Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~33~
THE PYROLYSIS OF PERFLUOROPOLYETHERS
Field of the Invention
This invention is in the fields of polymer and
fluorine chemistry.
05 Background
Preparation of saturated perfluoropolyethers
has traditionally been limited because of the lack
of versatile synthetic techniques. A successful
synthesis is the polymerization of perfluoro-
epoxides, particularly hexafluoropropylene oxide andtetrafluoroethylene oxide. W. T. Miller, U. S~
Patent 3,242,218. This synthetic procedure involves
a three-step scheme for production of the polymer
involving oxidation of perfluoroolefins to per~luoro-
epoxides, followed by anionic polymerization to acylfluoride terminated perfluoropolyethers and then
replacement of the acyl fluoride end groups with
perfluoroalkyl groups by decarboxylation reactions
or by chain coupling photolytic decarboxylation
reactions.
T~e procedure, however, allows little control
over the molecular weight distribution o~ the
product. Typically, a distillation cut is taken if
a specific molecular weight range is needed. When
tetrafluoroethylene oxide is polymerized, little if
any low molecular weight fluids are obtained; the
majority of the product is a higher molecular weight
solid. Conversely, the polymerization of perfluoro-
propylene oxide gives only a liquid; no products
: ' - , ' ' ' ' :
:
3~
--2--
are isolated with a su~ficiently high molecular
weight to be solid.
An alternate synthetic method for the pro-
duction of perfluoropolyethers involves the ultra-
05 violet photolysis of tetrafluoroethylene and/orhexa~luoropropylene in an iner-t solvent in the
presence of oxygen, D. Sianesi and R. Fontanelli,
British Patent 1,226,566. The multistep process
yields an acyl fluoride terminated polymer which
contains unstable peroxidic linkages in addition to
difluoromethylene oxide and tetrafluorethylene oxide
(or hexafluoropropylene oxide) repeating units.
Treatment of the polymer at elevated temperatures
and with fluorine gas gives a stable polymer con-
taining only perfluoroalkyl end groups. Once again,
it is very difficult to control the molecular weight
of the polymer product. The product can be sepa-
rated into various fractions based on vapor pres-
sure.
There exists a need ~or a convenient means to
alter the molecular weight of a perfluoropolyether
polymer.
Disclosure of the Invention
This invention pertains to a method of cleaving
~5 per~luoropolyethers to give lower molecular weight
polymers. ~he method comprises pyroiysis of a
perfluoropolyether, condensation and collection of
vaporized lower molecular weight ~ragments o~ the
perfluoropolyether.
In one embodiment o~ the method, the pyrolysis
is carried out in an apparatus which separates the
--3--
high molecular weight polymer from the desir~d lower
molecular weight fraction on the basis o~ vapor
pressure ~e.g. a distillation apparatus). The
perfluoropolyether to be pyrolyzed is placed into a
05 crucible or other vessel (pyrolysis vessel) which is
located in a heating zone of the apparatus. Pre-
ferably, the apparatus has means -for introduction o~
and exit of a gas and an inert gas is passed over
the polymer throughout the procedure. The polymer
is heated to pyrolysis temperature, about 350-600C,
preferably 500-600C, by raising the temperature in
the heating zone and the polymer is maintained at
that temperature to allow low molecular weight
~ragments to vaporize and distill out of the pyroly-
sis vessel. The low molecular weight products are
collected upon condensation generally in a collec-
tion vessel attached to the condensing zone of the
distillation apparatus.
The pyrolysis can be carried out using a pure
perfluoropolyether, generally in solid form.
Additives such as select metal oxides can be added
to the perfluoropolyether in order to catalytically
reduce the temperature required for pyrolysis.
; Carbon-carbon cross-links present in the polymer
to be pyrolyzed may be eliminated by adding MaOH or
KOII prior to pyrolysis. These age~ts break the
cross-links pre~erentially at the temperatures used
; (about 50G-60~C)~
The pyrolysis may be done in the presence of
f].uorine gas so that when the polymers are cleaved,
; the radicals resulting from bond breakage are capped
with ~luorine. Alternatively, the recovered lower
.
