I~ ~r~
U~ ~ ~ ' i ~D
~1 ~il`
u~ ~ ~ ~ ~ ~ ~ ,io
~ ~ u~ ~ u~ u~ ~ o ~ ~o o
~ ~1~ _~ ~ ~1` i~
H U~ ~D ~ O C~l O CO ~ ~ ~ ~
~ ~ r` i~ I" I~ I` ~
.~ . - _ _
~ C~l O CO
~ Q~t.) ~-1 . ~i ~ 7'1
-~1 E3 a) o ~ i r`
~ . . . __ ~ _ . ~ ._ __ _ ~
u~ ~ v ~ ~ a~
___ - _- __ .
C~
~c~ ~r
3 83 8`'
. . ..
m m m m ~r:
__ -
~ m m : :: :r m
. __ _ .~
~ ~ .
O. I~ co a~ o
_I ___ ____ N , ._
-
'. '
,
,
;79~7~
-- 10 --
_ _ .... __ .__ _. ,
~-- N o ~ ~ N C
m ~ _6 ~ c
, ~ 5: ~ m 1` ~ ~ ~ ~ ~ m O
u~ Ln ~ n u~_ ~ ~ ~ u~ o
9.1 co _ a~ ~ U:> a~ ~ ~ ~ o o~ ~3
m r~ ~ _ m
~; ~ ~ ~ ~r mu7 ~ ~ ~
i~ 5~ m m ~ m ~ m m ,~ m w D: m ~:
~ ~ ~ ..
u~ ~ ~n ~ w u~ ~ ~ ~ u
u~ cn I ~ ~ c~ u~ In co ~r ~o o N ~
~ O ~ ~D ~ m ~ co ~ a~ r~ ~ ,~
~t- ~~C~ ~ ~ ~ ~1~
__ ~ ~ . . ., . _. .
q ~ o~ ~ u~ ~ ~o
l ~ Ir~ --I ~ r~ n ~D N 1` U) OD .
U~ ~ _1,1 ~1 _1~1 ~ ,_i
_ ~ --
~ ~ ~ ~ oo ~ a~ c:~
~ ~ _l ~ _l
.~ _, . __-- .
~ ~D U~O ~D
~o) ~î~
~1 ~3 0 _1 Cl~ r~
__ . ~__ ... __ _
E~ ;~ a~ ca m~)
. ...~ ~ . - ' .,
v ~ .
~r ~`
N . . _ _ _
x m m m~:: m
. ~ _. . -r_ _ _ ~ .
ec ::c m
.._ _ ~ _ _
~ ~ ~ N .... _ _
,: .
-
- ~,
,
3~ " ",c~7~,~3
-- 11 --
A _ ~: . _ ~ _ , __.__. t _ ~
~ X ` ~N _ ~`I r
_ _ 5~ co ~ m ~ c
m ~ ,1l ~q,.,.,
:: U~ Ul ~ ~ ~ ~D ~ m $
. ~;t ~ r~t ~ ~ _ l ~ t~ ~t ~ E~ ~ ~ E3 ~ O
9~ S~ ~ D ~ I~ DO ~t
~ Cl 5 ~t N 1~ m ~ m m ~ a~
~ t~mm^ I~m ~u, _ ^. _~O
z ~ ~ , u, ~ :q ~ ~t~t a~
~ N ~ I t 5 '~ ~ Ut Ui t :~:
. . ~ ~ 0--r 0-~
l~ ~ U~ O ~ 0~
_ o t~ 'n ~D ~ 1~ r~
t ~D~ O` ~ ~ro ut
r~ _~ un ~ S~ ~D
--a~ n ~ o~ ~P~ , ~r ~D c~ o
~_ ,~ r- ~ ~; t~
.~ ___ . O~
c~t ~ O~; l l l l tn
~_ U~
, ._ _
~ æ' ~ m c,~ ~ ~
. ,..___ . __ ~.. ~ .._ ~
~ ~ ~t
~ C~'t ~
8~
. _ ,.._ _ .,
x m m m :: D:~
,, _ ... __
x-l :~ m :1~ 'F' ~
. ._ _ . _ _
~ ~ _ ~ .~ o _~
,
'~ ' ' ' ~ ,
:
- . . . .
