Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~28S934
-- l --
FC 248~X
~POLY-4-AMINOPYRROLE-2-CARBOXAMIDO DERIVATIVES AND PROCESS
FOR THEIR PREPARATION"
The present invention relates to poly-4-aminopyrrole-2-
-carboxamido derivatives, to a process for their prepara-
tion and to pharmaceutical compositions containing them.Distamycin A is a well known compound having the following
formula
HOC-l NH ,NH2
0~ ~ NH-CH2-CH2_C NH
CH3
Literature referring to distamycin A includes, for example,
Nature 203, 1064 (1964).
The invention provides, as a first obJect, distamycin A
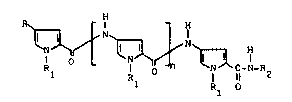
derivatives having the following general formula (I)
1 R~ O ~ ~ C-N-R2 (I)
1 Rl O
wherein
n is zero or an integer of 1 to 4;
R is a) -NHR3, wherein R3 is
a') -CONtNO)R4, ln which R4 is Cl-C4 alkyl either
unsubstituted or substituted by halogen; or
b') -CO(CH2) -R5, in which R5 is halogen, oxiranyl,
-nethyloxiranyl, aziridinyl, cyclopropyl or the residue of an
alicyclic d,~-unsaturated ketone or lactone,
and m is zero or an integer of 1 to 4;
b) -N - R wherein either R6 and R7 are the same and
.
~W4
are each oxiranemethyl, aziridinemethyl, or C2-C4
alkyl 2-substituted by halogen or by a group -OS02R8,
wherein R8 is C1-C4 alkyl or phenyl, or one of R and
R7 is hydrogen and the other is as defined above;
c) -N02;
d) -NH2; or
e) -NH-CH0;
each group Rl is, independently, hydrogen or C1-C4 alkyl;
R2 is a C1-C6 alkyl group terminating with a basic or acidic
moiety or with a free or glycosilated hydroxy group, with
the provisos that
(i) R is not as defined abovo under a) and b) ~h~n n i~ 1
and, at the sa-~ tiue, R2 is -CH2-CH2- ¢ NH
(ii) R is neither -N02 nor -NH2 nor -NH-CH0 when R2 is a
group -alk-C~NR,R~ , wherein alk is C1-C6 alkyl and
each of R' and R", independently, is hydrogen or
methyl, or when, n being zero or 1, R2 is a group
-(CH2)p-N _ CH3 wherein p is 2 or 3; and
tiii) R is not -NH2 when, at the same time, n is zero or 1,
each Rl is methyl and R2 is a group -CH2-CH2-COOH.
The invention includes also the pharmaceutically acceptable
salts of the compounds of formula (I), sub~ect to the above
provisos, as well as all the possible isomers covered by the
formula (I), both separately and in mixture.
- :
lZ8~4
As a second object, the present invention
provides pharmaceutical compositions comprising a pharma-
ceutically acceptable carrier and/or diluent and, as the
active substance~ a compound of the following formula (IA)
1 ~ ~ C-N-R2 (IA)
Rl O
wherein
n is zero or an integer of 1 to 4;
R ls a) -NHR3, wherein R3 is
a') -CON(NO)R4, in which R4 is C1-C4 alkyl either
unsubstituted or substituted by halogen; or
b') -CO(CH2)m-R5, in which R5 is halogen, oxiranyl,
methyloxiranyl, aziridinyl, cyclopropyl or the residue of
an alicyclic~~,~-unsaturated ketone or lactone, and
m is zero or an integer of 1 to 4;
b) -N ~ R wherein either R6 and R7 are the s&me and are
each oxiranemethyl, aziridinemethyl, or C2-C4 al~yl
; 2-substituted by halogen or by a group -OS02R8,
wherein R8 is C1-C4 alkyl or phenyl,or one of R6 and
R is hydrogen and the other is as defined above;
c) -N02;
d) -NH2; or
.f e) -NH-CHO;
. . .
.
.. . . . : : . . . .
1.285934
-- 4
each group Rl i~, independ~ntly, hydrogen or
Cl-C4 a}kyl;
R2 i~ a Cl-C6 alkyl group terminating ~ith a basic or
acidic moiety or with a free or glycosilated hydroxy
group, with the provisos that
(i) R is not as defined above under a) and b) when n
i~ 1 and, at the same time, R2 i8 -CH2-CH2-C~NH
and
ti~ R is neither -N02 nor -NH2 nor -NH-CH0 when R2 is
a group -alk-C~NH,R~ wherein alk is Cl-C6 alkyl
and each of R' and R", independently, is hydrogen
or methyl.
The invention includes in its second ob~ect also the
pharmaceutical compositions containing, as the active
substance, a pharmaceutically acceptable salt of a compound
of formula (IA), subject to the hereinbefore specified
provisos,as well as any possible isomer, or mixture of
isomers, covered by the formula (IA).
With reference to both the above formulae (I) and (IA)
preferred features of the various substituents are as
follows.
When R4 is unsubstituted C1-C4 alkyl, methyl and ethyl
are preferred, in particular methyl.
When R4 is C1-C4 alkyl substituted by halogen, the halogen
is, preferably, chlorine or bromine; in this case preferred
R4 values are chloroethyl and fluoroethyl.
Preferred n values are zero, 1 and 2.
When R5 is halogèn, it is, preferably, chlorine or
bromine.
When ~ is the residue of an alicyclic ~ unsaturated kebDne or lacbone,
it is, e.g., a group ~ or, respectively, a group ~
. . . , .:
, : ',' ', :.
~.2859~4
Preferred R5 values are oxiranyl ( P~); 1-aziridinyl ( ~ ~ );
cyclopropyl ( ~ ); a group ~ or a group ~
Preferred m values are zero, 1 or 2.
A R6/R7 C2-C4 alkyl group 2-substituted by halogen is,
s preferably, 2-chloroethyl.
A R6/R7 C2-C4 alkyl group 2-substituted by a group -OS02R8
g P 2 2 OS02R8, wherein R8 is
Cl-C4 alkyl, preferably methyl.
Preferably each group Rl, independently, is C1-C4 alkyl,
in particular methyl and, most preferably, all groups R
are methyl.
Sub~ect to the above provisos, when R2 is a C1-C6 alkyl
group terminating with a basic moiety, the C1-C6 alkyl is,
preferably, C1-C4 alkyl, in partlcular ethyl or n-propyl,
and the basic moiety is, for instance, an amino group; a
mono- or di-C1-C6 alkyl amino group, e.g. di-C1-C4-alkyl-
-amino; an amidino group; a group -N=N-N ~ CH3 ; or a
nitrogon containine heterocyclic
ring such as, o.g., imidazolyl, ioidazolinyl, tetrahydro-
pyrimidinyl and oxazolidinyl. These specific heterocyclicsare the preferred ones every time a nitrogen
containing heterocyclic ring is mentioned
in this specification.
Preferred R2 Cl-C6 alkyl groups terminating ~ith a basic
moiety are, e.g., subject to the above provisos
-(CH2)p-N ~CH3 ~ -(CH2)p-c ~NH ~ -(CH2)p C _ N~
H
: :. . . .
'' ~ ~ '' ' .'
.. ..
12859;~4
-~CH2) -N ~ , -(CH2)p-C~ ] ' -tCH2)P-C~ ~
~(CH2) -C~ ~ and -N=N-N~ , wherein p is an integer
of 1 to 4.
When R2 is a Cl-C6 alkyl group terminating with an acidic
moiety, the C1-C6 alkyl is,preferably,C1-C4 alkyl, in
particular ethyl or n-propyl, and the acidic moiety is,
preferably, a carboxy group.
Preferred R2 Cl-C6 alkyl group ter~inating with an acidic
moiety is, e.g., a group -(CH2) -COOH wherein p is an
integer of 1 to 4.
When R2 is a Cl-C6 alkyl group terminating with a free
hydroxy group it is, e.g., a group -(CH2)p-CH20H wherein p
is an integer of 1 to 4.
~hen R2 i8 a Cl-C6 alkyl group teroinating ~ith a glyco-
silated hydroxy group, it is, e.g., a group -(CH2) -CH2-0-D
~herein p i8 as defined above and D is a sugar_or amino-
sugar residue.
The sugar residuo may be, e.g., a glucose, oannose or ri-
bose residue; the amino-sugar residue uay bo, for instance,
a daunosaoine residue ~hich oay be optionally salified, e.g.
~ith acotic, tri~luoroacetic or hydrochloric acid.
The pharmaceutically acceptablo salts of tho coopounds of
formula (I) and (IA) include both the salts ~ith pharoa-
ceutically acceptable acids, eithor inorganic acids such as,
e.g., hydrochloric, hydrobromic, nitric and sulfuric, or
organic acids such as, e.g., acetic, trifluoroacetic, citric,
and ethanesulfonic,
tartaric, maleic, fumaric, methanesulfonic Jand the salts
: - . - ,
.. ..
-
,. . ~ .
12859~4
~ith phar~aceutiCally acceptable bases, either inorganic
bases ~uch as, for instance, alkali metal, e.g. sodium
or potassiu~, or alkalino-earth metal, e.g. calcium or
magnesium, or zinc or aluoiniu~, hydro~ides, or organic
bases, such as, e.g;, aliphatic anines as, e.g., methyl-
a~ine, diethylaoine, trimothylaoino, ethylamine, and
hoterocyclic aoines as, e.g., piperidino.
Salts of the compounds of formula (I) or (IA) with acids
may be, e.g., the salts of the compounds of formula (I) or
(IA) wherein R2 is a Cl-C6 alkyl group terminating with a
basic moiety with an acid, e.g. one of those hereabove
speci`fied.
Salts of the compounds of formula (I) or (IA) with bases
may be, e.g., the salts of the compounds of formula (I) or
(IA) wherein R2 is a C1-C6 alkyl group terminating with an
acidic moiety with a base, e.g. one of those hereabove
specified.
A specific class Or coopounds of for-ula (I) according to
the in~ention (hereinafter class A) aro tho coupounds of
foroula (I) ~herein, sub~ect to proviso (i) abo~e,
n is zero or an integer of 1 to 4;
- -: :-. '.- . , - ' , . . .
.
:: . - : . ........ . . ...... .
. . - . : . - .
~.285934
R is a) -NHR3, wherein R3 is
a') -CON(NO)R4, in which R4 is C1-C4 alkyl either
unsubstituted or substituted by halogen; or
b') -CO(CH2) -R5, in which R5 is halogen, oxiranyl,
methyloxiranyl, aziridinyl, cyclopropyl or the residue of
an alicyclic~,B-unsaturated ketone or lactone,
and m is zero or an integer of 1 to 4; or
b) -N~ R wherein either R6 and R7 are the same and
are each oxiranemethyl, aziridinemethyl, or C2-C4
alkyl 2-substituted by halogen or by a group
-OS02R8, wherein R8 is C1-C4 alkyl or phenyl, or
one of R6 and R7 is hydrogen and the other is as
defined above;
each group R1 is, independently, hydrogen or C1-C4 alkyl;
R2 is a C1-C6 alkyl group terminating with a basic or
acidic moiety or with a free or glycosilated hydroxy group,
and the pharmaceutically acceptable salts thereof.
The preferred oeanings of tho various substituents in this
class are the sa-e indicated before in this specification
with reference to the for-ulae (I) and (IA).
A preferred group of coopounds in tho anbit of the said
class A are the compounds of foroula (I) ~horein subject
to proviso (i) above,
n i8 zero, 1 or 2;
R is a) -NHR3 ~herein R is
a') -CON(NO)R4 ~herein R4 is Cl-C4 alkyl substituted
by halogen, or
b') -CO(CH2) -R5 ~herein R5 is halogen, oxiranyl,l-
aziridinyl, cyclopropyl, or the residue of an alicyclic ~ un~
saturated lactone, and o is zero, 1 or 2; or
1285g34
b) -N ~R6 , ~herein R6 and R7 are the same and are each
oxiranemethyl, l-aziridinemethyl, or a C2-C4 alkyl group
2-substituted by halogen or by a group -OS02R8 wherein
R8 is Cl-C4 alkyl;
each group R1 is, independently, Cl-C4 alkyl;
R2 is a Cl-C6 alkyl group terminating with a basic moiety,
and the Ralts thereof with pharmaceutically acceptable acids,
in particular ~ith hydrochloric acid.
In the above preferred group of compounds a R4 Cl-C4al-
kyl group i~, preferably, methyl or ethyl; a halogen atom is,
preferably, chlorine; the residue of an alicyclic ~,~-unsaturabed lactQne
is, preferably, a group ~ 0 ; a C2-C4 alkyl group in R6~R7
is, preferably, ethyl; when R6 and R7 are a C2-C4 alkyl group
2-substituted by halogen, they are, preferably, 2-chloro-
ethyl; when R6 and R7 are a C2-C4 alkyl 2-substituted by a
group -OS02R8 where R8 is Cl-C4 alkyl, they are, preferably,
methanesulfonyloxyethyl; a C1-C4 alkyl group for R1 is,
preferably, methyl; in the R2 substituent the Cl-C6 alkyl
group is, preferably, Cl-C4 alkyl, in particular ethyl or
n-propyl, and the terminal basic moiety is, preferably, amino;
mono- or di-C1-C6- alkylamino; amidino; a group -N=N-N~CH3;
or a nitrogen containing hetero-
cyclic ring; particularly preferred R2 values are those
specified before, in particular, -(CH2) -N~CH3 and
25 -(CH2) -C~ wherein p is an integer of 1 to 4, especially
NH2
2 or 3.
