Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~3~
-- 1 --
ABATEMENT OF VAPORS FROM
GAS STREAMS BY_SOLIDIFICATION
BACKGROUND OF THE INVENTION
1. Field o th~ Invention
The present invention relates to a process
and apparatus for abatement of warm melting point
vapors from gas streams.
2. Background Art
Manufacturing plants which have ~ryogenic
liquids available for use in plant processes are
finding it advantageous to use boiling cryogenic
liquids as the heat transer medium in the recovery
~f volatile materials from effluent gas streams.
Oft~n t~e ~olatile materials to be recovered require
an excessively low separation temperature or the gas
streams from which the volatile materials are to be
r~covered are generated intermittently and therefore
it is ~ot practical to use mechanical refrigeration
~o provide the hea~ transfer capability for
condensi~g the volatile materials out of the gas
streams.
The heat exchange between the effluent gas
str~am and the cryogenic liquid may be direct or
indirect. The heat exchange method is typically
indirect if it is desired to maintain ~he cr~ogenic
materi31 at pressure andior free from contamination
so the vaporized cryogen can ~e re-used in other
processes.
A commonly used process for indirect heat
transfer employs a shell and tube heat exchanger,
wherein a li~uid or partially vaporized cryogen
1~131iV()
flows through one side of the exchanger and the
effluent gas flows through the other side of the
exchanger. Direct hea~ exchange between the liquid
cryogen and the effluent gas can be accomplished
using any method by which the liq~id cryogen
contacts the ef luent gas, such as a ~o~nward spray
of the liquid cryogen upon an upwardly rising volume
of gas. U.K. Patent No. 1,582,955 to R.W. Watso~ et
al., entitled: CONDENSATION OF THE VAPOR OF A
VOLATILE LIQUID, describes processes like those
discussed above in more detail.
The following patents and abandoned patent
application disclose subject matter related to the
present invention U.S. Patent ~o. 4,150,494 to
R. D. ~othchild, entitled Methods and Apparatus for
Rec~veri~g Sol~ents; U.S. Patent ~o. 4,237,700 to
R. D. Rothchild, entitled Methods and Apparatus for
~ro~iding Refrigeration; U.S. Patent ~o. 4,122,684
to M, J. Clarkson, et al., entitled Method For The
Recovery o~ Volatile ~iguids; U.S. Patent ~o.
4,46~,g0~ to F. ~. Steigman, e~titled Process for
the Transfer O~ Refrigeration; U.S. Patent No.
4,551,9~1 to R. Banerjee, entitled Heat Exchange
Methods and Apparatus; U.S. Patent No. 3,535,345 to
R. 8. Eghert, entitled Method of Producing Phthalic
Anhydride, and published patent disclosure, Federal
Republic of Germany, P 24 11 601.6, X. M. Pohl,
entitled Process For The Reduction of Emissions
During The Storage and Loading of Volatile Liquids
And Device For Carrying Out Such Process.
With the exception of U.S. Patent 3,535,345
to R. B. Eghert, the above processes utilize a
~X~336
-- 3 --
cryogenic liquid ~a liquid with a normal boiling
temperature below about -200F~ as the heat transfer
medium in the recovery by condensation of volatile
materials from gas streams.
~ he methods and means disclosed in the
above-referenced cryogenic technology are
deliberately restricted to avoid the solidification
o~ volatile materials in the incoming gas stream
upon defined heat exchange surfaces (which would
foul the exchange surfaces). One approach to the
fouling problem has been ts eliminate the defined
heat exchange surfaces by directly contacting ~he
incoming gas stream or its refluxed condensate with
vaporizing cryogen. This approach causes loss of
cryogen pressure and contamination of the cryogen by
constituents of the gas stream. Thus, the vaporized
cryogen used in this direct contact approach is
typically vented to a~mosphere. The
non-recoverabili~y of ~he cryogen for use as a clean
gas at pressure adds greatly to process cost and
adds to the volume of gas containing volatiles which
is vented to atmosphere. The relatively large gas
vol~me of ~he vaporizing cryogen tends to loft
condensate droplets of the volatiles to be recovered
out of the condenser, subverting the process
objective which is volatil0 abatement or recovery.