:
I -
,
- '
-- 12 --
. . __ ' ,. __ _ _ ...
t`l ~ m m O-- ~C a~ ~1
~ I~ ~ " m ~ ~ ~ ,~
1- ,11 Y ~ ~r ~ 11 f'~ ~ ~ ~J ~
r~ ~ ~ 1~ ~~ ~r-- ~:
tN ~ ~ m ~ ~ ~ m p~ ~ m 8
~ ~~ ~ ~ ~ ~ ~ co ~ CD ~
~ _ ~ ~ o ~ ~ ~7 u~ o ~n ~
a) ~ _~ ~ ~ _ ~ ~ U~ O u~ m ~3
tq ~ ~ o cn ~ ~ m ~ t~l
1~ ~ ~ m ~ ~ 00 ~ ~ m ~) E~ t~
~ _~ ~ ~ ~ ~ CO ^ CO
z: m ~: . ~ _ ~ ~q ~ _ ~ . D:
~ ~ ~ 1~ ~ m ~ ~ m ~ ~ ~ m
~ ~ ~ ~ ~ r~ ~ ~ ~ ~ m
u~ ra u~ e u~ ~ ~ N 1~ ~`1
cs~ ~D I~D o ~ u) I ~ m ~ ~ m ~
~ ~p ~ C'~ ~ u~ ~ ~ ~ ~ L~ ~ N ~r ~ ~ N
r~ ~ ~ N m t~ 5~ ~ ~ ~ ~ ~ ~ ~m
~` ~_ 6` ~
_, ~1 ~ _1 o
~_~^` ~ _~ ~ _~ ~D
-~ ~ c~ r~ ~D ~ ~9
_ ~ o ~ 1~ o~r ao co ~ ~
,~ o ~1 o ~ o ~1 o ~1 o
1- r~ _1 ~ ~ ~ ~1 ~ ~
. . -~ ~ .---- . ,,
c~ o ~ a~ In ~`I N_1 E3 -~ ~ co In t~l ~
~ _ _ _ ~ _ ~. _ ._
~ ~ ~ ~ t~ ~ C~
._: ~ ..____ . . ,,, ,
_... _ .,._ _ ~_ _
x t: _ m _ m
._ _... _ _
x~ ~ Y ~ ~ ~
.. ._. ___
~o ~ ~ er u~
~,z
_ . _. . . __
- ` '` ~
--
3 7
- 13 ~
. . _ _ E ~ ~ ~: 5 __ ~ ~ ~ ~
_ ~ ::C ~ ` N ~q ~ ~1 ~D Ei U~ ~r ~ 3
. ~ --Nm ~ a~-- ~
tV) ~ N ~ ~ ~ ~ ~ ~ O
~3 m ~ 1~ ~ m W m ~ C I ~ C~ m
.J~ ~, ` ~ ~ ::C .IJ _ _ ~. U~ N N :r:-- N O
~ R. u~ ~_ ~--~_~ ~--~m_ ^~5:
ut ~~ 5 co ~ o, o. ~ m u7 ~
: ~ ~ ~ a~ tg m~-
~ ~ ~ ,~ ~
U, ~r ~ ~r m co ~ ,~ --N t~l ~ r- C~ 11) ~ a~ r- m
~ ~ u7 a~ 1~ ~1` ~ ~ 11 m m ::c ~ CO ~ 1` :c
.,. ~ 0~1~ Ol~D~ -- O~C~
. __ ~ .
U~ ~ ~D ~r
l U~ U~ ~ ~ ~ ~C> .
~ ~ ~ ~ ~r _1~
l~ ~ ~ ~ C~
~ ~ ~o ~
~ ~0:~ .... _ , ~.
V ~0~ ~ ~ ~ ~
___ _I ,~ U~ U~
~ _ . _ ____ _ ._ _ . .. _ _.
: ~ ~ ~q r~ ~ m
. __ _ ~
. m . .
. _
X~ ~ Y ~ ~
_ _ - _ . .. ~
L ~3 -
~ ~,,Z __ _ . _ _
.
.
.