~.
- . :
.. -
: :. . :
- . . . .
.
. .
~. . . ..
1285934
-- 10 --
Specific examples of preferred compound~ of the above class A
ar~:
B--~N-methyl-4- ~ -methyl-4-(3-methyl-3-nitrosoureido )
pyrrole-2-carboxamido~ pyrrole-2-carboxamido~propionamidine;
B-~N-methyl-4-~N-methyl-4-~3-(2-chloroethyl)-3-nitrosourei-
do ~-pyrrole-2-carboxamid~ pyrrole-2-carboxamidQ7propiona-
midine;
3-~N-methyl-4-~N-methyl-4-~3-methyl-3-nitrosoureido ~pyrrole-
-2-carboxamidQ7pyrrole-2-carboxamido~propyl-dimethylamine;
3-lN-methyl-4-LN-methyl-4-l3-(2-chloroethyl)-3.-nitrosourei-
do ~pyrrole-2-carboxamido~pyrrole-2-carboxamido~propyl-
-dimethylamine;
3- ~ -methyl-4-LN-methyl-4-~-methyl-4-(3-methyl-3-nitroso-
ureido)pyrrole-2-carboxamido]pyrrole-2-carboxamido~pyrrole-
-2-carboxamidoJpropyl-dimethylamine
.. . . ~ . ............... .. . . .
. ~ .
. . . ~ : . .
~2859~4
-- 11
~-~N-methyl-4-Lrl-methyl-4-~N-methyl-4-~3-~2-chloroethyl)
-3-nitrosoureid~ pyrrole-2-carboxamido~pyrrole-2-carboxa-
mido7pyrrole-2-carboxamido~propyl-dimethylamine;
N-deformyl-N-lN-methyl-4-(3-met~yl-3-nitrosoureido)pyrrole-
-2-carboxamido~Distamycin As
N-deformyl-N-LN-methyl-4-~3-(2-chloroethyl)-3-nitrosourei-
d~ pyrrole-2-carboxamido~Distamycin A;
3-~N-methyl-4-lN-methyl-4- ~ -methyl-4-~N-methyl-4(3-methyl-
-3-nitrosoureido)pyrrole-2-carboxamldo~pyrrob -2-carboxa-
mido]pyrrole-2-carboxamido]pyrrole-2-carboxamido~propyl-
-dimethylamine;
3-~N-methyl-4-lN-methyl-4-LN-methyl-4-~N-methyl-4~3-(2-
-chloroethyl)-3-nltrosoureld~ pyrrole-2-carboxamido~pyrrole-
-2-carboxam~do~pyrrole-2-carboxamidoJpyrrole-2-carboxamido7
~ropyl-dimetAylamine;
~-~N-methyl-4-~N-methyl-4-(oxiranecarboxamido)pyrrole-2-
-carboxamido/pyrrole-2-carboxamido~propionamidine;
3-~N-methyl-4-~N-methyl-4-(oxlranecarboxamldo)pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine
20 3-lN-methyl-4-L~-methyl-4-~N-methyl-¢-(oxiranecarboxamido)
,- py.role-2-carboxamidolpyrrole-2-carboxaml~o~pyrrole-2-carbo-
~ x~mido~pre?yl-dime~hylamine)
,
- - - ,
~, .- . ,
-, -:
.
, : - . ,
- . , : . :~, . . -, , ,. -
-: - ' . , - -: . ~
~285934
iefor~lyl-N-~N-methyl-4-(oxiranecarboxamido)pyrrole-2-
-carboxamido7~is~amycln A;
3-~N-methyl-4-L~;-methyl-4-LN-methyl-4-~1-methyl-4-(oxira-
necar~oxamido)pyrrole-2-carboxamido7pyrrole-2-carbox~mido7
pyrrole-2-carboxamido~pyrrole-2-carboxamidoJpropyl-dime-
thyiamine r
N-methyl-4- ~ -methyl-4-(cyclop.opyica~x~mido)py~1e-2_
-carboxamido7pyrrole-2-carboxamldo~propionamidine;
3-[N-mcthyl-4-~N-methyl-4-(cyclopropylca~un~do)pyrro1e-2-
-carboxamldo~pyrrole-2-carboxamido~propyl-dlmethylamine;
3-~N-methyl-4-~N-methyl-4- ~ -methyl-4-(cyclopropylca~x~ami~o)
pyrrole-2-carboxamldo~pyrrole-2-carboxamldo~pyrrole-2-carbo-
xamid~ propyl-dimethylamine;
N-deformyl-N-~N-methyl-4~cyclop~opylcarbluf~o)~ 1e-2-
-carboxamid~ Distamycin A;
3-~N-methyl-4-L~-methyl-4-LN-methyl-4- ~ -methyl-4-(cyclopro
pylcarbox~do ) pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido~pyrrole-2-carboxamid~ propyl-dime-
thylamlne;
~-~N-methyl-4-~N-methyl-4- ~ethyloxlraneca~ do)pyrro1e-2-
-carbo~mldo7pyrrole-2-carbox-mldo~proplon-~ldln-;
..... :
- - . , .: . -
. .
- ,
1285g~4
3-~N-methyl-4-~N-methyl-4-~thyloxiranecarboxamido)pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~propyl-dlmethylamine;
3-lN-methyl-4-~N-methyl-4-~N-methyl-4~$nethyloxiran~car~oxamido)
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carbo-
S xamido~propyl-dlme~hylamine;
;~'-deformyl-~ -methyl-4-~methyloxi x ~car~do)pyrrole-2-
-carboxamidQ~Dlstamycln A;
3-~N-methyl-4-Lh-methyl-4-~1-methyl-4-/N-methyl-4-~Snethyloxi-
ranecar~oxamido)pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido~pyrrole-2-carboxamido~propyl-dime-
thylamine;
B-~N-methyl-4-~N-methyl-4-(2~*~oroet~ylcar~oxamido)pyrro1e-2-
-carboxamido7pyrrole-2-carboxamldo~proplonamldlne
3-lN-methyl-4-~N-methyl-4-(2-Ghlo;oethylc~rx~lddo)pyrrole-2-
-carboxamido~pyrrole-2-carboxamldo~propyl-dimethylamine;
3-lN-methyl-4-~N-methyl-4-~N-methyl-4-(2~oroethy1car~ox~do)
pyrrole-2-carboxamldo~pyrrole-2-carboxamldo~pyrrole-2-carbo-
xamldo~propyl-dlme~hylamine
N-deformy~ ;-methyl-4-(2-chlor~et.~rlca~do)pyrr~1~2-
-carboxamid~ Dlstamycln A;
3-rN-methyl-4-Lh-methyl_4_ ~ _methyl_4_~N_methyl_4~2 ~ 10r~
e~ylcar~ox~do)pyrrole-2-carboxamldo~ pyrrole-2-carboxamldo~
pyrrole-2-carboxamido~pyrrole-2-carboxamido~propyl-dime-
thylamine;
- , - . - - - ..... -
.
. : ~ : - . . . -
5934
- 14 -
~-~N-methyl-4-[~-methyl-4-l;-(aZiridine)carboxarido7?yrrole-2-
-carboxamido~pyrrole-2-carboxamido~propionamidine
3-~N-methyl-4-[N-methyl-4-~.-(aziri~Lne)car~oxar.ido7pyr~ole-2-
-carboxamido~pyrrole-2-carboxamido]propyl-dimethylamine;
3-lN-methyl-4-~N-methyl-4-~N-methyl-4~ 7.iridir.e)car~oxa.~ o,7
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carbo-
xamido~propyl-dime~h~lamine;
N-deformyl-N-~N-methyl-4-~-(aziridine)ca~x~mido~pyrrole-2-
-carboxamidQ7Distamycin A;
3-~N-methyl-4-~'-methyl-4-~N-methyl-4- ~ -methyl-4 ~1-(aziri-
dine)car~nidoJpyrrole-2-carboxamldo7pyrrole-2-carboxamido7
pyrrole-2-carboxamido~pyrrole-2-carboxamldoJpropyl-dime-
thylamine;
~-~N-methyl-4-[N-methyl-4~ bis(2-chloroe'~iæ~no)~p~le-2-
15 -carboxamido/pyrrole-2-carboxamldo~proplonamidine;
3-~N-methyl-4-~N-methyl-4-/N,~ls(2-chloroethyl~lno~?yr ole-2-
- -carboxamldoJpyrrole-2-carboxamldo~propyl-dlmethylamine;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-~N,-~-bis(2-chlo~ ~ylæmino)~
pyrrole-2-carboxamldo~pyrrole-2-carboxamido~pyrrole-2-carbo-
xamldo~propyl-dlme~hylamlne;
N-deformyl-N-~.N-methyl-4-~,N-bis(2-chloroethylc.~no),tpyrrole-2-
-carboxamid~ Distamycin A;
3-rN-methyl-4-/~-methyl-4-LN-methyl-4-LN-methyl_4_~N,N-bis(2-
~oroethylamino~pyr~Ole-2-carboxamido7Pyrrole-2-carbox~mido7
pyrrole-2-carboxamido~pyrrole-2-carboxamidoJpropyl-dime-
thylamine~ and the pharoaceutically acceptable salt~
thereof, especially the hydrochlorides.
. . . ~ . .
.
~28sg34
- 15 -
Amother specific class of compounds of formula (I) according
to the invention (hereinafter class B) are the compounds of
fa,rmula (I) ~herein, subject to the above provisos (ii) and
(iii), n is zero or an integer of 1 to 4;
R is -N02, -NH2 or NHCHO;
each group Rl i8, independently, hydrogen or Cl-C4 alkyl;
R2 is a Cl-C6 alkyl group terminating vith a basic or acidic
moiety or with a free or glycosilated hydroxy group, and the
pharmaceutically acceptable salts thereof.
In the above class the preferred meanings for the various
substituents are the same indicated before in this specifica-
tion with reference to the formulae (I) and (IA).
A preferred group of compounds in the ambit of the said class
B are the compounds of formula (I) wherein subject to the above
provisos (ii) and (iii), n is zero or an integer of 1 to 4;
R is -NOz, -NH2 or -NHCHO;
each group R1 is, independently, C1-C4 alkyl;
R2 is a Cl-C6 alkyl group terminating ~ith a substituent
selected from the group consisting of a~ino; mono- and
di-Cl-C6 alkylamino; a group -N=N-N ~CH3 ; a nitrogen
containing heterocyclic ring; -COOH;
-CH2-OH; and -CH2-0-D ~herein D is a sugar- or amino-sugar-
residue,
and the pharmaceutically acceptable salts thereof.
In the above preferred group of compounds a C1-C4 alkyl group
for R1 is, preferably, methyl, and the C1-C6 alkyl group of
the R2 substituent i8, preferably, Cl-C4 alkyl, in particular
' ~`' , " ' ., . :' '" , :
~285934
- 16 -
ethyl or n-propyl. Particularly preferred groups R2 are
-(CH2)p-N ~ CH3 ;
-(CN ~ -C~ ~ 2 p ~ ~ ; -(CH2)p-C ~ ~ ;
H H H
2 p ~ ~ ; -(CH2) -COOH; -(CH2)p-CH20H
and -(CH2) -CH2-0-D ~herein p i8 an integer of 1 to 4,
especially 2 or 3, and D ~8 as defined abovo.