~ any of the disadvantages encountered by
direct contact of the vaporizing cryogen with the
i~coming gas stream (from which volatiles are ~o be
removed) can be a~oided by imposi`ng a suitable
i~termediary hea~ exchange fluid between the
vaporizing cryogen and the incoming gas stream.
~33~
Several of the references cited above disclose the
use of such an intermediary heat exchange fluid.
The intermediary fluid can be indirectly cooled by
the cryogen to a bulk temperature above its melting
point and then may undergo either direct or indirect
heat exchange with the incoming gas stream from
which volatile components are condensed, at
tem~eratures exceeding the volatile component
melting points. This technique avoids fouling of
the defined surfaces of any indirect heat exchangers.
When the chilled intermediary fluid is
directly contacted with ths incom;ng gas stream, the
above ref erences recommend use of a liquid
condensate of one or more of the volatile
- constituents of the incoming gas stream as the
chilled fluid. The references also recommend the
removal of any materials which freeze at high
temperatures Ssuch as moisture at 32F) from both
the intermediary fluid and ~he incominy gas stream
prior to implementation of the volatiles recovery
~ethod, to avoid freezing of such materials and
fouling of indirect heat exchange surfaces between
the chilled intermediary fluid and the vaporizing
cryogen. It has also been suggested that during,
indirect heat exchange between the intermediary
cooling fluid and the vaporizing cryogen, the
temperature of the warm end of the heat exchanger
(where the intermediary fluid first enters the
exchanger) be controlled to be at~,,least 32F to
prevent freezing out of any moisture at this
location in the exchanger.
~ ~3 6~
There are applications wherein volatile
abatement (to particularly low concentrations) is
required or wherein the gas stream entering the
volatiles reduction operation comprises a volatile
material which tends to freeze out (solidly) at
relatively high temperatures (such as water at
32F~. Such applica~tions are not adequately
addres~ed by the referenced cryogenic ar~.
Particularly in volatiles abatement situations, the
specified concentration of volati~es permissible in
the gas stream exiting the volatiles reduction
operation requires an exiting gas stream temperature
which is bel~w the melting poin~ of one or more of
the volatile constituents. Such a process will
necessarily form the solid phase of one or more of
the incoming volatile constituents.
In addition, many of the cryogenic
processes for volatiles reduction reguire continuous
operation. The methods of volatiles reduction from
gas streams disclosed in the cited art require at
lea~t one of the following: 1) a pretreatment of
the gas stream to remove moisture and/or other high
melting point ~olatiles; 2) limitation of the heat
exchange temperature within at least a portion of
the volatiles recovery operation to temperatures
above the warmest melting point o ga~ stream
volatile materials; 3) duplication o~ heat exchange
eyuipment to permit rege~eration ~solids removal
from) of heat exchange surfaces.. Substantial
economies both in terms of capital equipment outlay
and/or in costs of operation can be achieved by
36~0
-- 6
eliminating the need for any of the three above
means within the cryogenic process for volatiles
removal.
SUMMARY OF THE INVENTION
The present invention provides a process
and apparatus for the reduction or abatement of warm
melting poin~ vapors from gas streams. The warm
melting point vapors are solidified by d;rect
contact between the gas stream and a chilled liquid,
a~d are subsequently removed from the chilled liquid
using conv0ntio~al liquid-solids separation
techniques. The chilled liquid must exhibit a vapor
pressure at the eo~tacting temperature which is low
enough to preclud~ unsafe or uneconomic levels of
the chilled liquid vapor in the processed gas
stream. The chilled liguid is recycled within the
process, wherein it is cooled using indirect heat
exchang~ with a cryogenic li~uid.