,
:, ' '': " '
- : . : ' ,
9 7 ~ ~ 3
_ ~ N . _ _ _ C
5 ~ ~ m o
. ~ ~ ~ m e ~ ~ ~ ~ o
g.~ ~ cO u~
u~ c~ m
~: ~~ ~ ~ ~ ~ ~
~D m ~c 1l ~^ 2^ ^ ~ m
,, I ~ ~ i, m u~
e ~ ~ x ~ e u~
~r ~ ~ ~ r o ~ a~
) ~ ~ ~ C~ t` N ~ _I
. 0 ~ O ~
_ . __
U~ U~oo 0~
` ~ U~ U~CO
~ ~ I-oo ~ ~ I l .
~ _ ~ "~r o~l~
----- ~ ---- ~ - ~ - - -
o ~ -- ~ 3 ~ ~
~
~ . .... ..
~ ~_ _ . .___ m .
~ ~ ~ :~ ~q D:
8 8
.
~ ~ ~ ~: :~ D::
_ . ._
X ~ ~ tc 5: ¢
. . . . ... . .. .
~ o Z ....... .. . _ . . U~
- ~ .
r~ L3
-- 15
-~ ~rl
~ ~ l l l l l
Cl
~1 ~ ~ _~ u)o
~D a~ ~rco ~=")c~
o c~ c~i co o In o cO o
U~ ~ ~ I` c~ ~ ~r ~ ~ u~
~ t~1 ~ C~_~ ,~,ll ~1
~ _ _. _ . _ . . . _ _
Ei ~/ ~ U N ~ O
_l ~ ".. __ _ ~_
;~ ~1 ` ~ v oa ' ~ ;~
... _ __ _.__
~ ~ ~q
~c~ c~ m m S m
. ~ . , .
~ c~ Y ~ ~ ~
~ _ ~ _ ....
~ ~ _ a) ,__ ~ o
- ~
7~i~3
~ 16 -
Among these compounds, compounds Nos. 1, 2, 3,
9, 12, 13, 15, 16, 18, 19, 21, 26, 27, 28, 29, 43, 44 and
45 are preferred. Compounds Nos. 1, 2, 15, 27, 28 and 29
are particularly preferred because they are effective at
low dosages. Compounds Nos. 15, 18t 21, 26, 27, 28, 29
- and 43 have high cytokinin activity. Compounds Nos. 1,
2, 3, 4, 6, 12, 13, 15, 29 and 43 have high growth promot-
ing activity and high root-formation promoting activity.
The agriculturally acceptable salts of the
compounds of formula (I) (to be generically referred to
as the active compound of the invention) have the ability
to increase the photosynthetic action and/or root-forming
action of plants and to promote the growth of the plants.
There is no particular limitation on the plants whose
growth can be promoted in accordance with this invention,
and they may include various agriculturally or horticul-
turally cultivated plants. Speci~ic examples include
cereal plants such as rice, wheat, barley, and corn,
leguminous plants such as soybean and adzuki bean; plants
having underground tubers or bulbs such as onion, garlic
and potato; vegetables grown for their edible roots such
as radish, sweet potato, beet and carrot; fruits such as
peach, persimmon, grape and apple; vegetables grown for
their edible fruits su~h as tomato and cucumber; vegeta-
bles grown ~or their edible leaves such as lettuce,cabbage, cauliflower and spinach and flowers such as
tulip and cosmos.
The active compound of this invention may be
formulated for application in any known form, such as a
wettable powder~ granules, an aqueous solution, an emulsi-
fiable concentrate or an aqueous suspension, using a
conventional agriculturally acceptable carrier or diluent.
There is no special restriction on the carrier or diluent
~sed in the formulation so long as they are agriculturally
acceptable. For example, talc, clay and bentonite may be
used as a carrier for a wettable powders and granules.
. .. ' . ~ ~ .
~ 17 ~
The aqueous solution is most preferred as the form in
which the active compound is applied.
The resulting formulations may contain the
active compound of the invention in an amount of 1 to 75
by weight, preferably 30 to 75% by weight. Such formu-
lations may further contain another conventional agricul-
turally active ingredient such as a fertilizer, herbicide,
insecticide or bactericide.