Examples of specific co~pounds of tho above class B are:
3-~N-methyl-4-~h-methyl-4-~h-methyl-4-Lh-methyl-4-nitro=
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-
-carboxamido~pyrrole-2-carboxamidQ7propyl-dimethylamine;
3-LIJ-methyl-4-~l-methyl-4- ~-methyl-4_ ~-methyl_4-L~-methyl-
-4-nitropyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-
-2-carboxamido~pyrrole-2-carboxamldo]pyrrole-2-carboxamidQ7
propyl-dimethylamine;
3-Lh-methyl-4-~h-methyl-4-~-N-methyl-4-~N-methyl-4- ~-methyl-
-4-/N-methyl-4-nitropyrrole-2-carboxsmido~pyrrole-2-csrboxa-
mido~pyrrole-2-carboxamidQ7pyrrole-2-carboxamidQ7pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine;
3-Lh-methyl-4-~h-methyl-4-LN-methyl-4-~1-methYl-4-aminopyr-
role-2-carboxamido~pyrroIe-2-carboxamid ~ pyrrole-2-carboxam.d~
pyrrole-2-carboxamido~propyl-dimethylamine;
'`' ' ' ' ~` ~''' `'` ' ' ' '.' ' '
~285934
- 17 _
3-Lh-methyl-4-~-methyl-4-~N-methyl-4-L~-methyl-4-~-methyl-
-4-aminopyrrole-2-carboxamido~pyrrole-2-carboxamidQ7pyrrole-
-2-carboxamido7pyrrole-2-carboxamidQ7pyrrole-2-carboxamido~7
propyl-dimethylamine;
3-~-methyl-4-CN-methyl-4-LN-methyl-4-~N-methyl-4-~N-methyl-
-4-~h-methyl-4-aminopyrrole-2-carboxamido~pyrrole-2-carboxa-
mido,~pyrrole-2-carboxamidQ7pyrrole-2-carboxamid~7pyrrole-2-
-carboxamid~ pyrrole-2-carboxamid~ propyl-dimethylamine;
3-~N-methyl-4- ~-methyl-4-[N-methyl-4-~N-methyl-4-formyl-
aminopyrrole-2-carboxamidQ7pyrrole-2-carboxamido~pyrrole-
-2-carboxamido~pyrrole-2-carboxamido~propyl -dimethylamine;
3- ~ -methyl-4-~1-methyl-4-CN-methyl-4-L~-methyl-4-Lk-methyl-
-4-formylaminopyrrole-2-carboxamidQ7pyrrole-2-carboxamido~
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carbo-
xamido7propyl-dimethylamine;
3- ~-methyl-4-~N-methyl-4- ~ -methyl-4- ~ -methyl-4-L~-methyl-
-4-~N-methyl-4-formylaminopyrrole-2-carboxamido~pyrrole-2-
-carooxamidoJpyrrole-2-carboxamid~ pyrrole-2-carboxamido~
pyrrole-2-carboxamido7pyrrole-2-carboxamido~propyl-dimethyl-
amine;
B-~,h-methyl-4-[N-methyl-4-~N_methyl-(4-formylamino)pyrrole-
-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamidQ7
ethyl-L2-imidazole];
B-fN-methyl-4- ~-methyl-4-~N-methyl-4-formylaminopyrrole-2-
-carboxamido-7pyrrole-2-carboxamido~pyrrole-2-carboxamido-7
ethyl/2-(2-imidazoline)~;
B- ~-methyl-4-LN-methyl-4- ~_methyl-4-formylaminopyrrole-2-
-carboxamido~pyrrole-2-carboxamid~ pyrrole-2-carboxamido~
ethyl- ~ -(3,4,5,6-tetrahydro-pyrimidine)~;
, .
.
.
- .
128S93~
- 18 -
B-~N-methyl-4-LN-methyl-4-LN-methyl-4-formylaminopyrrole-2-
-carboxamido]pyrrole-2-carboxamido~pyrrole-2-carboxamido/
ethyl-L2-(4 5-dihydro)oxazole~;
B-LN-methyl-4-L'N-methyl-4-~h-methyl-4-formylaminopyrrole-2-
S -carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
propionic acid;
B-Lh-methyl-4-~i-methyl-4-~N-methyl-4-formylaminopyrrole-2-
-carboxamid ~pyrrole-2-carboxamid~ pyrrole-2-carboxamido7
propyl alcohol;
3-CN-methyl-4- ~-methyl-4-L~-methyl-4-formylaminopyrrole-2-
-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
?ropane-1-~(3-amino-2 3 6-trideoxy- d -L-lyxo-hexapyranosy ~ xy~
trifluoroacetate;
and when appropriate the phanmaceutically acceptable salt~ thereof especially
the hydrochlorides.
The invention also provides a process for the preparation of
a compound of formula (I) tho said process co~prising:
(A) reacting a compound of formula (II)
_ (II)
H2N- ~ CONH- 2
wherein Rl and R2 are as defined abovo e~cept that pro~iso
(iii) does not apply and q is an intogor of 1 to S ~ith
-: , . ~ .
. .
12859~4
-- 19 --
a co~pound of formula (III)
02N ~ (III)
N
Rl
wherein Rl is as defined above and Z is a leaving group,
so obtaining a compound of formula (I) wherein R is -N02;
or
(B) reducing a compound of formula (IV)
02N ~ 1 ( ~V)
~herein n, R1 and R2 are as defined abovo, except that n i8
not zero or 1 ~hen each R1 i8 methyl and R2 i8 -CH2-CH2-COOH,
0 80 obtaining a compound of formula (1) ~herein R i8 -NH2; or
(C) formylating a compound of formula (V)
Rl ~ ~ 0 ~ ~ ~ 2 (V)
Rl
~herein n, Rl and R2 aro as defined above, except that
proviso (iii) does not apply, 80 obtaining a coopound of
lS formula (I) ~herein R i8 -NH-CH0; or
:- ,. . .. . . .
12~359~4
- 20 -
(3) reacting a compound of formula (V) wherein R1, R2 and n
are as defined above, with a compound of formula (VI)
o
Z'-C-N(NO)-R4 (VI)
wherein R4 is as defined above and Z' is a leaving group,
so obtaining a compound of formula (I) wherein R is
-NHR3 and R3 is -CON(NO)R4, wherein R4 is as defined
above; or
(E) reacting a compound of formula (Y), wherein R1, R2 and
n are as defined above, with a compound of formula (VII)
Z-CO-(CH2) -R5 (VII)
wherein R5, m and Z are as defined above, so obtaining
a compound of formula (I) wherein R is -NHR3 and R3 is
-CO(CH2) -R5, wherein m and R5 are as defined above; or
(F) reacting a compound of formula (V), wherein R1, R2 and
n are as defined above, with a compound of formula (VIII)
X-CH-CH (VIII)
\01
wherein X may be hydrogen, C1-C2 alkyl or halomethyl, to
give a compound of formula (IX)
N ~ N ~ ~ O-N-R
wherein R1, R2 and n are as defined above and each X has
- : ' ' : ', ' ' : .: `
1285934
- 21 -
the meaning corresponding to the meaning of X in the
compound (VIII), and transforming a compound of formula
(IX) into a compound of formula (I) wherein R is -N _ R '
wherein R6 and R7 are as defined above; and, if desired,
modifying the R2 moiety in a compound having the above
formula (I)~subject or not to the above provisos,in order
to obtain a compound of formula (I) with a different R2
moiety and/or, if desired, salifying a compound of formula
(I) or obtaining a free compound from a salt and/or, if
desired, separating a mixture of isomers of formula (I)
into the single isomers.
The leavlng group Z in the compounds (III) and (VII) may
be, for example, a halogen atom, e.g. chlorine or bromine,
or an imidazolyl or phenoxy group.
lS The leaving group Z' in the compound~ (VI) may be, for
instance, an azido group or a trlchlorophenoxy or succini-
mido-N-oxy group.
The reaction between a compound of formula (II) and a
compound of formula (III) is preferably carried out in the
presence of a solvent and, preferably, using an excess of
the compound of formula (III), e.g. from about l.l to about
2 moles of compound (III) per l mole of compound (II).
The so1vent proferab1y 1s sn lnert organ1c so1vent chosen
:
:.. : , '; ' ........ , , . , .. : .
- : ;. ~ .. ' .: ,. : ................. , , . -
:~ . . ., . ,-: . .
128~;934
- 22 -
from dialkylsulfoxides, e.g. dimethylsulfoxide , aliphatic
acid dialkylamides, e.g., dimethylformamide, heterocyclic
amines like pyridine, aliphatic alcohols,and also water.
A particularly preferred solvent is dimethylformamide.
The reaction temperature may range from about -50C to
about 50C. The time required for the reaction may vary
approximately within the range from 0.5 to 24 hours.
The reduction of a compound of formula (IV~ may be, e.g.,
carried out by catalytic hydrogenation according to known
procedures, using, for instance, palladium on charcoal,
platinum, rhodium or raney nickel, as the catalyst.
Reduction may be, for example, carried out at room tempera-
ture and under atmospheric pressure in an inert solvent
such as, e.g., ethanol, methanol or dimethylformamide, in
the presence of 10% palladium on charcoal.
The formylation of a compound of formula (V) may be, e.g.,
carried out with the mixed anhydride of formic and acetic
acid, optionally in the presence of a tertiary amine, such
as, for instance, pyridine, triethylamine or dimethylani-
line, as reported, e.g., in U.K. patent specification no.1,061,639; or with N-formyl imidazole, obtained from
carbonyl imidazole and formic acid, according to, e.g.,
J. Org. Chem. (1985) 50, 3774-3779; or with formamide and
ethylformate according to, e.g., Gazz. Chim. Ital. 99,
632, (1967).
- .
1285~34
- 23 -
The reaction between a compound of formula (V) and a
compound of formula (VI) is preferably carried out in
the presence of a solvent and, preferably, using an excess
of the compound of formula (VI), e.g. from about 1.1 to
about 2 moles of compound (VI) per 1 mole of compound (V).
The solvent preferably is an inert organic solvent chosen
e.g. from dialkylsulphoxides e.g. dimethylsulphoxide,
aliphatic acid dialkylamides, e.g. dimethylformamide or
dimethylacetamide, phosphoric acid triamide or hexamethyl-
phosphoramide,or.fOr example, dioxane or dimethoxyethane.Dimethylformamide (DMF) is a particularly preferred solvent.
The reaction temperature may ran8e from about -10C to
about 25C, although 0C is a particularly preferred
temperature.
The time required for the reaction may vary within the
range from about 0.5 to about 6 hours.
Also the reaction between a compound of formula (V) and a
compound of formula (VII) is preferably carried out in the
presence of a solvent and, preferably, using an excess of
the compound of formula (VII), e.g., from about 1.1 to
about 2 moles of compound (VII) per 1 mole of compound (V).
The solvent preferably is an inert organic solvent chosen
from dialkylsulfoxides, e.g. dimethylsulphoxide , aliphatic
acid dialkylamides, e.g., dimethylformamide, heterocyclic
amines like pyrldine, allphatic alcohols and also water.
. .
' . -' ' . ' , , ' '" ' '
~285934
- 24 -
A particularly preferred solvent is DMF.
The reaction temperature may range from about -50C to
about 50C. The time required for the reaction may vary
approximately within the range from 0.5 to 24 hours.
When in the compound of formula (VIII) X is halomethyl, it
is,preferably, chloromethyl or bromomethyl.
The reaction between a compound of formula (V) and a
compound of formula (VIII) is preferably carried out in the
presence of a solvent and, ?referably, using an excess of
the compound of formula (VIII), e.g. from about 25 moles to
about 50 moles of compound (VIII) per 1 mole of compound (V).
The solvent can be, e.g., water, an aliphatic alcohol, e.g.
methanol or ethanol, an aliphatic carboxylic acid such as,
e.g., acetic acid, an aliphatic acid dialkylamide, e.g.
dimethylformamide, or a dialkylsulphoxide, e.g. dimethyl-
sulphoxide, dioxane or dimethoxyethane. Methanol is a
particularly preferred solvent.
The reaction temperature may range from about -20C to
about 25C.
The time required for the reaction may vary within the
range from about 2 to about 48 hours.
The transformation of a compound of formula (IX) into a
compound o~ formula (I) wherein R is a group -N_ R
wherein R6 and R7 are as previously defined, may be carried
out through reactions commonly used in the organic chemistry.
.
'' -' ~, ' .~ ' :
~285934
_ 25 -
Thus, for example, a compound of formula (IX) wherein each
group X is hydrogen or C1-C2 alkyl may be reacted with an
halogenating agent such as, e.g., a haloqen, e.g. chlorine
or bromine, or a thionyl halide, e.g. thionylchloride, to
give a compound of formula (I) wherein R is a group -N _~ ,
wherein each R6 and R7 is C2-C4 alkyl 2-substituted by
halogen, e.g. chlorine or bromine. I
Similarly, a compound of formula ~IX) wherein X is hydrogen
or C1-C2 alkyl may be reacted with a sulfonic acid of
formula R8S03H, wherein R8 is as defined above or, most
preferably, with a reactive derivative thereof such as, e.g.,
the corresponding sulfonyl halide, e.g. chloride, or anhy-
dride, to give a compound of formula (I) wherein R is a
group -N ~ R wherein each R6 and R7 is C2-C4 alkyl 2-substi-
tuted by a group -0-S02R8 wherein R8 is as defined above.
On the other hand, a compound of formula (IX) wherein each
group X is halomethyl, e.g. chloromethyl or bromomethyl
may be reacted with a base to give a compound of formula
(I) wherein R is a group -N ~ R wherein each R6 and R7 is
oxiranemethyl.
The base may be either an inorganic base such as, for
instance, an alkali metal, e.g. sodium or potassium,
hydroxide, or an alkaline-earth metal, e.g. calcium or
magnesium, hydroxide, or an organic base such as, for
instance, an aliphatic amine, e.g. trimethylamine, or a
heterocyclic amine, e.g. pyridine, piperidine, morpholine
or methylmorpholine.
- . . ~ ,: ,
-` . : : . .
12859~4
- 26 -
- R6
Other compounds of formula (I) wherein R is a group -N ~ R
may be prepared from a compound of formula (IX) through
reactions well known in the organic chemistry and following
known procedures.
The modifications of the R2 moiety in a compound having
the formula (I), sub~ect or not to the above proviso, in
order to obtain a compound of formula (I) with a different
R2 moiety, may be carried out according to known methods.