?
., . :
,
~3~
molecular weight fractions may be treated with
elemental fluorine after pyrolysis to ensure satura-
tion and terminal group capping with fluorine.
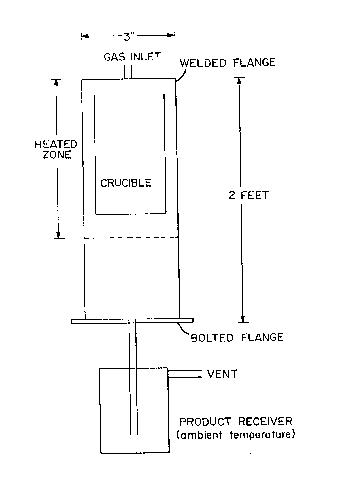
~rief Description of the Drawings
05 The Figure illustrates a simple apparatus for
conducting the pyrolysis procedure of the invention.
Best Mode of the Invention
The pyrolysis procedure of this invention can
be carried out in a variety of apparati. A simple
design is shown schematically in the Figure. It
consists of a nickel tube with one removable flange
(A) sealed to the reactor using an O-ring (TeflonT
O-ring3. Suspended in the chamber near the top is a
nickel crucible (B) which is used to hold the per-
fluoropolyether which is to be pyrolyzed. A furnace
(C) is placed around the nickel tube in the viclnityof the lower one-half of the crucible. A gas diluent
enters through the top inlet (D) and exits along with
the pyrolyzed fluid through (E). A collection vessel
is attached to the bottom of the vessel to collect and
hold the fluid. If fluorine is used as the pyrolysis
gas, a fluorine scrubber is at-tached downstream from
the collection vessel.
The perfluoropolyether is placed in the nickel
crucible. The temperature at the bottom of the
crucible is raised to about 35~-600C, preferably
500-600C and held at that temperature (the tempera-
ture at the top of the crucible can be variable
depending on the location of the heater). The polymer
3~
--5--
is refluxed until a sufficient number of bonds are
broken to allow the lower molecular weight fragments
to distill out of the pyroylsis vessel. A stream of
inert gas (e.g. nitrogen) sweeps the fragment vapors
05 from the reactor into the collection vessel. Fluorine
gas or a fluorine gas/inert gas mixture can be used in
place of the inert gas as described in detail below.
Many other reactor designs can be used success-
fully. ~irtually any type of distillation apparatus
which can be heated to 500C preferably in the pre-
sence of fluorine gas and hydrogen fluoride, can be
used. In general, a suitable apparatus comprises:
i) a sample vessel (pyrolysis vessel) located
in a heating zone;
ii) a condensing zone;
iii) a collection vessel connected by passage-
way to the condensing zone; and
iv) means for introduction of a gas.
The pyrolysis procedure of this invention is
applicable for all saturated perfluoropolyethers.
This techni~ue can be employed to crack perfluoro-
polyethers of any molecular weight including high
molecular weight solids and low molecular weight low
viscosity fluids. Because perf~uoropolyethers are
oxidatively stable at 500C and because bond breakage
occurs preferentially over oxidation, inert gases,
such as helium, can be used or ~trong oxidi~ers, such
as oxygen, air, or fluorine, work satisfactorily.
~ pyrolysis temperature of 500C appears to be
optimal to pyrolyze approximately 1/2 pound of
1~8~3~J
~6--
perfluoropolyether each hour (e.g. in a 3" pyrolysis
tube). If the pyrolysis is carried out in ~luorine, a
delivery rate of approximately lcc/min is needed for
each gram pyrolyzed. The procedure can be performed
05 at ambient pressure for most applications but it
should be recognized that an increase in pressure can
be used to lower the molecular weight distribution
while a decrease in pressure has the opposite effect.
~his pressure dependence ~5 observed since the
pyrolysis products distill from the high temperature
zone.
The yield obtained is a ~unction of the mclecular
weight distribution. Due to the random nature of bond
breakage, a loss in yield occurs only when a fra~nent
is formed which is too small to be of any use. If the
average molecular weight is known and if the lowest
useable molecular weight is identified, then an
approximate yield can be estimated using simple
statistical methods. For example if an average
molecular weight of 5000 is desired, approximatPly 87
of the sample will have a molecular weight above 700.