The formulations may desirably contain a sur-
factant. The amount of the surfactant is, for example,0.02 to 20% by weight, preferably 0.1 to 5% by weight,
depending upon the form of the formulation to promote the
adsorption and penetration of the active ingredients.
Preferred surfactants may include nonionic surfactants
such as polyoxyethylene alkyl ethers ~e,g. polyoxyethylene
lauryl ether), and anionic ~urfactants such as lauryl
sulfonate triethanolamine salt.
The active compound of this invention may be
applied by any metbods known per se. For example, it may
be sprayed onto the stalks and leaves of mature plants,
or poured onto parts near the roots. Seeds are preferably
immersed in a solution containing the active compound of
the present invention.
The rate of application of the active compound
Of the invention varies~ for example, with ~he type of a
plant to be treated, the stage of plant growth, and the
manner and timing of application. For example, for
spraying onto the stalks and leaves of a plant, the rate
of application of the active compound of the invention
normally ranges from 3 to 1,500 g, preferably from 10 to
1,000 g, per hectare of the cultivated area. An aqueous
solution is a preferred type of formulation for foliar
application, and may contain the active compound of the
invention in a concentration of 10 to 100,000 ppm,
particularly 20 to 50~000 ppm~
Generally, ~he good time of applying the active
3~7~3
-- 18 ~
compound of the invention is when photorespiration of the
plant is at its peak. For example, it is desirably
applied at some point during a period from the reproduc-
tive growth to the harvesting time. To some plants,
however, application during vegetative growth may provide
more desirable effects. In other words, there is no
specific limit to the timing of application.
In the case of pouring the a~tive compound of
the invention, it is usually advantageous to apply an
aqueous solution containing the active compound of the
invention in a concentration of 0.1 to 300 ppm, preferably
0.5 to 100 ppm, to parts near the roots of a plant at a
rate of 5 to 100 m3, preferably 10 to 50 m3, per
hectare.
When the active compound of this invention is
used to treat plant seeds, it is suitable to immerse the
seeds in an aqueous solution normally containing 0.05 to
1,000 ppm, preferably 0.1 to 300 ppm, for about 1 to
about 48 hours, preferably about 3 to about 24 houes.
To use the active compound of the invention for
root formation or anchoring of rice seedlings, it is the
general practice to pour an aqueous solution of the
active compound onto the roots of the rice seedlings
before transplantation.
The method, time and rate of application and
the expected effect of the active compound of the in-
vention on typical plants are summarized in Table 2.
' ~ ' ~ ' ' ~','- '- , "
-
.
t7~ 3
19
Table 2
-
Meth~d of Time of Rate of Expected
Crop application application a~pl atl_ effect
-
rice, wheat, foliar from 40 days 50-1000 g/ha increased
barley before heading harvest
to 10 days
after heading
soybean foliar from 10 da~s 20-300 g~ha increased
(leguminous before flower- harvest
plants) ing to 20 days (increased
after fl~ering number of
~)
onion, garlic foliar early stage of 20-~00 g~ha buIb swelling
tulip bulb swelling
potato~ foliar early stage of 20-300 g/ha tuber growth
tuber growth and increased
. yield
peach, foliar flcwering stage 3-300 g/ha size increase
persimmon, to 10 days of fruits,
grape, apple before harvest increase of
sweetness,
maintenance
of freshness
wheatl barley foliar 2- to 3-leaf 3-300 g/ha growth
stage promotion
ri~e seed i~mersed for 0.1-300 pem promotion of
treatment 24 hours after growth and
immersion in root formation
water for 2 days
wheat, barley seed immersed for 0.1-300 ppm prcmotion of
treatment 24 hours growth and
root formation
tomato, seed immersed for 0.1-300 ppm promotion of
lettuce treatment 24 hours grGwth and
root formation
rice seedling pouring from the 2-leaf 0.5-50 mg2 promo~ion of
stage to the /1800 cm growth and
time before ~1-100 ppm) root formation
transplantation
tamato pouring seedling stage 0.1-10 mg growth
seedling seedling promotion
~1-100 p~m~
- .
. . .
. . .