Examples of such modifications include e.g.:
10 (a') in a R2 moiety which is a C1-C6 alkyl group terminating
with an amidino group (-C~NH ) to convert the amidino
group into a heterocyclic ring which ma~ be, e.g., a
2-imidazole ( ~ ~ ) or 2-imidazoline (~N ~ ) ring, or
into a carboxy~group; H
15 (b') in a R2 moiety which is a C1-C6 alkyl group terminating
with a -COOH group to convert the -COOH into -CH20H;
and
(c') in a R2 moiety which is a C1-C6 alkyl group terminating
with a free hydroxy group, to convert the free hydroxy
into a glycosilated hydroxy group.
As regards the modifications under (a') above, the conver-
sion of the amidino group into the 2-imidazole ring may be,
e.g., carried out by reaction with, e.g., aminoacetaldehyde
dimethylacetal according to known procedurelwhile ethylen-
25 diamine may be, e.g., used for converting the amidino into
. - . ~, . .. . . .
- .
. ~ ,, . - -.. , : .. . . ..
. . - , , . . , . : , . . .
.. . . :. .. ,- . .. .. , ~
~28S934
- 27 -
2-imidazoline; conversion into other heterocyclics may be
carried out in similar way by known methods.
Basic hydrolysis, e.g. with sodium hydroxide in methanol
at reflux temperature, may be, e.g., used to convert the
amidino group into carboxy.
The transformation of the carboxy group into -CH20H as per
item (b') above may be, e.g., conducted by reduction in a
conventional way, for instance using NaBH4 as the reducing
agent.
Conventional etherification proceduresmay be followed for
converting the free hydroxy group into a glycosilated
hydroxy group as per item (c') above.
Obviously, the above indicated modifications at
the R2 moiety may be carried in absence of interfering
groups on the rest of the formula (I)-molecule.
Otherwise,.possibly present interfering groups need to be
preliminarly protected and then reinstated, in a conven-
tional way, after the modification on R2 has been completed.
The salification of a compound of formu}a (I) and the
preparation of a free compound from a salt may be carried
out according to known methods.
Conventional procedures, such as, e.g., fractional crystal-
lization and chromatography, may also be used for the
optional separation of a mixture of isomers of formula tI)
into the single isomers.
: - .:- - -. - - . . -
.
1285~34
- 28 -
The compounds of formula (II) may be obtained following
known procedures, e.g. those reported for preparing
distamycin derivatives in, e.g., Gazz. Chim. Ital. 97,
1110 (1967).
In particular, for instance, a compound of formula (II)
wherein ~ is 1 may be obtained reducing a com?ound of
formula (X)
0 N
N CONH-R2 (X)
wherein R1 and R2 are as defined above.
A compound of formula (II) wherein q is 2 may be obtained
by reacting a compound of formula (II) wherein 9 is 1 with
a compound of the above formula (III) so obtaining a
compound of formula (XI)
02N ~ ONH ~ CONH-R2 (XI) `
Rl 1
wherein R1 and R2 are as defined above, which is then, in
its turn, reduced.
In analogous way, subjecting a compound of formula (II)
wherein q is 2 or, respectlvely, 3 or 4 to the same above
reaction with a compound (III) and subsequent reduction,
there is obtained a corresponding com?ound of formula (II)
wherein q is 3 or, respectively, 4 or 5.
.: ' '~ , .
-
.
iZ85~334
- 29 -
The reduction of the nitro derivatives such as, e.g., the
above compounds (X) and (XI), may be carried out as indi-
cated before for the reduction of the compounds (IV).
The conditions for the reaction between compounds (II) and
compounds (III) have been already indicated previously in
this specification.
The compounds of formula (III) are known compounds or may
be prepared by known methods from known compounds.
The compounds of formula (IV) may be obtained through
reaction between compounds (II) and compounds (III).
The compounds of formula (V) may be obtained from the
reduction of the compounds (IV).
The compounds of formula (VI) are known compounds and may
be prepared, for example, according to J. Med. Chem. (1982),
25, 178-182.
The compounds of formula (VII) and (VIII) are known compounds
too, or may be prepared by known methods from known compounds.
In particular, for instance, the compounds of formula (VII)
are either commercially availabie products or may be pre-
pared through activation of the parent carboxy-derivatives
in a conventional way.
The compounds (VIII) are, generally, commercially available
products.
The compounds of formula (X) may be obtained reacting a
compound of formula (III) with a compound of formula (XII)
, , . . ~, . :. ' ~
~285934
- 30 -
R2-NH2 (XII)
wherein R2 is as defined above, followin~e.g.,the u~
conditions described in the organic chemistry for the
acylation of the amines.
The compounds of formula (XII) are known or commercially
available compounds.
As already said, object of the invention are also pharma-
ceutlcal compositions containing a compound of the above
formula (IA) as the active substance.
A specific class of coopositions according to the in- -
vention ~hereinafter classC) are phsr~aceutical composi-
tions comprising a pharmaceutically acceptable carrier
and/or diluent and, as the actiYe substance, a compound
of the above formula (IA) ~herein, subject to the above
proviso (i), n is zero or an integer of 1 to 4;
R is a) -NHR3, wherein R3 is
a') -CON(NO)R4, in which R4 is C1-C4 alkyl either
unsubstituted or substituted by halogen; or
b') -CO(CH2) -R5, in which R5 is halogen, oxiranyl,
methyloxiranyl, aziridinyl,cyclopropyl or the residue of an
alicyclic ~,B-unsaturated ketone or lactone,
and m is zero or an integer of 1 to 4; or
b) -N _ R wherein either R6 and R7 are the same and
are each oxiranemethyl, aziridlnemethyl, or C2-C4
alkyl 2-substituted by halogen or by a group
-OS02R8, wherein R8 is C1-C4 alkyl or phenyl, or
one of R6 and R7 is hydrogen and the other is as
defined above;
1285934
- 31 -
each group R1 is, independently, hydrogen or Cl-C4 alkyl;
R2 is a Cl-C6 alkyl group terminating with a basic or
acidic moiety or with a free or glycosilated hydroxy
group,
or a pharmaceutically acceptable salt thereof.
In the above class preferred meanings for the various
substituents are the same as those previously indicated
with reference to the formulae (I) and (IA).
A preferred group of coopounds of for~ula (IA) in the
a~bit of the abo~e class C are the compounds of foroula
(IA) ~herein, subject to the above pro~iso (i),
n is zero, 1 or 2;
3 3
a') -CON(NO)R4 ~herein R4 i~ Cl-C4 alkyl substituted
b~ halogen, or
b') -CO(CH2) -R5 ~herein R5 is halogon, osiran~l,
l-aziridinyl, cyclopropyl, or the residue of an alicyclic
a,~-unsatNrated lactone, and m is zero, 1 or 2; or
,: . - : .
:., , .... . i . : . - :
: - - . -: . .
12859;~4
b) -N ~R6 , ~herein R6 and R7 are the same and are each
oxiranemethyl, 1-aziridinemethyl, or a C2-C4 alkyl group
2--substituted by halogen or by a group -OS02R8 wherein
R8 i8 Cl-C4 alkyl;
each group Rl i9, independently, Cl-C4 alkyl;
R2 is a Cl-C6 alkyl group terminating ~ith a basic moiety,
and the salts thereof ~ith pharmaceutically acceptable acids,
in particular with hydrochloric acid.
In the above preferred group of compounds a R4 Cl-C4al-
kyl group is, preferably, methyl or ethyl; a halogen atom is,
preferably, chlorine; the residue of an alicyclic ~,~-unsaturated lactone
is, preferably, a group ~ 0 ; a C2-C4 alk~l group in R6/R7
is, preferably, ethyl; ~hen R6 and R7 are a C2-C4 alkyl group
2-substituted by halogen, they are, preferably, 2-chloro-
ethyl; when R6 and R7 are a C2-C4 alkyl 2-substituted by a
group -OS02R8 ~here R8 i8 C1-C4 alkyl, they are, preferably,
methanesulfonyloxyethyl; a Cl-C4 alkyl group for Rl is,
preferably, methyl; in the R2 substituent the Cl-C6 alkyl
group is, preferably, Cl-C4 alkyl, in particular ethyl or
n-propyl, and the terminal basic moiety is, preferably, amino;
mono- or di-Cl-C6- alkylamino; amidino; a group -N=N-N~CH3;
or a nitrogen containing hetero-
cyclic ring; particularly preferred R2 ~alues are those
specified before, in particular, -(CH2) -N~CH3 and
25 -(CH2) -C~ wherein p is an integer of 1 to 4, especially
P NH2
2 or 3.
': ' ' ,~ '
' , ~ ' ' :
~285g34
- 33 -
Examples of preferred specific compounds of formula (IA)
in the ambit of the ~bove class C may be the same spe-
cific compounds indicated before a~ preferred for the
class A, especially in the form of salts with hydroclo-
ric acid.
Another specific class of compositions according to the
invention (hereinafter class D) are pharmaceutical compo-
sitions comprising a pharmaceutically acceptable carrier
and/or diluent and, as the active substance, a compound
of the abovo formula (IA) wherein, subject to the above
proviso (iv), n is zero or an integer of 1 to 4;
R is -N02, -NH2 or -NH-CH0; each group Rl is, independently,
hydrogen or Cl-C4 alkyl; R2 is a Cl-C6 alkyl group termina-
ting with a basic or acidic moiety or with a free or glyco-
silated hydroxy group; or a pharmaceutically acceptable salt
theroof.
Also in the above class D the preferred meaning~ for the
various substituents are the same indicated before with
reference to the formulae (I) and (IA).
A preferred group of compounds of formula (IA) in the ambit
of the said class D are the compounds of formula (IA)
wherein
n is zero or an integer of 1 to 4;
. ' ' " ` ` ~ ` ' . `. `' ~ ' ' ' ' `' ' ', `
.
l2ass34
R is -NO2, -NH2 or -NHCHO;
each group Rl is, independently, Cl-C4 alkyl;
R2 is a C1-C6 alkyl group toroinating ~ith a substituent
~elected fro- the group consisting of a-ino; ono- and
di-Cl-C6 alkyla-ino; a group -N=N-N~ CH3 ;
a nitrogen containing heterocyclic ring; -COOH;
-CH2-OH; and -CH2-O-D ~herein D is a sugar- or a-ino-sugar-
residue,
and the pharoaceuticallr acceptable salts thsreof
In the above preferred group Or co-pounds a Cl-C4 alk~l group
for Rl is, preferably, ethyl, and the Cl-C6 alkJl group of
the R2 substituent is, preferably, Cl-C4 alkJl, in particular
ethyl or n-propyl Particularly preferred groups R2 are
-(CH2) -N ~ CH3 ;
-(CH2)--C~~ ; -(CH2)p-C ~ ; -(CH2)p--C ~ ~ ;
H H H
~N
2 p ~ O ~ ; -(CH2)p-COOH; -(CH2)p-CH20H
and -(CH2) -CH2-O-D ~herein p is an integor of 1 to 4
especially 2 or 3, and D is as defined abo~e
.
. . . ~ ' , .
. , , : . .
~285934
- 3s -
Examples of specific compounds of formula (IA) contained
as the active substance in the pharmaceutical compositions
of the above class D are:
3- ~-methyl-4-(N-methyl-4-nitropyrrole-2-carboxamido)pyrrole-
-2-carboxamid~ propyl-dimethylamine;
3-CN-methyl-4- ~-methyl-4-~N-methyl-4-nitropyrrole-2-
-carboxamidQ7pyrrole-2-carboxamido~pyrrole-2-carboxamido~
propyl-dimethylamine;
3-L7t-methyl-4-rN-methyl-4-~N-methyl-4-~N-methyl-4-nitropyr-
role-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxa-
mldo~pyrrole-2-carboxamido~propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-~h-methyl-4-L~-methyl-4-~N-methyl- '.
-4-nitropyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-
-2-carboxamid~ pyrrole-2-carboxamid~ pyrrole-2-carboxamidQ7
propyl-dimethylamine;
3-Lh-methyl-4- ~-methyl-4-CN-methyl-4- ~-methyl-4- ~-methyl-
-4- ~ -methyl-4-nitropyrrole-2-carboxamido~pyrrole-2-carbo-
xamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-
-2-carboxamid~ pyrrole-2-carboxamido~propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-~h-methyl-4-CN-methyl-4-aminopyr-
:: ~ . - .................... ..