As mentioned, virtu~lly any perfluoropolyether
can be pyrolyzed to give lower molecular weight
polymers by the method of this invention. For
example, polyhexa~luoropropylene oxide, when heated to
500C, randomly breaks apart giving low ~iscosity
~luids. Other examples include per~luoro(polyethylene
oxide) ~olyme;s, ~er~luoroethylene oxide/pr~pylene
oxide copolymers, per~luoro(polytetramethylene oxide)
and per~luoropolycyclohexyl oxide polymers.
Perfluoropolyethers are chemically well suited
~or this reaction since they do not depolymeriæe by
.' ' ' ' :
~' ' ' .
3~2~3
eliminating monomer units in a sequential manner as
many polymers do. Additionally, perfluoropolyethers
can break down completely at elevated tempera-tures in
both an inert a-tmosphere and in an oxidizing atmos-
05 phere without leaving a nonvolatile residue.
Although -the discussions to this point have dealt
wlth the pyrolysis of perfluoropolyethers in a neat
form, the reac-tions can be carried out with other
materials present. For exarnple, perfluoropolyethers
prepared via direct fluorination may contain NaHF2
or NaF because NaF can be added as a HF scavenger.
See Canadian Patent Application Serial No. 522,462,
filed November 7, 1986, entitled "Perfluorination of
Ethers". Mixtures containing NaF/NaHF2 concentra-
tions as high as 90% can be successfully pyrolyzed.
In addition, additives such as metal fluoride (e.g.
titanium fluoride and aluminum fluoride) or metal
oxide (e.g. aluminum oxide) can be added to cataly-
tically reduce the temperature required for pyrolysis.Further, sodium hydroxide or potassium hydroxide can
be blended into the polymer prior to pyrolysis to
improve the linearity of the fluid produced by
breaking any incidental cross-links which may be
present in the higher molecular weight polymer.
The pyrolysis procedure can be perforrned in the
presence of 1uorine gas. If cleavage occurs in the
presence of elemental fluorine~ the radicals resulting
from bond breakage are capped with fluorine. The
thermal cracking of perfluoropolyethylene oxide in the
C
1~3~;~9
presence of fluorine gas primarily leads to carbon-
carbon and carbon-oxygen bond cleavage. The carbon-
carbon bond, beiny the weaker of the two, is broken
preferentially as illustrated by the following
05 equations:
CF30(CF2-CF2 O)nCF3
3 2 2 )m CF2 ~ CF2-0(CF2-CF2-o) ( -CF
F2
3 2 2 0)mCF3+CF3-o(CF2-CF2-0) CF
If carbon-oxygen bond is broken, an unstable
acyl fluoride is formed which decomposes to give a
perfluoro-alkyl terminal group as depicted by the
following reaction sequence:
CF-O(CF2-CF2-O)nCF3 ?
o(cF?-CF -0~ CF2-cF2-~-o(cF2 CF2 )n-(m+l) 3
2F2
3 2 2 )mC2Fs ~ CF30(CF2-CF2_o) ( )CF
~ COF2
It is often desirable to carry out the pyroly-
sis in an inert atmosphere to avoid having to
handle hot fluorine gas. T~is can be done success-
fully ana usually results in acyl fluoride termi-
i ~0 nated polymers which contain a slight degree o~
unsaturation resulting in very slight discolora-
tions. The acyl fluoride end groups and unsatu-
ration can be easily eliminated by treatment
;
;. ' ,
~83~
g
of the polymer with fluorine gas at 110C aftar
pyrolysis.
The invention is illustrated further by the
following example.
05 Example 1
The nickel crucible shown in the Figure was
filled with 700g of perfluoropolyethylene oxide
solids. The crucible (nickel tube, outside diameter
2~ inches, length 12 inches) was placed in the
nickel pyrolysis tube (outside diameter, 3 inches;
length 2 feet) and was purged with several volumes
of nitrogen prior to heating. The crucible was
heated to the pyrolysis temperature (500C) over a
two hour period and was maintained at that tempera-
ture for approximately three hours to ensure thatall of the polymer is thermally cracked. The lower
molecular weight fragments distilled out of the
crucible which was held at a temperature of approxi-
mately 350C at the top. A purge of nitrogen
(50cc/min) through the pyrolysis tube swept the oil
vapors rom the reactor into a collection vessel.