~ y using solidification rather than
condensation o the warm melting point vapors, the
temperature at whioh the ~apors are removed is
significantly lower, thereby reducing the vapor
pressure of both the warm melting point vapors and
the chilled liguid used to remove th~m rom the
effluent gas stre~m. These lower vapor pressures
resul~ i~ a processed gas stream containing re~uced
warm melt~ng point vapor and reduced chilled liquid
components. Selection of a chilled liquid in which
the solidified warm melting point vapors have
mi~imal solubility is helpful in reducing ~he warm
melting point ~apor content of the recycled chilled
liquid (~hereby further reducing the warm melting
~X83~0
-- 7
point vapor content of the processed gas stream) and
in reducing the viscosity of the chilled liguid in
some cases, thereby improving fluid flow
characterîstics in the chilled liquid recycle loop.
As used in the presen~ specification and
claims, the ~ollowing terms shall have the following
meani~gs:
Contacting temperature: the temperat~ure of
the chilled liquid as it is brought into contact
with the incoming gas stream to the volatiles
reduction process. The contacting temperature is
determined by the specific volatiles content
limi~ations ~laced on the gas stream exiting the
volatiles ~e~uction process.
Warm melting point vaporæ: the
constituents of the gas stream incoming to the
~ola~iles reduc~ion process whose mel~iny poi~t~ are
abave the intermediary cooling liquid co~tacting
temperature, and which thus solidify ~pon contacting
said liquid. Typi~al hydrocarb~n warm melting point
vapors, which are intended ~o be exemplary but not
limiting, include benzene (normal meeting point
(nmp) - 41F), carbon tetrachloride (nmp o 10~) and
1,1,1 trichloro ethane (nmp =.-24F~. Typical non-
hydrocarbon warm meetin~ point vapors, intended to
be exemplary but not limiting, include water (nmp =
3~F), bromine (nmp =19F) heavy water, D20, (nmp
- 39F), hydrazine, NH2NH2 (nmp - 36F), and
hydrogen peroxide, H202, (nmp = 31F).
Also included as non-hydrocarbon warm
meeting point vapors, intended to be exemplary but
not limiting, are vapors of relatively low-molecular-
~33~0~3
-- 8 --
weight metallic compounds such as nickel carbonyl,Ni~C0)4, (nmp = -13F), selenium oxyfluoride,
SeOF2, (~mp = 40F), and vanadium tetrachloride,
VC14, (nmp = -18F).
Chilled liquid: ~he liquid used to
solidify at least a portion of the ~apors/volatlles
in the incomi~g gas s~ream with which the chilled
liquid is contacted. ~ote that some vapors/
volatiles may be condensed upon contact with the
chilled liquid incidental to the warm melting point
vapors which are solidified. The chilled li~uid
melting point must be lower than t~e melting point
temperature of any of the warm melting point vapors
intended to be removed or a~ated from the incoming
gas stream. and should typical}y be substantially
below such vapor melting poin~s, to mi~imize the
potential buila u~ of froze~ ~solidified3 chilled
liguid on the vaporizing cryoyen indirect heat
exchange surface. The wtual bulk ~emperature of
the chilled liguid will typically be maintained at
or sligh~ly below ~he contacting temperature. The
vapor pressure of the chilled li~uid at the
contacting temperature must be sufficiently low to
preclude unsae levels o~ chilled liquid in the gas
stream exiting the process. In addition, the
viscosity of ~he chilled li~uid at the bulk
temperature should be sufficiently low to permit it
to be pu~ped and to ~fficiently contact the gas
stream within the volatiles reduction process.
Examples of suitable chilled liquids, not intended
to be limiting, ~nclude ethyl alcohol (nmp - -179F~
a~d acetone (~mp - -140F). The volume
83~()()
concentrations of these substances in an
~guilibrated, one atmosphere, ~50F processed gas
stream would be approximately 0.0~0 percent and 0.44
percent, respectively.