: '' ` ' ' ' ' ` '
~285934
_ 36 -
role-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-carboxa-
mido~pyrrole-2-carboxamidQ7propyl-dimethylamine;
3-/N-methyl-4-~N-methyl-4-aminopyrrole-2-carboxamidolpyrrole-
-2-carboxamido~propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-CN-methyl-4-aminopyrrole-2-carbo-
xamido]pyrrole-2-carboxamido~pyrrole-2-carboxamido~propyl-
-dimethylamine;
3- ~-methyl-4-~N-methyl-4-[N-methyl-4-~N-methyl-4- ~ -methyl-
-4-aminopyrrole-2-carboxamido]pyrrole-2-carboxamido7pyrrole-
10 -2-carboxamidolpyrrole-2-carboxamido;rpyrrole-2-carboxamido?
propyl-dimethylamine;
3-CN-methyl-4- ~-methyl-4- ~ -methyl-4-~N-methyl-4-/N-methyl-
-4-CN-methyl-4-aminopyrrole-2-carboxamido~pyrrole-2-carboxa-
mido~pyrrole-2-carboxamido~pyrrole-2-carboxamido7pyrrole-
-2-carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-formylaminopyrrole-2-carboxamido~
pyrrole-2-carboxamido~propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-LN-methyl-4-formylaminopyrrole-
-2-carboxamido]pyrrole-2-carboxamido~pyrrole-2-carboxamidoJ
propyl-dimethylamine;
3-~N-methyl-4-~N-methyl-4-CN-methyl-4-LN-methyl-4-formyl-
aminopyrrole-2-carboxamido~pyrrole-2-carboxamido]pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine;
' 3-/N-methyl-4-C,N-methyl-4-CN-methyl-4-~N-methyl-4-[N-methyl-
-4-formylaminopyrrole-2-carboxamidolpyrrole-2-carboxamido~
pyrrole-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-
-carboxamido~propyl-dimethylamine;
,: - . ~ - :, .: .
., . , - . . . ..
~285934
3-rN-methyl-4-~N-methyl-4-LN-methyl-4- ~ -methyl-4-[N-methyl-
-4-LN-methyl-4-formylaminopyrrole-2-carboxamido]pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido7
pyrrole-2-carboxamidoJpyrrole-2-carboxamido7propyl-dime-
thylamine;B- ~-methyl-4- ~-methyl-4-~N-methyl-(4-formylamino)pyrrole-
-2-carboxamido]pyrrole-2-carboxamido~pyrrole-2-carboxamido7
ethyl-~2-imidazole~;
B-/N-methyl-4-~N-methyl-4-~N-methyl-4-formylaminopyrrole-
-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
ethyl-L2-(2-imidazoline)~; -
B-~N-methyl-4-~N-methyl-4-~N-methyl-4-formylaminopyrrole-2-
-carboxamido,7pyrrole-2-carboxamido~pyrrole-2-carboxamido~
ethyl-/2-(3,4,5,6-tetrahydro-pyrimidine),';
B-LN-methyl-4- ~-methyl-4-~N-methyl-4-formylaminopyrrole-2-
-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamidoJ
ethyl-~2-(4,5-dihydro)oxazoleJ;
B-LN-methyl-4-~N-methyl-4-~N-methyl-4-formylaminopyrrole-2-
-carboxamido~pyrrole-2-carboxamido7pyrrole-2-carboxamido~
propionic acid;
B-LN-methyl-4-~N-methyl-4-~N-methyl-4-formylaminopyrrole-
-2-carboxamido~pyrrole-2-carboxamido]pyrrole-2-carboxamido~
propyl alcohol;
3-/N-methyl-4-/N-methyl-4-/N-methyl-4-formylaminopyrrole-2-
-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~ -
propane-1-/(3-amino-2,3,6-trideoxy- d -L-lyxo-hexapiranosyl)
oxy~trifluoroacetate,
~i
.
~ ' . ~--` `.
~285~34
anld ~here appropriate the pharmaceutically acceptable salts
thereof, especially the hydrochlorides.
The compounds of formula (IA), active principle in the
phar~aceutical coopositions of the invention, may be
prepared by kno~n methods, e.g. those reported in the U.K.
patents Nos. 1,009,797 and 1,061,639, as well as those
described before in this specification for the preparation
of the compounds of formula (I).
The compounds of the invention of foroula (IA) can be useful
as antiviral and antineoplastic agents.
They sho~, e.g., a re-arkable effectiveness in interfering
~ith the reproductive aetivity of the pathogenic viruses
and proteet tissuo eells fro- viral infoetions.
For exaople they shov aetivity against DNA viruses such as,
for instanee, herpes, e.g.horpes simplex and herpes zoster,
viruses, and Adenoviruses, and against retroviruses such as,
for instanee, Sareooa viruses, e.g., ~urine sarcoma virus, and
Leukemia virusos, e.g. Friend leukemia virus. Thus, for exam-
ple, herpes, eoxsaekie and respiratory syneytial viruses ~ere
tested in fluid oediu- as follo~. Serial t~ofold dilutions
of the compounds fro- 200 to 1.5 meg/nl ~ere distributed in
duplieate 0.1 ol/~oll in 96 ~ell8 oieroplates for tissue
culturo.
Cell su8pension8 (2x105 cells/ol), uninfeeted, for cytotoxi-
city control, or infected ~ith about 5xlO TCID50 ofvirus/cell were immediately added 0.1 ~ ell. After 3-5 day
incubation at 37C in C025X, the cell eultures ~ere evalua~ed
by microseopieal observation and ~axiou- Tolerated Dose (~xTD)
- . . .
- ,
~285~34
- 39 -
a~ ~ell as Minimum Inhibiting Concentration (MIC) were
determined MxTD is maxi-um concentration of the compound
~hich permits a gro~th of monolayers similar to the controls
in density and in morphology ~IC is minimum concentration
S ~hich determines a reduction of cytopathic effect
in comparison ~ith the infected controls
Coopounds ~ere considered active ~hen their activity index
calculated by the ratio MxTD/~IC ~as ~ 2
Thus, for example, for the coopound of the invention 3-~N-
--ethyl-4-~N--ethyl-4-rN-methyl-4-rN-methyl-4-nitropyrrole-
-2-carboxamido~pyrrole-2-carboxa-ido~pyrrole-2-carboxamido~
pyrrole-2-carboxa-ido~propyl-di~ethyla-ine (internal code
FCB 24558) in vitro te~ts indicate an activity index of about
8 on herpes simplex infected Hep # 2 cells and of about 4 on
coxackie B infected Hep ~ 2 cells For dista-ycin A
the same test8 indicate an activity index of about 4 on herpes
simplex infected Hep ~ 2 cells and an activity index ~1 on
coxsackie B infected Hep # 2 cells
The coopounds of the invention of for-ula (IA) sho~ also
cytostatic properties to~ards tumor cells 80 that they can be
useful, e g , to inhibit the gro~th of various tumors, such as,
for instance, carcinomas, e g ma-mary carcinoma, lung carci-
noma, bladder carcinoma, colon carcinoma, ovary and endometrial
tumors Other neoplasias in ~hich the co-pounds of the inven-
tion could find application are, for instanco, sarcomas, e gsoft tissue and bone sarcomas, and the hematological mali-
gnancies such as, e g , leukenias
The compounds of the invention can be ad-inistered
by the usual routes, for example, parenterally,
,
12859~4
- 40 -
e.g. by intravenous injection or infusion,
intramuscularly, subcutaneously, topi6ally or orally.
The dosage depends on the age, weight and conditions o~ t~
patient and on the administration route.
S For example, a suitable dosage for administration to a~ult
humans may range from about 0.1 to about 100 mg pro dose
1-4 times a day.
As already said, the pharmaceutical compositions of the
invention contain a compound of formula (IA) as the active
substance, in association with one or more pharmaceutically
acceptable excipients.
The pharmaceutical compositions of the invention are usually
prepared following conventional methods and are administered
in a pharmaceutically suitable form.
For instance, solutions for intravenous injection of
infusion may contain as carrier, for example, sterile water
or,preferably,they may be in the form of sterile aqueous
isotonic saline solutions.
Suspensions or solutions for int`ramuscular injections may
contain,together with the active compound,a pharmaceutically
acceptable carrier, e.g. sterile water, olive oil, ethyl
oleate, glycols, e.g. propylene glycol, and if desired, a
suitable amount of lidocaine hydrochloride.
In the forms for topical application, e.g. creams, lotions
or pastes for use in dermatological treatment, the active
.
. - . .
~Z85g34
- 41 -
inKredient may be mixed with con~entional oleaginou~ or
em~ ifying excipients.
The solid oral for~s, e.g. tablet~ and capsules; may contain,
together ~ith the actiYe compound, diluents, e.g., lactose,
dextrose, saccharose, cellulose, corn ~tarch and potato starch;
lubricants, e.g. silica, talc, ~tearic acid, magnesium or
calciu~ stearate, and/or polyethylene glycols; binding agents,
e.g. ~tarches, arabic gu~s, gelatin, ~eth~lcellulose, carboxy-
~ethyl cellulose, polyvinylpyrrolidono; disaggregating agents,
e.g. a starch, alginic acid, alginates, sodiuo starch glycolate;
effervescing ~ixtures; dyestuffs; s~eeteners; Yetting agents,
for instance, lecithin, polysorbate~, laur~lsulphates; and, in
general, non-toxic and pharuacologically inactive substances
used in phar~aceutical for~ulations. Said phar-aceutical prepara-
tions may be manufactured in a kno~n anner, for exa-ple by
means of ~ixing, granulating, tabletting, sugar-coating, or
film-coating processes.
The invention provides also a process for producing a
pharmaceutical composition as described above, the process
comprising formulating an effective amount of the active
substance of formula (IA) with a pharmaceutically acceptable
carrier and/or diluent.
Furthermore, according to the invention there is provided
a method of treating viral infections and tumors in a
patient in need of it, comprising administering to the
said patient a composition of the invention.
The abbreviations DMSO, THF, CDI, DMF, DCC, DCU and ACOH
stand, respectively, for dimethylsulfoxide, tetrahydrofuran,
carbonyldiimidazole, dimethylformamide, dicyclohexylcarbo-
diimide, dicyclohexylurea and acetic acid.The following examplesillustrate but do not limit the
invention.
i285g34
- 42 -
Example 1
To a stirred aqueous solution of ~,N-dimethylaminopropyl-
amine (2.03 g in 40 ml of water) and sodiu~ bicarbonate
(3.36 g) at room tem?erature a solution of
N-methyl-4-nitropyrrole-2-carboxylic acid chloride (~ g)
in S ml of oenzene w~s added_The resulting mixture was stirred fo.
2 hours at room ~emperature, saturated with sodium chlorice
and extracted with benzene (2 x 50 ml). The dried orgar.ic
extracts were concentrated in vacuo and the residue was
crystallized from light petroleum ether to yield 3.5 g of
pure 3-LN-methyl-4-nitro-pyrrole-2-carboxamido~propyl-
-dimethylamine, white needles, m.p. 118-120C.
N.M.R. (DMS0-d6): ~ 1.60 (2H, m); 2.12 (6H, s); 3.23 (2~, t);
3.20 (2H, m); 3.88 (3H, s); 7.37 (lH, d);
8.08 (lH, bd); 8.35 (l:i, bt).
Example 2
The compound of example 1 (3.4 g) was dissolved in ethanol
(40 ml) and diluted hydrochloric acid (20 ml) and -educed
over a Pd catalyst (5% on carbon) under H2 pressure (50 ?si)
in a Parr apparatus. Water (20 ml) was added and the ca~alyst
filtered off. The resul~ing solution was concentra~ed and
the residue was dissolved in water (40 ml). Sodium bicar-
bonate (4 8) was added,followed by a solution of N-methyl-
-4-nitropyrrole-2-carboxylic acid chlor$de (2.8 g) in 20 ml
of benzene. The resulting mixture was stirred for about 2
-
- . . :
~285934
- 43 - 25521-119
hours at room temperature and then was extracted with chloroform.
The dried organic extracts were concentrated in vacuo and the
residue was purified by column chromatography (CHC13 75,
EtOHg5% 25, NH40H 0.6) to give 4.7 g of 3-~N-methyl-4-
(N-methyl-4-nitropyrrole-2-carboxamido)pyrrole-2-carboxamido~
propyl-dimethylamine as a yellow solid, m.p. 178-180C.
N.M.R. (CDC13)~ : 1.74 (2H, m); 2.30 (6H, s); 2.49 (2H, t):
3.44 (2H, m); 3.88 (3H, s); 3.99 (3H, s);
6.58 (lH, d); 7.21 (lH, d); 7.38 (lH, d);
7.6 (lH, br); 8.80 (lH, bs).
By analogous procedure, the following compounds can be
obtained:
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-nitropyrrole-2-
carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~propyl-
dimethylamine, m.p. 175C (dec.);
N.M.R. (DMSO-d6)~: 1.63 (2H, m); 2.22 (6H, s); 2.38 (2H,t);
3.16 (2H, dt); 3.80 (3H, s); 3.85 (3H,s);
3.87 (3H, s); 3.97 (3H, s); 6.80-7.30
(6H, m); 7.59 (1H, d); 8.04 (1H, t);
8.16 (lH, d); 9.84 (1H, bs); 9.95 (1H,bs);
10.26 (1H, bs);
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-~N-methyl-4-nitropyrrole-2-
carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-
carboxamido~propyl-dimethylamine, m.p. 195C (dec.);
- ~ ' ; ' ' :
,
l.Z855~34
- 44 -
.~.R. (D~IS0-d6) S: 1.64 (2H, m); 2.13 (6~, s); 2.27 (2~,t);
3.20 (2H, dt); 3.80 (3H, s); 3.85 (3H,s);
3.88 (3H, s); 3.98 (3H, s); 6.82 (lH,d);
7.04 (2H, m); 7.18 (lH, d); 7.26 (2~, d);
7.58 (l~,d); 8.18 (lH, d); 8.02 (l~
9.86 (lH, s); 9.94 (lH, s); 10.25 (lH,s);
~-~N-methyl-4- ~-methyl-4-nitropyrrole-2-carboxamido~pyrrole-
-2-carboxamido~propionamidine hydrochloride;
~-~N-methyl-4-~N-methyl-4- ~-methyl-4-nitropyrrole-2-carbo-
xamid~ pyrrole-2-carboxamido~pyrrole-2-carboxamido~propio-
namidine hydrochloride;
~-~N-me~hyl-4-~N-methyl-4-~N-methyl-4-~N-methyl-4-nitro-
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyr.ole-2-
-carboxamido~pyrrole-2-carboxamidoJproplonamidine hydrochlo-
ride.