~1.. ~ t 3~ 3 ~ ~ ~3
- 20 -
~ y applying the agriculturally acceptable salt
of the compound of ~ormula (I) to a plant in accordance
with this invention, the photosynthetic action and root-
forming action of the plant can be greatly increased, and
consequently, the growth of the plant can be promoted.
For example, the active compound of this invention can
promote the root formation of C3 plants such as rice,
wheat, barley, beet, sweet potato~ potato and onion and
C4 plants such as corn and sugarcane, nurse their sound
seedlings, promote their growth in the early stage, and
increase the harvest of the crops. Furthermore, it can
increase the sweetness and size of fruits such as apple,
persimmon, peach, orange and lemon, promote their color-
ation, and maintain their freshness. It can further
promote size increase of bulbs of flowers such as tulip,
and promote the flowering of cosmos, etc~
For application of the active compound of the
invention to plants, it may be formulated into a wettable
powder, an aqueous solution~ a suspension in water or
2~ oil, etc~ Typical formulation examples are given below.
Formulation Example 1
Aqueous solution:-
Fifty grams of compound No. 15, 10 g of polyoxy-
ethylene oleyl ether and 10 g of triethanolamine lauryl
sulfate and 180 ~ of pure water are mixed to prepare an
aqueous solution containing 20% of compound No. 15.
Usually, i~ is used after it is diluted to 100 to 2000
times.
Formulation Example 2
Wettable powder:-
Fifty grams of compound No. 29, 2 g of sodium
dodecylbenzenesulfonate, 1 g of polyoxyethylene alkyl
aryl ether, 10 g of talc, and 37 g of bentonite are
uniformly mixed and pulverized to give a wettable powdPr
containing 50~ of compound No. 29.
The excellent plant growth promoting activity
'7t.
-- 21 --
of the active compounds of this invention are demonstrated
by the following Test Examples~
Test ExamE~le 1
Photosynthesis using protoplasts:-
~heat (Variety: Norin No~ 61) was cultivated
. for 10 days in vermiculite as soil in a phytotron kept at
25C in the day time under natural light and 20C at
night. Protoplasts w~re isolated from the wheat by a
conventional method [see Plant Physiol. ~1978), 62,
313-319]. The effect of the protoplasts on photosynthesis
was examined in the folloiwng manner using an oxygen
electrode~
The protoplasts were incubated for 1 minute
with the test compound in a rPaction solution [50mM
HEPES-ROH buffer ~pH 7.6), 004M sorbitol, lmM EDTA, IomM
sodium hydrogen carbonate], and then light ~100,000 lux)
was irradiated to initiate photosynthesis. The activity
was examined in comparison with a non-treated lot ~con-
taining no test compound~O The results are shown i~
20 Table 3.
9 ~
- 22 -
Table 3
Compound Concentation Increase in .
NoO (mM) photosymthesis ~*)
3 1 f~ T
13 n +-t~+
18 n ++ .
23 ~ +-t -:
2 6 n +++
2 9 r + +~ ~:
41 ~ ++
42 . ++~
(*) -: Decreased from that in the non-treated lot,
+: Same as
-+: 0-5~ increase from n
ff: 5-10% increase from n
~ : 10-15% increase from n
++~+: More than 15% increase from n
- ' -' - '- .: .
'
', ' ' ~"', "` :'
'.
-- 23 --
Test Example 2
Cytokinin activity was examined by using cucum-
ber in accordance with the method of R. A. Fletcher and
D. McCullagh [Planita9 101, 88 (1971)].
Seeds of cucumber (a green ground crawling
species with long nodes) were immersed in water for 3 to
4 hours, and then sown in fully watered nursing boxes (30
x 20 x 3 cm) containing vermiculite at a rate of 200 per
box. The seeds were nursed for 5 days at 28C in the
dark, and the emerged yellow cotyledons were cut off
under pale green light. Twenty pairs of the cotyledons
were put in a Petri dish having a diameter of 9 cm con-
taining 5 ml of 2mM phosphate buffer (p~ 6 r O ) containing
each of the test compounds shown in Table 4 in a concen-
tration of 10 ppm. The Petri dish was maintained in thedark at 28C for 15 hours, and then placed under day
light fluorescent lamp (3000 to 4000 luxes) for 4 hours.