Unsafe levels of the chilled liquid vapors
'in the gas stream exiting the process:
concentrations of chilled liquid vapors which exceed
applicable health or flammability standards
designated to protect persons and property.
Health standards are available from sources
such as OSHA, t~e EP~ and other gover~mental
agencies which provide health and safety standards.
Exemplary of such health standards, not intended to
be limiting, are the threshold limit values (TLV) of
substances as issued by the American Conference of
Governmental ~ndustrial Hygienists (usually provided
on the basis of concentration in air, but which can
be converted to concentration in a nonhazardous
gas). The T~V values represent conditions under
which it is believed that ~early all workers may be
repeatedly exposed day after day without adverse
effect. The amount and nature o~ the information
availabl~ ~or establishing a TLV varies from
substance to substance and is subject to updating.
Thus, the latest documentation should be consulted
in determining ~he most recent guidelines for the
control of health hazards.
Explosive concentration limits (LEL) and
flammability information are available from sources
such as the "Fire Protection Guide on Hazardous
Material~" publi~hed by the ~ational Fire Pro~ection
Association.
``` ~L2~336~
-- 10 --
Uneconomical levels of chilled liquid
vapors: concentrations of chilled liquid in the gas
stream exiting the volatiles reduction process which
cause the present process to be more expensive than
alternate technologies for achieving the same
recovery or abatement resùlt.
Cryogenis Liquid: a liguified atmospheric
component such as nitrogen, oxygen or argon,
supplied at a temperature ~elow about - 200~F.
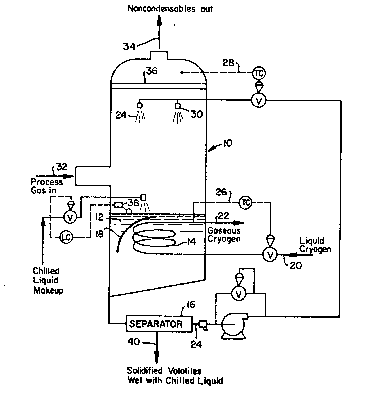
DESCRIPTION OF THE DRP.WINGS
Fig. 1 illustrates a preferred embodiment
of apparatuæ, in accordance with the present
i~e~tion, for removal of warm melting point vapors
from a proces~ gas stream by solidification
(freezing-out).
The device enabling heat exchange between
the intermediary chilled liquid and vaporizing
cryogen (illustrated as a coil of tubing) is
contained within the vessel used for direct con~act
heat exchange between the incoming gas stream and
the intermedi~ry chilled liquid.
Fig. 2 illustrates a second apparatus
preferred embodimen~ similar to that shown in
Fig. l; however, the device used to remove heat from
the int.ermediary chilled liguid is located in a
separate sealed vessel, to increase protection of
the heat exchange surface of the device from the
deposition o~ solidified warm melting point vapors.
...~ .
DESCRIPTION OF THE PREFE~ED EMBODI~5ENT~:
The pre~rred process of the present
invention comprises directly contacting a gas stream
6~)
containing ~apors/volatiles to be aba~ed with a
chilled liguid which is not a constituent of the
vapors to be abated. The contac~ing chilled liquid
is supplied at a temperature ~elow the melting point
of at least one of the constituents to be removed
from the vapor stream. The contacting liquid must
therefore have a melting point below the melting
point of the vapors/volatiles to be removed by
solidiication. The contacting liguid must also
haYe a vapor pressure at the contacting temperature
which is low enough to preclude unsafe levels of its
vapor in the processed gas stream. In addition, the
contacting liquid should have a vapor pressure at
the contacting temperature which does not result in
uneconomical levels of its vapor i~ the processed
gas stream. Chilled liq~id which is carried out o~'
the process along with the solidified warm melting
poin~ vap~rs (which are separated from the bulk of
th~ chilled liquid and removed from the process)
mus~ be replaced during operation of the process,
thereby re~resenting a process cost.