Example 3
N-methyl-4-~N-methy}-4-nitropyrrole-2-carboxamido/pyrrole
-2-carboxamido7propionamidine hydrochloride (900 mg)
dissolved in 150 ml of ethanol, 75 ml of water and 9 ml of
2N HCl was hydrogenated in a Parr apparatus for 45 minutes
at 45 ~si of H2 at room temperature over a Pd catalyst
(10% on carbon). The catalyst was filtered off and the
flltrate were evaporated under vacuum to yield 930 mg of
crude ~-~N-methyl-4-~N-metnyl-4-aminopyrrole-2-carboxamido~
pyrrole-2-carboxamido~propionamidine dihydrochloride. The
, . . . . . . . .
.: . .
.
~- , . , , - . . .
,
1285~34
- 45 -
residue wasdissolvedin methyl alcohol (60 ml), cooled ~o
-20C and treated with 12 ml of ethylenepxide. After 15
minutes the temperature was allowed to rise and the mixture
is left at room tem?erature overnight. ~he solution was
evaporated to dryness affording, after chromatography on
SiO2 washed with HCl, 800 mg of ?ure ~ -methyl-4-/~- -
-methyl-4-L~,~'-bis-(2-hydroxyethylamino)~pyrrole-2-carDo-
xamido7pyrrole-2-carboxamido7propionamidine hydrochloride;
Mass spectrum: m/e 419 (~ ); 420 (M ~l);
H-N.;~.~. (dimethyl-d6 sul~oxide), ~ : 2.63 (2H, t);
2.90-3.8C (lOH, m); 4.55 (2H, br); 6.30 (lH, d);
6.52 (lH, d); 6.92 (lH, d); 7.12 (lH, d);
8.20 (lH, t); 8.70 (2H, bs); 9.01 (2H, bs);
9.63 (lH, s);
U.V. (~tOH 95~ max 245, ~= 16,352
~ max 292, ~= 15p70
By analogous procedure the following compounds can be
obtained:
- , :. . , , . : ,
1285934
N-deformyl~ -methyl-4-/N,~-bis (2-hydroxyethyiamino)~
pyrrole-2-carooxamidQ7Distamycin A hydrochloride;
~-lN-methyl-4-/~-methyl-4- ~,N-bis (2-hydroxye~hyla~ino)~
~yrrole-2-carboxamido7pyrrole-2-carboxamido7propyl-
-dimethylamine hydrochloride;
3- ~-methyl-4-~N-methyl-4-L~-methyl-4-LN,~i-bis(2-hydroxy-
ethylamino)7pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido~propyl-dimethylamine hydrochloride;
3-LN-methyl-4-/N-methyl-4-~N-methyl-4- ~-methyl-4-LN,N-
-bis(2-hydroxyethylamino)]pyrrole-2-carboxamidoJpyrrole-
-2-carboxamido~pyrrole-2-carboxamidQ7pyrrole-2-carboxa-
midoJpropyl-dimethylamine hydrochloride.
- . : .
- : ~
lZ8S9~4
- 47 -
Example 4
A stirred soLution of ~-~N-methyl-4-LN-methyl-4-~N~N-
-bis~2-hydroxyethylamino)~pyrrole-2-carboxamido/pyrrole-
-2-carboxamido7propionamidinehydrochloride (717 mg) in dry
5 nyridine (10 ml) was cooled with an ice bath, treated under
nitrogen atmosphere with a solution of methansulphonylchlo-
ride in pyridine (1.27 M, 2.7 ml) and stirred at 5 ~ for 45
minutes. After quenching with methyl alcohol, the whole was
allowed to warm to room temperature and evaporated to dryness.
The crude product was chromatographed on silica yieldlng 440
mg ofl3-~N-meth~1-4-LN-methyl-4-LN,N-bi~2-chloroethylamino)/
pyrrole-2-carboxamido7pyrrole-2-carboxamidoJ propionamldine
hydrochloride.
1H-NMR (dimethyl-d6sulfoxide) ,5 2.63 (2H,t~; 3.30-3.80(lON,~);
3.78 (3H,8); 3.81 ~3H,s); 6.42 ~lH,d); 6.55 (lH,d); 6.92 (lH,d);
7.17 (lH,d~ 8.20 (lH,t); 8.70 (2H,bB) g.O2 (2H,bs); 9.68
(lH,s);U.V.(EtoH 9g%): A maX 245,~=17,373; ~ max 293,~= 15,450.
By analogous procedure the following compound~ can be obtained:
B-~N-methyl-4-CN-oethyl-4-rN-l~ethyl-4--~N,N--bis(2-chlorQethyl-
anino)]pyrrole-2-carboxamido~pyrrolo-2-carboxa-ido~pyrrole-
-2-carboxa-ido~propionamidi~e hydrochloride;
N-defornyl-N-~N-oethyl-4-CN,N-bis(2-chloroethyla-ino)~pyrro-
le-2-carboxanido~Distamycin A.hydrochloride,
NNR (D~SO-d6) S :2.63 (2H,t); 3.20-3.9 (lOH,o); 3.80-3.85
(3H,s); 6.46 (lH,d); 6.58 (lH,d); 6.90-7.30 (6H,-);
8.20 (lH,t); 8.73 (2H,br); 9.00 (2H,br); 9.70 (lH,bs);
9.90 (2H,s)
:
.
1285934
- 48 -
N-deformyl-N-~N-~ethyl-4-~N-methyl-4-CN,N-bi~(2-chloro-
ethylamino)lpyrrole-2-carboxamido]pyrrole-2-carboxamido3
Di~ta~ycin A.hydrochloride;
N-d~formyl-N-CN-methyl-4-LN-methyl-4-rN-~ethyl-4-~N,N-bis
(2-chloroethylamino)~pyrrole-2-carboxamido~pyrrole-2-
-carboxamido]pyrrole-2-carboxaoido3Distamycin A.hydrochlo-
ride;
3- ~-methyl-4-~*-methyl-4-~N,N-bis(2-chloroethylamino)Jpyrrole-
-2-carboxamido~pyrrole-2-carboxamid ~ propyl-dimethylamine hydro-
chloride:
3-LN-methvl-4-LN-methyl-4-~N-methyl-4-/N,N-bis~2-chloroethyl-
amino)~pyrrole-2-carboxamido7pyrrole-2-carboxamldo/pyrrole-2-
-carboxamido~propyl-dymethylamine hydrochloride.
3-~N-methyl-4-LN-methyl-4-LN-methyl-4~N-methyl-4~N,N-bis(2-
-chloroethylamino~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido7pyrrole-2-carboxamido~propyl-dimethylami-
ne hydrochloride.
- . - . . - . - .
~28sg~4
-- 49 ~
r xample 5
To an ice-cooled solution of B-/N-methyl-4-(N-methyl-4-
-aminopyrrole-2-carboxamido)pyrrole-2-carboxamido7propio-
namidine dihydrochloride (0.404 g) in 5 ml of D~F and
320 mg of 2,4,5-trichlorophenyl-N-methyl-N-nitrosocarbamate
/prepared according to J. Med. Chem. 25, 178 (1982)/ , a
solution of diisopropylethylamine (0.164 ml) in 8 ml of
DMF was added dropwise. The resulting solution was
stirred 1 hour at 0C. The reaction mixture was concen-
trated under vacuum and the residue was purified by
column chromatography to yield 251 mg of B-~N-methyl-4-
-fN-methyl-4-(3-methyl-3-nitrosoureido)pyrrole-2-
-carboxamido1pyrrole-2-carboxamido~propionamidine hydro-
chloride.
15U.V.(EtoH 9~ max
241 21,611
293 28,207
I.R. (KBr): ~ cm 3500-2800; 2500-2200; 1450; 970; 650
N.M.R. (DMS0-d6) S: 2.59 (2H, m); 3.15 (3H, s); 3.48 (2H,m);
3.79 (3H, s); 3.85 (3H, s); 7.01-7.31
(4H, m); 8.61 (2H, br); 8.97 (2H, br);
9.91 (2H, b); 10.61 (lH, bs).
By analogous procedure the following compounds can be obtained:
~-rN~methyl-4-~N-methyl-4-~3 (2-chloroethyl)-3-nitrosourei-
do ~-pyrrole-2-carboxamid~ pyrrole-2-carboxamld~proplona-
midine hydrochloride,
N.M.R. (DMS0-d6) 5 : 2.61 (2H,t); 3.50 (2H,a); 3.69 (2H,t);
3.81 (3H,s); 3.87 (3H,s); 4.19 (2H,t);
6.90-7.25 ~4H,-); 8.19 (lH,t);
8.55-10.72 (6H,-);
.
lZ85934
- 50 -
3-~N-methyl-4-~N-methyl-4-[3-methyl-3-nitrosoureido¦
pyrrole-2-carboxamido]pyrrole-2-carboxamido]propyl-
-dimethylamine hydrochloride;
3-rN-methyl-4-CN-methyl-4-r3-(2-chloroethyl)-3-nitro-
soureido~pyrrole-2-carboxamido~pyrrole-2-carboxamidoJ
propyl-dimethylamine hydrochloride,
N.M.R. (DMS0-d6) ~ : 1.84 (2H,m); 2.70 (6H,s); 2.90-3.90
(6H,m); 3.81 (3H,s); 3.87 (3H,s);
4.18 (2H,t); 6.85-7.30 (4H,m); 8.15
(lH,t); 8.93-9.75 (3H,m);
3-CN-me thyl-4-CN-methyl-4-cN-methyl-4-(3-methyl-3-nitro-
soureido)pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido~propyl-dimethylamine hydrochloride,
N.M.R. (DMS0-d6)~ : 1.80 (2H,m); 2.53 (6H,s); 2.78 (2H,m);
3.18 (3H,s); 3.20 (2H,m); 3.80 (3H,s);
3.85 (3H,s); 3.88 (3H,s); 6.85-7.25 (6H,m);
8.10 (lH,t); 9.85-10.70 (3H,m);
3-CN-methyl-4-cN-methyl-4-~N-~ethyl-4-c3-(2-chloroethyl)-3-
-nitrosoureido]pyrrole-2-carboxamido]pyrrole-2-carboxamido]
pyrrole-2-carboxa-ido¦propyl-dimethylamine hydrochloride,
N.M.R. (DhS0-d6) S: 1.98 (2H,m); 2.65 (6H,s); 2.90 (2H,t);
3.81 (3H,s); 3.87 (3H,s); 3.89 (3H,~);
4.09 (2H,t); 6.85-7.30 (6H,m); 8.12 (lH,t);
9.80-10.8 (3H,m);
'' ' ' .
-' , , .'
1285934
- 51 -
N-deformyl-N'-lN-methyl-4-(3-methyl-3-nitrosoureido)pyrrole
-2-c~rboxamido~Distamycin A.hydrochloride;
N-deformyl-~-¦N-methyl-4-~3-(2-chloroethyl)-3-n1trosourei-
d~ pyrrole-2-carboxamido~Distamycin A.hydrochloride;
3-/N-methyl-4-~N-methyl-4- ~ -methyl-4-~N-methyl-4(3-methyl-
-3-nitrosoureido)pyrrole-2-carboxamido~pyrrole-2-carboxa
mido~?yrrole-2-carboxamido]pyrrole-2-carboxamido~propyl-
-dlmethylamlne hydrochlorlde,
N.~.R. (D~S0-d6)~ : 1.90 (2H,o); 2.73 (6H,s); 3.19 (3H,s);
3.82 (3H,s); 3.84 (3H,s); 3.85 (3H,s);
3.86 (3H,s); 6.90-7.30 (8H,-); 8.13
(lH,t); 9.88-10.70 (4H,-);
3-~k-methyl-4-lN-methyl-4-~N-methyl-4-[N-methyl-4~3-(2-
-chloroethyl)-3-ni~rosoureid~ pyrrole-2-carboxamld~ pyrrole-
-2-carboxam~do~pyrrole-2-carboxamido~pyrrole-2-carboxamidoJ
~ropyl-~imethylamine hydrochloride~
N.M.R. (~Ld6/CDC13) ~: 1.90 (2H,m); 2.60 (6H,s); 2.85 (2H,t); 3.15-4.00
(16H,m); 4.22 (2H,t); 6.80-7.30 (8H,m); 8.00 (IH,t); 9.63 (lH,bs); 9.70
(IH,s); 9.77 (LH, s); 10.48 (lH,s);
n-~-methyl-4-rN-methyl-4-(oxiranecarboxamido)pyrrole-2-
-carboxamido/pyrrole-2-carboxamido]propionamidine hydro-
chloride;
3-[N-methyl-4-~N-methyl-4-(oxira~ecarboxamido)pyrrole-2-
-carbox~r,id ~ py.role-2-carboxamido~propyl-dimethylamine
hydrochloride;
3-~Nhmethyl-4-[N-methyl 1 ~Nhmethyl 1 (oxlnlYKarb~ul~do)~ le-2_
-carbo~do~pyrrole-2-carboxamido]pyrrole-2-carboxamid~propyl-
-dimethylanine;
,
1285934
- 52 -
~xample 6
~o a solution of (2R,3R)-3-methyl-oxirane-carboxylic acid
~765 mg)in dry THF (20 ml), cooled to -20C, N-methylmorpholine
- (0.825 ml) and then pivaloyl chloride (0.920 ml) were added.