609g of a light oil was collected (87~ yield) which
was slightly discolored and contained some acyl
fluoride terminal groups. Treatment of the oil with
pure ~luorine at 110C in an ambient pressure
reactor gave 581g of a clear, nonreactive, light oil
~overall ~iéld of 83~).
le 2
The apparatu5 of Example 1 was charged with
650g of a medium viscosity perfluoropolyethylene
~331~9
--10--
oxide fluid (viscosity at 100F was approximat~ly
100 centistokes). The crucible was placed in the
nickel pyrolysis tube which was purged with several
volumes of nitrogen and pressured with 2S0 psi of
o5 nitrogen. A nitrogen purge through the pressurized
vessel was maintained as the crucible was heated to
the pyrolysis temperature (500-600C). This
temperature was maintained for approximately 3 hours
which allowed the lower molecular weight fragments
to distill out of the crucible and into a collection
vessel as they were produced. Approximately 580y of
a light oil was recovered having a viscosity of
15-20 centistokes at 100F.
Example 3
-
The apparatus of Example I was filled with 725g
of a perfluorinated 70:30 ethylene oxide:propylene
oxide copolvmer~ The crucible was placed in the
nickel pyrolysis tube, was loaded in the pyrolysis
apparatus, and was purged with several volumes of
nitrogen prior to heating to 500C. The lower
molecular weight fragments distilled out of the
xeactor as they were produced giving rise to 630g of
a pale yellow oil which contained some acyl fluoride
terminal groups. Titration of the oil with a 1
molar NaOH solution (phenothalein end point) showed
that approximately 25~ of the terminal groups were
reacti~!e acyl 1uoride groups. Treatment of the o'l
at 110C in pure fluorine gave 610g of a clear,
chemically inert, light oil which was shown to be a
perfluoro(ethylene oxide-propylene oxide) copolymer
by F nmr.
~118
Example ~
Approximately SOOg of perfluoropropylene oxide
solids (prepared by direct fluorination of propylene
oxide) were placed in a nickel crucible which was
05 positioned in the nickel pyrolysis vessel depicted
in Figure I. Following purging with several volumes
of nitrogen, the apparatus was heated to 500C over
a 2 hour period and was maintained at that tempera-
ture for approximately 3 hours as the solids were
thermally cracked. The lower molecular weight
~ragments distilled out of the crucible which was
held at a temperature of approximately 350C at the
top~ Approximately 430g of a yellow oil was reco-
vered in the collection ~essel. Treatment of the
fluid with pure fluorine for several hours (110C)
gave a medium viscosity fluid with a 19F nmr indis-
tinguishable from that of a Rrytox fluid with a
comparable viscosity. (Krytox is the trademark of a
perfluoropolyether fluid based on
hexa~luoropropylene oxide which is marketed by Du
Pont).
Industrial Applicability
Perfluoropolyether fluids, due to their ext~eme
stability and chemical inertness, are useful for
many applications such as hydraulic fluids, sol-
vents, lubricants, sealants, etc. However, their
uses are currently numbered due to synthetic limi-
tations which prevent the preparation of a fluid
with the proper molecular weight distribution. The
pyrolysis method of this invention can be used to
produce low molecular perfluoropolyether fluids from
831~9
-12-
high molecular weight solids (or fluids). By
incorporating this pyrolysis technology with
existing polymerization or direct fluorination
technologies for producing perfluoropolyethers,
05 essentially all molecular weight ranges of perfluoro
polyethers can be made in fairly high vields.
Equivalents
Those skilled in the art will recognize, or be
able to ascertain using no more than routine experi-
mentation, many equivalents to the speci~ic embodi-
ments o~ the invention described herein. Such
equivalents are intended to be encompassed by the
following claims.