The abo~e preferred process enables the use
of a temperature di~erential between the selected
chilled liquid and the warm mel~ing point vapor~
which is directly designed to optimize vapors/
volatiles reduction to meet specific standards such
as the TL~ standards applied to the gas stream
exiting the vola~ s recovery process. The chilled
liquid to be used can be selected based on physical
and chemical data a~ailable from reference sources
commonly used in the art.
- ~.2~
- 12 -
In a less preferred process of the present
invention, the chilled liquid is ~he condensate of
any of the condensables in the incoming gas stream
whose melting points are below the contacting
temperature. The melting points of the condensate
must be sufficiently lower than the melting points
of the warm melting point vapors to be removed that
the warm.melting point vapors ar~ solidified upon
direct contact wi~h the condensate. In addition,
the amount of the chilled liquid vapors remaining in
the gas stream must not be unsafe and should not be
uneconomical. When the chilled liquid is the
condensate of co~densables in the incsming gas
stream, the efficiency of ssparation of the
solidified warm melting point vapors from the
chilled liquid is less critical in terms of
maintaini~g a given amou~ of chilled liquid within
~he process. Chilled liquid which is removed from
the process along with the solidified warm melting
point vapors is replaced at leas~ in part by
additional condensate generated during processing of
the incoming gas stream, thereby reducing
incremental processing costs. Any additional
chilled liquid required must be supplied as make up
directly to the process.
The apparatus used ~o facilitate the
process of the present invention is best described
in combination with a description of the process.
The contacting of a gas ~tream containing
warm melting point vapors with a chilled liguid is
typically done in a spray tower 10, as shown in
Fig. 1, ~ince ~uch a tower is not as susceptible to
/36~)0
- 13 -
fouling by solidified warm melting point vapors.
The contacting device is not limited to a spray
towex, however; ~ plate column, for example, might
be used if the flushing action of the contacting
chilled liquid is sufficient to prevent fouling.
Another option would be to bubble the effluent gas
through the contacting chilled liquid.
As shown in Fig. 1, the process gas 32
containing the warm melting point vapor is fed into
the spray tower 10, wherein it is directly contacted
by chilled liquid 24 impinged upon the process gas
from the spray nozzles 30. The contact of the
process gas with the chilled liquid results in
solidification or free~ing out of the warm melting
poi~t vapors, which solidif.ied vapors are carried
along with the chilled liguid into the pool of
chilled liquid and solidified vapors 12 at the
bottom portion of the spray tower 10.
The pool 12, comprising the slurry o
contactin~ liquid and frozen vapors, is kept at the
contacting temperature (or slightly below contacting
temperature) by an immersed refrigeration coil 14.
Liquid cryogen or vaporizing cryogen provides the
cooling medium inside the coil 1~. Typically liquid
cryogen 20 would enter the refrigeration coil 14 and
vaporized cryogen 22 would exit the coil. The hea~
exchange device need no~ ~e a coil as shown, but can
be any indirect heat exchange device known in the
industry so long as use of the device does not cause
build up of solidified warm melting point vapors on
the heat exchan~ surface of the device to an extent
which renders the device inoperable.
A shroud or screen 18 can be used to
prevent the solidified warm mel~ing point vapors
from impinging and layering upon the coil 14. The
solidified warm melting point vapors are removed
from the slurry 12 by the separator 16 and sent on
for further processing as necessary, depending o~
the final desired use of the solidified vapors. The
separator 16 may be any suitable apparatus known in
the art, such as a flo~ation device, a settling
tank, a filter, a centrifuge, etc. The chilled
liguid 24 separated from the solidified warm melting
point vapors is recycled to the spray nozzles 30 in
the spray tower 10.