Five pairs of the cotyledons which turned green were
extracted with acetone, and the amount of chlorophyl per
unit weight of the raw cotyledons was calculated~ The
results are shown in Table 4.
~, 3 ^~ 7~j~3
- 24 -
Table 4
Com- Concentra- Weight ratio Amount of .
pound tion of the cotyl- chlorophyl
No. (ppm) edons to the ~the ratio
non-treated to the
area are(.))
Non- _ 100 100
treated (205 mg/5) (70.8 kg/g)
_
107 199
21 10 106 186
18` 10 105 182
26 10 101 17~ .
28 10 103 177
13 10 105 165
27 10 106 2~4
29 10 99 199
43 10 105 185 .
t*): Rel~tive proportion when the value in the
non-treated area is taken as 100.
' -
- 25 -
The active cornpounds in accordance with this
invention show a high effect in an actual field test in
spite of having nearly the same cytokinin activity as
benzyladenine. This is considered to be because the
active compounds of this invention are well absorbed and
transferred to the plant body. Another cause would be
that the cytokinin activity of the active compounds of
this invention is suitable for the growth promotion of
the plants. The active compounds of this invention have
the advantage of being much better soluble in water than
zeatin and benzyladenine.
The foregoing is considered to be the reason
why the active compounds show a higher effect in a field
test than in a basic indoor test.
Test Example 3
Upland farm soil was put in Wagner pots ~lf5000
a) and a chemical fertilizer ~N: 0~2 g, P: 0~48 g, K:0.32
g) was applied as a base fertilizer at a rate of 2 g/pot.
On ~arch 5, soybean (variety: Okuhara ~S-l)
which had been immersed for 20 hours in each of the test
chemicals in each of the concentrations shown and washed
with water were sown at a rate of one per pot, and grown
in a greenhouse. In the non-treated area, the soybean
was immersed in water for 20 hours. On March 15, 10 days
after the sowing, the seedlings were reaped, and the dry
weights of the stalks and leaves and the underyround
portion were measuredO
The results are shown in Table 5.
.. . .
~ ' ' .
'7~i9
- ~6 -
Table 5
. _ _
. _ _ _ _ .
Ratio to the non-treated area
Com- Concent-
pound ration _ _ _
No. (ppm) Dry weight of the Dry weiqht of
stalks and leaves the root
._ _
Non- 100 100
treated _ (1.0 g~plant~ port on
1 100 158 217
1~8 213
2 100 160 210
168 243
3 100 160 190
16~ 133 :
13 100 123 143 -
158 217
100 1~ 227
146 193
29 10~ 1~9 210
157 205
12 100 152 177
165 217
6 100 160 233
150 183
4 100 162 200
165 200
43 100 158 195
157 199
. ._ . .
(*): Relative proportion when the value for the non-
treated area is taken as 100.
'
~:-; ' ' ' ' : . -
'
~9~ 3
- 27 -
Test Example 4
A liquid preparation containing each of the
active compounds shown in Table 6 in a concentration of
50 ppm was sprayed onto clusters of grape ~variety:
~yoho) three days before harvest until they were ~ully
wet. After the lapse of days indicated in Table 6, the
number of berries which dropped from the clusters after
the harvest was measured. One area consisted of three
clusters each of which had about 80 berries.
The resutls are shown in Table 6.
Table 6
_
Compound Cumulative total ~%~
NQ.
.. _
0 day 5 days 7 days 9 days 11 days
Non- 0 6 15 26 41
treated
~ ,
3 0 0 0 15 24
13 0 0 0 4 17
0 0 0 ~ 18
26 0 0 0 7 1~
29 0 0 0 3 11
43 0 0 0 10 16
Test Example 5
Paddy soil was filled in Wagner pots ~1/50U0
a), and a chemical fertilizer (nitrogen content 10%~
phosphorus content 24%, potassium content 16%) was
applied to the soil as a base fertilizer at a rate of
2 g/pot. On May 25, 6 rice seedlings ~variety:
"koshihikari") were transplanted in each pot, and
cultivated in a green house. On July 18 (15 days before
.. - . . - -
, - ' ' ~ .' - .