The resulting suspension was stirred at -20C for 20 minutes,
then the whole was added to a cooled solution of 2.6 g of
3-lN-methyl-4-L~I-methyl-4-~1\,'-methyl-4-aminopyrrole-2-
-carboxamido7pyrrole-2-carboxamido7pyrrole-2-carboxamido~
propyl-dimethylamine dihydrochloride in DMF (50 ml) and
10 ~SaHC03 (0.4 g). The mixture was stirred for 30 minutes at
0C, and then for 4 hours at room temperature. Solvents
were evaporated in vacuum to dryness, and the residue
chromatographed on SiO2 (solvent CHCl3 lOO~CH30H 100/HCl2N 1)
to yield 1.4 g of 3-~N-methyl-4-LN-methyl-4-~N-methyl-4-
15 -~3-methyl-(2R,3~)oxiranecarboxamidoJpyrrole-2-carboxamidoJ
pyrrole-2-carboxamido7pyrrole-2-carboxamid 7propyl-dimethyl-
amine hydrochloride.
N.`l.~. (DMS0-d6) ~: 1.25 (3H, d); 3.3 (lH, m); 3.60 (1'~, d);
/J = 4.7 Hz (cis)~.
2C 3y analogous procedure the following compounds can be obtained:
N-~efo.myl-~-~.i-metryl-4-(oxiraneCarboXamido)pyrrole-2-
-carboxamido~is.amycin A.~ydrochloride;
3- ~ -methyl-1-/N-methyl-4- `J-methyl-4-L~-methyl-4-(oxira-
.~ecarbox~m do)pyrrole-2-carboxamido7?yrrole-2-carboxami~o
25 ?yrrole-2-carboxamido~pyrrole-2-c~rboxamidoJpropyl-d~me
~hyl~ine hydrochloride;
lZ~59~4
- 53 - 25521-119
~-fN-methyl-4-~N-methyl-4-(cyclopropylcarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propionamidine hydrochloride;
3-~N-methyl-4-~N-methyl-4-(cyclopropylcarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine hydro-
chloride;
3-~N-methyl-4-~N-methyl-4-rN-methyl-4-(cyclopropylcarboxamido)
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
propyl-dimethylamine hydrochloride;
N-deformyl-N-~N-methyl-4-(cyclopropylcarboxamido)pyrrole-2-
carboxamido~Distamycin A.hydrochloride;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-~N-methyl-4-(cyclopropylcar-
boxamido) pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine hydro-
chloride;
~-~N-methyl-4-~N-methyl-4-(3-methyloxiranecarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propionamidine hydrochloride;
3-~N-methyl-4-~N-methyl-4-(3-methyloxiranecarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido7propyl-dimethylamine hydro-
chloride;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-(3-methyloxiranecarboxamido)
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~
propyl-dimethylamine hydrochloride;
N-deformyl-N-~N-methyl-4-(3-methyloxiranecarboxamdio)pyrrole-2-
carboxamdiQ7Distamycin A.hydrochloride;
3-~N-methyl-4-~N-methyl-4-rN-methyl-4-~N-methyl-4-(3-methyloxi-
ranecarboxamido)pyrrole-2-carboxamido~pyrrole-2-carboxamido~
pyrrole-2-carboxamido~pyrrole-2-carboxamidQ7propyl-dimethylamine
X
12~34
- 54 - 25521-119
hydrochloride
~-~N-methyl-4-~N-methyl-4-(2-chloroethylcarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido7propionamidine hydrochloride;
3-~N-methyl-4-~N-methyl-4-(2-chloroethylcarboxamido)pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine hydro-
chloride;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-(2-chloroethylcarboxamido)
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamidoJ
propyl-dimethylamine hydrochloride;
N-deformyl-N-~N-methyl-4-(2-chloroethylcarboxamido)pyrrole-2-
carboxamido~Distamycin A.hydrochloride;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-~N-methyl-4-(2-chloroethyl-
carboxamido)pyrrole-2-carboxamido~pyrrole-2-carboxamidoJpyrrole-2-
carboxamido/pyrrole-2-carboxamido/propyl-dimethylamine hydro-
chloride;
~-~N-methyl-4-~N-methyl-4-~1-(aziridine)carboxamido/pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propionamidine hydrochloride;
3-~N-methyl-4-~N-methyl-4-~1-(aziridine)carboxamido~pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine hydro-
chloride;3-~-methyl-4-~N-methyl-4-~N-methyl-4-~-(aziridine)carboxamido~
pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carbox-
amido~propyl-dimethylamine hydrochloride;
N-deformyl-N-~N-methyl-4-~1-(aziridine)carboxamido7pyrrole-2-
1285~33~
- 55 - 25521-119
carboxamido~Distamycin A.hydrochloride;
3-~N-methyl-4-~N-methyl-4-~N-methyl-4-~N-methyl-4- 1-(aziridine)
carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido~pyrrole-2-
carboxamido~pyrrole-2-carboxamido~propyl-dimethylamine hydro-
chloride.
~1
.
1285934
- 56 -
ExamDle 7
To a ~tirred aqueous solution of 3-CN-~ethyl-4-~N-~ethyl-~-
-[N-oethyl-4-aminopyrrole-2-carboxamido~pyrrole-2-carboxa-
~ido]pyrrole-2-carboxa-ido]propyl-dimethylaoine dihydrochlo-
ride (2.3 g in 200 ml of ~ater) and sodiuo bicarbonate
(1.5 g),a solution of N-oethyl-4-nitropyrrole-2-carboxylic
acid chloride (1.0 g) in 25 ol of THF ~as added at room
teoperature.
The resulting mixture ~as refluxed under stirring for 2 hours.
The reaction ixturo ~as evaporated under vacuu-, the residue
~as taken up ~ith ~ater~ tho pH ~as set to 13 ~ith NaOH 2N and
extraction ~as oade ~ith a 70:30 ixture of CHC13 and methanol.
The dried organic extracts ~ere concentrated in ~acuo and the
residue ~as purifled by colu-n chro-atography (CHC13 70,
MeOH 30, NH40H 1) to give 2.6 g of 3-~N--ethyl-4-CN-methyl-4-
-~N-methyl-4-CN-oethyl-4-nitropyrrole-2-carboxa-ido~pyrrole-2-
-carboxamidolpyrrole-2-carboxa-ido~pyrrole-2-carboxa-ido]
propyl-dimethyla-ine as yello~ solid, m.p. 195~C (dec.);
N.M.R. (DMSO-d6) 5 1.64 (2H,o); 2.13 (6H,s); 2.27 (2H,t);
3.20 (2H,dt); 3.80 (3H,s); 3.85 (3H,s);
3.88 (3H,s); 3.98 (3H,s); 6.82 (lH,d);
7.04 (2H,m); 7.18 (lH,d); 7.26 (2H,d);
7.58 (lH,d); 8.18 (lH,d); 8.02 (lH,t);
9.86 (lH,s); 9.94 (lH,s); 10.25 (lH,s).
8y analogous procedure the follo~ing co-poundg can be obtained:
3-~N-oethyl-4-[N--ethyl-4-~N--ethyl-4-CN--ethyl-4-[N-oethyl-4-
-nitropyrrolo-2-carboxaoido]pyrrole-2-carboxaoido]pyrrole-2-
-carboxamido~pyrrole-2-carboxamido~pyrrole-2-carboxamido]
propyl-dimethyla-ine,
,- - - .
.
- . ` : ,
~285934
- 57 -
N.~.R. (DNS0-d6) 5: 1.68 (2H,c); 2.3Z (6N,s); 3.81 (3H,s);
3.88 (9H,b~); 3.97 (3H,s); 6.8-7.3
(8H,m); 7.60 (lH,d); 8.04 (lH,bt); 8.18
(lH,d); 9.85-10.27 (4H,b,ss,NH), and
3-[N-~ethyl-4-rN-~eth~1-4-CN--eth~1-4-cN--ethyl-4-cN-cethyl-
-4-CN-oeth~1-4-nitropyrrolo-2-carboxa-ido~p~rrol~-2-carboxa-
~ido~p~rrole-2-carboxa-ido]pyrrole-2-carboxa-ido~pyrrolo-2-
-carboxa-ido~pyrrole-2-carboxa-ido3propyl-di-eth~la-ine.
N.N.R. (DNS0-d6) ~: 1.68 (2H~-); 2.32 (6H,s); 3.81 (3H.s);
3.88 (9H,bs); 3.97 (3H,s); 6.8-7.3
(8H,o); 7.60 (lH,d); 8.04 (lN,bt);
8.18 (lH,d); 9.85-10.27 (4H,b,ss,NH).
- -" . ': ' . ' ' : ' ~, ,
1285934
- 58 -
XxamDle 8 -4- t N-methyl-
The compound 3-tN-methyl-4-tN-methyl-4-~N-methylT4-nitro-
pyrrole-2-carboxamido]pyrrole-2-carboxamido~pyrrole-2-
-carboxamido~pyrrole-2-carboxamido]propyl-dimethylamine
(800 ~g) ~as dissolved into a mixture of CH30H (70 ml),
H20 (30 ml) and lN HCl (3 ol) and reduced over a Pd cata-
ly~t (lOX on carbon) under H2 pressure (50 psi).
The catalyst ~as filtered off, the resulting solution was
concentrated in vacuo and the residue ~as crystallized from
ethyl acetate/ethanol to give 760 og of 3-~N-methyl-4-¦N-
-methyl-4-tN-methyl-4-CN--ethyl-4-aminopyrrole-2-carboxamido]
pyrrolo-2-carboxamido~pyrrole-2-carboxa-ido~pyrrole-2-carboxa-
mido~propyl-dimethylaoine.dih~drochlorido,
N.N.R. (D~S0-d6) S: 1.88 (2H,o); 2.72 (6H,s) 3.81 (3H,s);
3.85 (3H,s); 3.86 (3H,s); 3.90 (3H,s);
6.9-7.3 (8H,m); 8.13 (lH,bt); 8.87 (lH,bs);
9.91 (lH,bs); 10.09 (lH,bs); 10.2 (3H,b,
NH )-
By analogous procedure the follo~ing compounds can be obtained:
3-~N-methyl-4-[N-methyl-4-aminopyrrole-2-carboxamido]pyrrole-
-2-carboxamido]propyl-dimethyla-ine.dihydrochloride;
3-CN-methyl-4-~N-methyl-4-cN-methyl-4-aminopyrrole-2-carboxa-
mido]pyrrole-2-carboxamido]pyrrole-2-carboxa~ido~propyl-
-dimethylamine.dihydrochloride;
'
.
~Z8~;934
ss
3--[N-methyl-4-[N-oethyl-4-[N-oethyl-4-~N-methyl-4-[N-
-clethyl-4-aminopyrrole-2-carboxamido]pyrrole-2-carboxamido~
pyrrole-2-carboxaoido~pyrrole-2-carboxamido~pyrrole-2-
-carboxaoido]propyl-dimethyla~ine.dihydrochloride; and
3-CN-methyl-4-cN-oethyl-4-~N-methyl-4-cN-methyl-4-[N-methyl-
-4-~N-methyl-4-a~inopyrrole-2-carboxamido~pyrrole-2-carboxa-
mido~pyrrole-2-carboxamido~pyrrole-2-carboxamidoJpyrrole-2-
-carboxamido~pyrrole-2-carboxauido~propyl-dimethylamine.
dihydrochloride.
ExamDle 9
Sodiuo bicarbonate (150 Dg) ~as added to a solution of 3-rN-
-methyl-4-tN-oethyl-4-CN-oothyl-4-rN-oethyl-4-a~inopyrrole-
-2-carboxaoido]pyrrole-2-carboxa-ido~pyrrole-2-carboxamido~
pyrrole-2-carboxaoido~propyl-dioethylamine.dihydrochloride
(500 og in 25ml of anhYdr0U8oethanolland the suspension was
stirred for 1 hour at rooo te-perature.
The ~hole ~as cooled do~n to -40-C and a solution of
N-formylimidazole in THF (prepared according to JOC (1985)
50, 3774-3779 starting froo 1.625 g Or CDI, 0.38 ol of formic
acid and 15 ml Of anhydrousTHF) ~as dropped in 15'.
The teoperature ~a~ madc to rise to 10C in ôO'.