An alternative apparatus for chilling the
con~acting liquid is shown in Fig. 2. The heat
transfer device, ag~in shown as a coil 14, is
located in a second insulated ~essel 42 which is
located downstream of the ~eparator 16. This
reduces the amount of ~ouling of the coil 14 by
solidified warm melting point vapor impingement.
Fig. 2 also shows apparatus for removal of
excess chilled liguîd which may be required if the
contacting liguid i ~he condensate o at least one
of the constituents of the incoming gas stream,
~i~ce t~e rate of condensation of the contacting
liguid constituent may Pxceed the rate at which
condensate leave~ the process with the solidified
volatiles stream 40. Apparatus for removal of
excess chilled liguid can also be used in
combination with the apparatus shown in Fig. 1.
I~ the duty cycle of the apparatus and the
properties of the chilled liquid tend to cause
` ~ .
~33~0~
- 15 -
excessive deposits of frozen chilled liquid on the
refrigeration coil 14, a second coil (not shown) may
be located in the pool at the bottom of the spray
tower/contactor 10, as shown in Fig. 1 or in the
alternate cooling vessel ~2 as shown in Fig. 2. The
second coil can be placed in use while the fouled
coil is defrosted either by the warmer bulk
temperature of ~he surrounding liguid or by the
action of warm cryogen vapor forced through the
fouled coil. It is preferred, however, to select a
chilled liquid which reduces or avoids the formation
of deposits of chilled liquid on the heat exchange
device, thus reducing total equipment costs. Once
the necessary contacting temperature has been
calculated, based on re~erence data available within
the art5 a list o4 potential chilled liquids capable
o~ providing the contacting temperature and of
mee~ing vapor pressure requiremen~s at ~hat
temperature can be tabulated. Minimal
experimentation using techniques known in the art
can be ~onducted to determine which of the chilled
liquids on the list will provide minimal deposits of
frozen chilled liquid on the heat exchange device
(such as a refrigeration coil).
EXAMPLE 1
The ~ollowing Example is presented to
further illustrate the invention and is not intended
to be limiting.
It is desired to abate carbon tetrachloride
(nmp - -10F~ from a gas stream comprising air and
carbon tetrachloride at atmospheric pressure. The
desired concentration of carbon tetrachloride in the
'I Z836~(~
16 -
gas stream exiting the volatiles recovery process i5O.017 volume percent, corresponding to a vapor
pressure of 0.0025 psia. The vapor pressure curve
for carbon tetrachloride indicates the required
vapor pressure corresponds to an equilibriated vapor
temperature of about -90F. Thus, the abatement
criteria will not be met by removing the carbon
tetrachloride at a temperature above -90F.
Referring again to Fig. 1, the following is
a process fox accomplishing the above objective.
The contacting chilled liquid 24, is comprised of
acetone liquid at about -90F. The process gas 32
is comprised of air containing carbon tetrachloride
wherein the concentration of carbon tetrachloride is
about 2.0 volume percent. Acetone has low
solubility in carbon tetrachloride at the contacting
temperature (about -90F) and its low melting point
0F) minimizes buildup on the immersed coil ~4.
A shroud or screen 1~ can be used to keep the carbo~
tetrachloride precipitate from building up o~ the
coil 14. The liquid cryogen 20 used ~o cool the
slurry 12 comprising acetone and solidified carbon
tetrachloride is liquid nitrogen, which enters the
coîl 14 at a temperature of about -32~F. The
acetone contacting liguid 24 is direc~ly contacted
with the incoming process gas 32 in the form of a
spray exiting nozzles 30. The direct contact
between ~he acetone contacting liquid 24 and the
incoming gas 32 causes solidification of the carbon
tetrachloride which is carried wi~h the acetone into
the bottom of the spray ~ower 10, to form a slurry
12 in the bottom of splay tower 10. The slurry 12
- 17 -
is processed through a liquid-solids separator 16
(of the kind previously discussed) whereby
solidified carbon tetrachloride wet with chilled
liquid 40 is removed from the acetone contacting
liquid. The separated acetone contacting liquid 24
is then pumped to the spray nozzles 30. One
temperature controller 26 maintains the pool of
slurry 12 tsmpera~ure by varying the liquid nitrogen
20 flow rate, while a second temperature controller
28 maintains the temperature of the leaving effluent
34 by varying the flow to the spray nozzles 30. A
demister pad 36 may be required to trap any
particulates and contacting liquid droplets which
are co~vected up by the leaving noncondensibles
stream 34~ The volume concentration of acetone i~
the gas 34 exiting the process is about 0.061 volume
percent, which value is well below the threshold
~imit value (TLV) and the lower explosive li~it
~LEL) for acetone.