.
- 28 -
heading), an aqueous solution o each of the test com-
pounds shown in Table 7 in the concentratiorls indicated
(containing 200 ppm of polyoxyethylenealkyl aryl ether as
a surfactant3 was sprayed onto the leaves of the rice
plants~ One area consisted of four pots.
On September ~0, the rice plants were reaped,
and the amount of refined rice was examined The results
are shown in Table 7. In the non-treated area, only a
mixture of the surfactant and water was used.
Table 7
,
Compound Dosage Yield of refined rice
No. (g/lOa) t~, based on the yield
the non-treated area)
Non- _ 100 (20.3 g/pot)
treated
1 10 126
122
2 10 114
125
134
l 129
(*): Relative proportion when the yield of the
non-treated area is taken as 100.
Test Example 6
Each of the test compounds in each of the dosages
shown in Table ~ was dissolved in 100 liters of waterf and
200 ppm of polyoxyethylene alkyl aryl ether was added as a
surfactant. On May 10 ~20 days before flowering), the
solution was sprayed onto the leaves of wheat (variety:
winter wheat "horoshiri"). On July 26, the wheat was
harvested, and the dry weight of the overground portions
(stalks and leaves) and the amount of harvest were
measured.
? ~ 7 ~ j~
- 29 -
In the non-treated area, only a mixture of
water and the surfactant was sprayed.
The test results are shown in Table 8.
Table 8
Compound Dosage Ratio to the non-treated area .
No. tg~lOa) _ t*) _
Dry amount of Yield
the overground
portions
Non- _ 100 100
treated tll~O kg/lOa) ~541 kg/lOa)
1 ~0 111 1 116
111 117 .
110 115
112 118 :
27 20 113 120
~ 50 113 119
~*~: Relative proportion when the weight or
yield of the non-treated area is aken as 100.
Test Example 7
On June 28, soybean (variety: "enrein) and corn
(dent corn) were sown in l-liter plastic pots filled with
volcanoic ash field soil, and germinated in a greenhouse
at 25C. Then, they were grown in a field~ A base
fertilizer was applied at a rate of 3 k~/lOa as nitrogen.
Polyoxyethylene alkyl aryl ether (100 ppm) as a
spreader was added to a liquid preparation of each of the
test chemicals indicated in Table 9 in a concentration of
300 ppm. The liquid preparation WAS sprayed by a sprayer
onto soybean in the stage of developing 2 main leaves and
to corn in the four-leaf stage so that the entire plants
... .
'' ' ', . '
~, ~. 7~3 7~
- 30 -
were fully wetted with the li~uid preparation. A mixture
o~ water and the spreader was applied to a non-treated
area.
Every seven days after the treatment, the dry
weight and the leaf area of 8 plant individuals were
measured, and in accordance with the following Watson's
equations, the relative growth rate (RGR) and the net
assimiation rate ~NAR~ expressing the rate of increase in
dry weight per unit area of the leaves of the individuals
were calculated.
Calculating equations:
RGR = 1 O dw ln 2-lnwl
NAR 1 dw ( 2 Wl) ClnF2 lnFl)
In these equations, wl represents the dry
weight in tbe first measurement, and wz represents the
dry weight in the next measurement; t2 ~ tl is the day
from the first measurement to the next measurement; and
(Fl - F2) represents an increase in the leaf area during
that period.
The results are shown in Table 9.
It is seen from the test results that compounds
Nos. 2 and 15 show higher RGR and NAR values than choline
chloride.
7~ 3
-- 31 --
Table 9
. .. . ___ _ .
Soybean Corn
Co~ _ .
pound RGR NAR RGE~ NAR
tg.g l~day 1~ (mg,~~2,day~1) ~g g~l day~l) (mg~~~21~day~1)
No~ 0 .077 53 .60 .070 40 .4
treated
2 0.10~ 77.7 0.135 61.4
0.100 75.0 0.140 60.6
Chol ne 0.09071.7 0.101 56.6
~Cn~Qari- . . .. __ . .. _ _ _ _
- - - :
, -.
- ~ . .