The resulting mixture ~as evaporated under vacuo and the resi-
due was chromatographed on SiO2 to give 460 mg of yello~ solid,
pure 3-~N-methyl-4-~N-methyl-4-~N-oethyl-4-rN-methyl-4-formyl-
a~inopyrrole-2-carboxaoido3pyrrole-2-carboxamido~pyrrole-2-
-carboxamido3pyrrole-2-carboxaoido~propyl-dimethylamine.hydro-
chloride,
-
.
1285g34
- 60 -
N.M.R. (D~S0-d6)S: 1.90 (2H,m); 2.72 (6H,s); 3.84 (12H,bs);
6.8-7.3 (8H,m); 8.12 (lH,bt); 8.13 (lH,d);
9.88 (3H,bs); 10.04 (lH,b~).
By analogous procedure the follo~ing co-pound8 can be obtained:
3-CN-oethyl-4-cN-~ethyl-4-formylaminopyrrole-Z-carboxamido~
pyrrole-2-carboxamido~propyl-dimethylamine.dihydrochloride;
3-CN-methyl-4-~N-methyl-4-cN-methyl-4-formylaminopyrrole-2-
-carboxamido]pyrrole-2-carboxaoido~pyrrole-2-carboxamido~
propyl-dimethylaoino.dihydrochloride;
3-CN-oethyl-4-~N--ethyl-4-~N-oethyl-4-cN-oethyl-4-~N-methyl-
-4-foroylaoinopyrrole-2-carboxa-ido~pyrrolo-2-carboxaoido]
pyrrole-2-carboxaoido~pyrrole-2-carboxa-ido~pyrrole-2-carboxa-
mido~propyl-dimethylaoin-.hydrochloride, and alao
3-~N-oethyl-4-~N-methyl-4-CN-oethyl-4-~N-methyl-4-~N-methyl-4-
[N-methyl-4-formylaoinopyrrolo-2-carboxa-ido]pyrrole-2-carboxa-
mido~pyrrole-2-carboxaoido~pyrrole-2-carboxa-ido~pyrrole-2-
-carboxaoido~pyrrole-2-carboxaoido~propyl-dimethylaoino.hydro-
chloride.
~28S934
- 61 -
ExamDle 10
A solution of Distamycin A-hydrochloride (2 g) in 60 ml of
methanol was treated ~ith aminoacetaldehyde dimethyl acetal
~0.5 ml) and allo~ed to stand at room temperature for 5 hours.
After then, additional lOX of aminoacetaldehyde dimethyl
acetal ~as added and the solution ~as refluxed for 16 hours.
The solvent ~as remo~ed under reduced pressure and the crude
product ~as dissolved in 100 1 of lN oxalic acid aqueous
solution and kept at 70C over 4 hours period.
The aqueous solution ~as evaporated to dryness and the solid
residuo ~as ~ashed fe~ times ~ith acetone. collected and
troated ~ith 3.SN HCl alcoholic solution excess.
The solvent ~as removod undor vacuuo and the crude product
taken up in acetone, filtered and collected to give 1.1 g
(52.5X) of B-[N-nethyl-4-CN-methyl-4-~N-methyl-(4-formyl-
amino)pyrrole-2-carboxa-ido]pyrrole-2-carboxamido~pyrrole-
-2-carboxamido~ethyl-C2-ioidasole]hydrochloride,
N.M.R. (D~S0-d6) S: 2.83 (2H,bt); 3.50 (2H,o); 3.82 (3H,s);
3.85 (6H,s); 6.80-7.25 (8H,n); 8.10
(lH,bt); 8.13 (lH,bs); 9.87 (2H,bs);
10.00 (lH,b 8) .
' ` ` ` ' ` ' ` .
lZ85934
- 62 -
ExamDle 1 1
A solution of Distamycin A.hydrochloride (200 mg) in 4 ml
of methanol was treated with ethylendiammine (0.1 ml).
The resulting solution was kept overnight at room tempe-
rature, and the whole evaporated in vacuo. The residue
was taken up with acetone (30 ml), stirred for 30' and
filtered to yield 20 mg of B-[N-methyl-4-~N-methyl-4-rN-
-methyl-4-formylaminopyrrole-2-carboxamido~prrrole-2-
-carboxamido~pyrrole-2-carboxamido~ethyl-~2-~2-imidazo-
line)~.hydrochloride,
N.H.R. (DMS0-d6)~: 2.68 (2H,bt); 3.52 (2H,bd); 3.76 (4H,bs);
3.80 (3H,s); 3.83 (6H,s); 6.8-7.3 (6H,m);
8.12 (lH,d); 8.27 (lH,bt); 9.90 (2H,bs);
10.11 (lH,bs).
~y analogou~ procedure the following compounds ean be obtained:
B-rN-methyl-4-CN-methyl-4-[N-methyl-4-formylaminopyrrole-2-
-carboxamido]pyrrole-2-carboxamido~pyrrole-2-carboxamido~
ethyl-C2-(3,4,5,6-tetrahydro-pyrimidine)~hydrochloride,
N.M.R. (DHS0-d6) ~: 1.75 (2H,m); 2.60 (2H,t); 3.00-3.70
(6H,m); 3.81 (3H,s); 3.84 (6H,s);
6.80-7.30 (6H,m); 8.12 (lH,d); 8.25 (lH,t);
9.90 (2H,s); 10.15 (lH,s); and
B-~N-methyl-4-~N-methyl-4-[N-methyl-4-formyla-inopyrrole-2-
-carboxamido~pyrrole-2-carboxamido]pyrrole-2-carboxamido~
ethyl-~2-(4,5-dihydro)-oxazole~,
N.~.R. (DHS0)S : 2.42 (2H,t); 3.42 (2H,m); 3.5-4.40 (4H,m);
3.80 (3H,s); 3.85 (6H,s); 6.80-7.30 (6H,m);
8.00 (lH,t); 8.15 (lH,d); 9.90 (2H,s);
10.00 (lH,~).
' '- ~' " ' ' -
E;xamDle 12
A solution of Distamycin A.hydrochloride (2 g) in 100 ml
of methanol and 8 ml of 20X NaOH (d 1.22) ~as refluxed
t`or 8 hours. To the cold solution were added 8 ml of 23X
aqueous HCl.
The resulting mixture ~as concentrated under vacuo: a
red solid precipitated, ~hich ~as filtered and dried into
a oven (40 under Yacuum).
The red solid (1.9 g) ~as dissolved into 66 ml of formamide
and 6.6 ml of ethylformate. The resulting solution ~as
refluxed for 2 hours. The ~hole ~as concentrated to dryness
undor high vacuum (0.1 m- Hg) and the oily residue purified
on silica (CHC13 15, NeOH 15, 2N HCl 0.3) to yield 920 mg
of B-[N-mothyl-4-CN--ethyl-4-~N--ethyl-4-formylaminopyrrole-
-2-carboxamido3pyrrole-2-carboxa~ido]pyrrole-2-carboxamido]
propionic acid,
N.~.R. (DHSO-d6) 5: 2.36 (2H,bt); 3.40 (2H,m); 3.80 (9H,bs);
6.8-7.3 (6H,-); 7.84 (lH,bt); 8.12 (lH,d);
9.89 (lH,bs); 9.94 (lH,bs); 10.20 (lH,bs).
Exam~le _13
The co-pound ~-~N-methyl-4-~N-mothyl-4-CN-methyl-4-formylami-
nopyrrole-2-carboxanido]pyrrole-2-carboxamido~pyrrole-2-
-carboxaaido~propionicacid (1.9 g) ~as dissolYed in 20 ml of
D~F and treated ~ith 600 mg of N-hydroxysuccinimide and 1.2 g
of DCC. The resulting mixture ~as stirred at room temperature
for 7 hours. DCU ~as filtered off and the DNF distilled out
.
~ ' " '' ' . ,
12859;~4
- 64 -
at 350Cunder reduced pressure. The waxy residue was
redi~solved in D~F and cooled down to 5C with an ice-bath.
A solution of 0.3 g of NaBH4 in 5 ml of water vas dropped
and the whole stirred at 5C for 2 hours. The excess of
NaBH4 was quenched with AcOH and the resulting ~ixture con-
centrated under vacuo.
The residue was purified on silica (CHC139,~eOH1) to give
0.52 g of B-~N-methrl-4-[N-methyl-4-CN-oethyl-4-formylamino-
pyrrole-2-carboxaoido]pyrrole-2-carboxa-ido]pyrrole-2-car-
boxamido]propyl alcohol,
N.~.R. (D~SO-d6) S: 1.67 (2H,-); 3.80 (3H,s); 3.86 (6H,s);
4.4 (lH,bt); 6.84 (lH,d); 6.91 (lH,d);
7.05 (lH,d); 7.21 (3H,m); 7.92 (lH,bt);
8.12 (lH,d); 9.85 (lH,bs); 9.88 (lH,bs);
10.02 (lH,bs).
~E~,
To a solution of 469 mg of B-[N-methyl-4-tN--othyl-4-~N-
-methyl-4-fornyla-inopyrrole-2-carboxa-ido]pyrrole-2-carboxa-
mido~pyrrole-2-carboxaaido]propyl alcohol in ~0 ml of DHF,
cooled to 0C and stirred under N2, ~ere added in one portion
536 mg of 1-desoxy-1-B-chloro-3,4-bis-trifluoroacetyl dauno-
sa~ine- To the resulting solution ~as dropped a solution of
385 mg of Ag (CF3S03) in 5 ~1 of D~F. The suspension was
stirred for 1 hour at 0C, then filtercd and the solution,
treated` with 10 ml of NaOH 2N, was stirred for 3 hours at 0C.
The whole was again filtered and the solution e~aporated to
.
~2859~4
- 65 -
dryness under vacuo. The residue was chro~atographed on
acidic ~1203 (CHC139, NeOH1) to give 230 ~g of ~hite solid
3--tN-sethyl-4-~N--eth~1-4-CN--ethyl-4-for-yla-inopyrrole-2-
-carboxa-ido~pyrrolc-2-carboxa-ido3pyrrole-2-carboxa-ido3
S propane-1-~(3-aeino-2,3,6- trideoxy-o(-L-lyxo-hesapyranosyl)-
-oxy]trifluoroacetate,
N-~.R. (D~SO-d6) 5 : 1.08 (3H,d); 1.74 (4H,~); 3.81 (3H,s);
3.85 (6H,s);4.83 (lH,bs); 5.40 (lH,d);
6.8-7.3 (6H,-); 7.95 (lH.bt); 8.12 (lH,d);
~ 8~3H,bt(NN3 )~; 9.85 (lH,bs); 9.88 (lH,bs);
10.04 (lH,b 8, ) .
- :
' '' ' ' ' - ' '', ' '' ' ' ' , ` "',' ~
~285934
- 66 -
Example 15
Tablets each ~eighing 0 250 g and containing 50 mg of the
actiYe substance can be manufactured as follow~
Composition (for 10,000 tablets)
3-~N-methyl-4-~N-methyl-4-rN--ethyl-4-[N-methyl-4-
-nitropyrrole-2-carboxamido}pyrrole-2-carboxamido~
pyrrole-2-carboxa-ido~pyrrole-2-carboxamido]propyl-
-dimethylamine 500 g
Lactose 1,400 g
Corn starch 500 g
Talc po~der 80 g
~agnesiuo stearate 20 g
The 3-tN-methyl-4-[N-methyl-4-[N-methyl-4-[N-methyl-4-nitro-
pyrrole-2-carboxa-idoJpyrrole-2-carboxamido]pyrrole-2-carboxa-
mido]pyrrole-2-carboxamido~propyl-dimethylamine, the lactose
and half the corn starch are mixed; the mixture i8 then forced
through a sieve of 0 5 mm oesh size Corn starch (10 g) is
su5pended in ~aro ~ater (90 1) and the resulting paste is
used to granulate the po~der The granulate is dried, commi-
nuted on a sieve of 1 4 mo mesh size, then the remainingquantity of starch, talc and magnesium stearate is added,
carefully mixed and processed into tablets~
.- . , ~ . .
.. . .
lX85934
- 67 -
Example 16
C'apsules, each dosed at 0 200 g and containing 20 mg of
the active subgtance can be prepared as follows
Composition for 500 capsules
B-[N-methyl-4-~N-methy}-4-CN-methyl-4-(4-formyl-
amino)pyrrole-2-carboxamido]pyrrole-2-carboxa-
mido~pyrrole-2-carboxamido]ethyl-r2-imidazole~
hydrochloride 10 g
Lactose 80 g
Corn starch 5 g
~agnesium stearate 5 g
This foroulation / encapsulated in t~o-piece hard gelatin
capsules and dosed at 0 200 g for each capsule
~xaoDle 17
Intraouscular injection 25 m~/ml
can be
An injectable pharoaceutical composition / anufactured
by dissolving 25 g of 3-[N--eth~1-4-tN-methyl-4-rN-oethyl-
-4-formylaoinopyrrole-2-carboxamido~pyrrole-2-carboxa-ido~
pyrrole-2-carboxaoido]propane-1-[(3-aoino-2,3,6-trideoxy-~-
-L-lyxo-hexapiranosyl)-oxy~trifluoroacetate in sterile
propyleneglycol (1000 ml) and sealing ampoules of 1-5 ml