A con~acti~g liquid level control system 38
is reguired for continuous operatio~ to replace
con~acting chilled li~uid los~ with the separated
solidified warm melting point vapors 40.
A~ alternative apparatus for chilling the
contacti~g liquid 24 is shown in Fig. ~. The device
for indirect heat exchange between the contacting-
liquid and the cryoge~, shown as a re~rigeration
coil 14, is located in a se~arate sealed vessel 4~.
In this case, the slurry 12 comprising acetone and
solidified carbon ~etrachloride is processed through
the separator 16 to remove the solidified carbon
tetrachloride from the acetone contacting liguid.
~33~00
- 18 -
The separated acetone contacting liquid 24 is
pressured or pumped to the cooling vessel 42 wherein
the acetone contacting liquid 24 is chilled by
indirect heat transfer with the vaporizing cryogen
passing through refrigeration coil 14.
EXAMPLE 2
; The following test was made to determine
the nature of a typical chilled liquid/solidified
warm melting point vapor mixture which would be
formed by the process of the present invention.
About one quart of ethanol was chilled to
-100F using dry ice in an open-mouth Dewar. After
it was determined that no dry ice crystals remained
in the bottom of the Dewar, a water mist was sprayed
down into the Dewar from a height of about 18 inches
usi~g a Spraying Systems Co. Hydraulic Atomi ing
Nozzle ~ 1/4 LNN-2 at 60 psig or one minute (2.5
gallon per hour rating at 60 psig~. The resulting
mixture was filtered through a 100 mesh ASTM screen
and the filtra~e was caught in a bucket below.
A bed of wet granules was retained on the
screen, which bed turned to slush as it warmed up.
Examination of the material in the bucket below
indicated no solids.
The above procedure was repeated using ASTM
scre~ns with increased opening size, to determine
the opening size which produces the most rapid and
complete separation without:permitting the passage
of crystals with the filtrate. No`granules were
observed to pass through ASTM screen sizes # 50 and
# 30. A few small granules were observed to have
passed through ASTM screen size # 20.
- 19 -
The amount of ethanol remaining on thegranules after fil~ering was not determined during
the abo~e testing, but could be determined ~y
standard analytical ~echniques or by doi~g standard
material balance calculations for measured water and
ethanol quantities at various steps during the
determination.
The process of the present invention
requires that the solidified warm melting point
vapors be separabl.e from the slurry of warm melting
point vapors a~d contacting chilled liquid. The
warm melting point vapors removed may comprise only
a portion of the total volatiles to be removed from
the incoming gas and other vola~iles to be removed
may be conderlsed and remoYed as part of the slurry
formed i~ the bottom of the spray tower. Such
co~densed volatiles may be separated from`the
contacting li~uid using standard li~uid-liguid
separation techni~ues prior to recycle of the
contacting liquid to the spray nozzles, or such
condensed volatiles may be used as a~ least part of
the contacting liguid.
The embodiments of the invention described
above are not intended to be limiting, as one
skilled in the art will recognize that numerous
substitutions, modifications and alterations are
permissible without departi~g from the spirit and
scope of the i~entio~, as demonstrated i~ the
following claims. ~.