Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
12~35~354
` GC227
--1--
HYDRAZIDE DERIVATIVES OF MONOCYCLIC
~-LACTAM ANTIBIOTICS
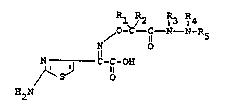
Compounds having the formula
IRl R2 R3 R4
o-C-~ -N- N-R5
N l_6 - 7
H2N ~ C~ NH ~ -SO3H ~
and pharmaceutically acceptable salts thereof t have
antibacterial activity. In formula I, and
throughout the specification, the symbols are as
defined below.
R1 and R2 are each independently hydrogen or
Alkyl of 1 to 4 carbon atoms, or Rl and R2 together
with the carbon atom to which they are attached
form a cycloalkyl ring;
R3 is hydrogen or alkyl;
R4 is hydrogen or alkyl, and R5 is hydrogen,
alkyl, phenyl, substituted phenyl, a 4,5,6 or
7-membered heterocycle (hereinafter referred to as
Rx), phenylalkyl, (substituted phenyl)alkyl,
o
Rx-alkyl, -e-NHYl [wherein Yl is hydrogen, alkyl,
phenyl, substituted phenyl, methylcarbonyl, tri-
fluoromethylcarbonyl, phenylcarbonyl, (substitutedpheny})carbonyl, carboxymethyl, methylsulfonyl,
phenylsulfonyl, (substituted phenyl)sulfonyl,
:~ aminocarbonyl, aminocarbonylamino, aminoethyl,
hydroxy, alkoxy, amino, alkylamino, dialkylamino,
phenylcarbonylamino, (subs~ituted phenyl)carbonyl-
S
~ : amino, l-pyrrolidinyl, or l-piperidinyl], -~-NHYl,
: ~ ~ O
Y2 [wherein Y2 is hydrogen, alkyl, phenyl,
substituted phenyl, Rx, alkoxy, formyl, carbonyl,
, .
:,
:
;~ ~
- . .
.: , . ,
-2- GC227
aminocarbonyl, aminocarbothio, methylaminocarbonyl,
methylaminocarbothio, trifluoromethyl, phenyl-
methyl, (substituted phenyl)methyl, phenyloxy-
methyl, (substituted phenyl)oxymethyl, cyano-
methyl, hydroxymethyl, alkoxymethyl, aminomethyl,methylcarbonylaminomethyl, aminocarbonylaminomethyl,
methylsulfonylaminomethyl, carboxymethyl, amino-
carbonylmethyl, alkoxycarbonylmethyl, Rx-alkyl,
hydroxyaminocarbonylmethyl, or azidomethyl],
10 S NH
-~-Y2, -~-Y3 [wherein Y3 is amino, alkyl, alkyl-
thio, carboxythio, alkoxycarbonylthio, or amino-
carbonylthio], -SO2-Y4 [wherein Y4 is alkyl, amino,
hydroxyamino, alkoxyamino, methylcarbonylamino, or
O
phenylcarbonylamino], -SO3H or -~-OY5 [wherein Y5
OY6
is hydrogen or alkyl and Y6 is hydrogen, alkyl,
carboxymethyl, or aminocarbonylmethyl]; or together
R4 and R5 are =CH-Y7 wherein Y7 is phenyl or
substituted phenyl;
R6 and R7 are the same or different and each
is hydrogen, alkyl, alkenyl, alkynyl, cyclo-
alkyl, phenyl, substituted phenyl or Rx, or one of
R6 and R~ is hydrogen and the other is azido,
halomethyl, dihalomethyl, trihalomethyl, alkoxy-
carbonyl, 2-phenylethenyl, 2-phenylethynyl,
carboxyl, -C~2Xl [wherein Xl is azido, amino
(-NH2), hydroxy, alkanoylamino, phenylcarbonyl-
: 30 amino, (substituted phenyl)carbonyl- amino, alkyl-
sulfonyloxy, phenylsulfonyloxy, (substituted
phenyl)sulfonyloxy, phenyl, substituted phenyl,
, . . , . . , , . . - . . .............. - . .
- . : .
,
~as~
GC227
--3--
cyano, -A-C-NX6X7' -S~X2, or -0-X2 (wherein A, X2,
X6 and X7 are as hereinafter defined)], -S-X2 or
--X2 [wherein X2 is alkyl, substituted alkyl,
phenyl, substituted phenyl, phenylalkyl, (sub-
stituted phenyl)alkyl, alkanoyl, phenylalkanoyl,
~substituted phenyl)alkanoyl, phenylcarbonyl,
(substituted phenyl)carbonyl, or heteroaryl-
IX3 IX3
carbonyl] -o-c-x4 or -S-f-x4 ~wherein one of
X5 X5
X3 and X4 is hydrogen and the other is hydrogen or
alkyl, or X3 and X4 when taken together with the
carbon atom to which they are attached form a
cycloalkyl group; and X5 is formyl, alkanoyl,
phenylcarbonyl, (substituted phenyl)carbonyl,
phenylalkylcarbonyl, (substituted phenyl)alkyl-
carbonyl, carboxyl, alkoxycarbonyl, aminocarbonyl
(N~2-C-), (substituted amino)carbonyl, or cyano
(-C_N)], or -A-C-NX6X7 (wherein A is -C~=C~-,
(CH2)n~, -CH2-O-, -CH2-N~-, or -CH2-S-CH2-, n is
0, 1 or 2, and X6 and X7 are the same or different
and each is hydrogen, alkyl, phenyl or substituted
phenyl, or X6 is hydrogen and X7 is amino, sub-
stituted amino, alkanoylamino or alkoxy, or X6 and
X7 when taken together with the nitrogen atom to
: which they are attached form a 4, 5, 6 or 7-
membered heterocycle).
Listed below are definitions of various
terms used to describe ~-lactams of this
;~ invention. These definitions apply to the terms
as they are used throughout the specification
(unless they are otherwise limited in specific
.
, ~ . - - . - - : . - -
12~S~S~
GC227
--4--
instances) either individually or as part of a
larger group.
The terms "alkyl" and "alkoxy" refer to both
straight and branched chain groups. Those groups
having l to lO carbon atoms are preferred.
The term "cycloalkyl" refers to cycloalkyl
groups having 3,4,5,6 or 7 carbon atoms~
The term "substituted alkyl" refers to alkyl
groups substituted with one, or more, azido, amino
(-N~2), halogen, hydroxy, carboxy, cyano, alkoxy-
carbonyl, aminocarbonyl, alkanoyloxy, alkoxy,
phenyloxy, (substituted phenyl)oxy, Rx-oxy,
mercapto, alkylthio, phenylthio, (substituted
phenyl)thio, alkylsulfinyl, or alkylsulfonyl groups.
The terms "alkanoyl", "alkenyl", and
"alkynyl" refer to both straight and branched chain
groups. Those groups having 2 to 10 carbon atoms
are preferred.
The terms "halogen" and "halo" refer to
fluorine, chlorine, bromine and iodine.
The term "substituted phenyl" refers to a
phenyl group substituted with 1, 2 or 3 amino
(-N~2), halogen, hydroxyl, trifluoromethyl, alkyl
(of l to 4 carbon atoms), alkoxy (of l to 4 carbon
atoms), or carboxyl groups.
The expression "a 4,5,6 or 7-membered
heterocycle" (referred to as ''Rx'') refers to
substituted and unsubstituted, aromatic and
non-aromatic groups containing one or more
nitrogen, oxygen or sulfur atoms. Exemplary
substituents are oxo (=0), halogen, hydroxy, nitro,
amino, cyano, trifluoromethyl, alkyl of 1 to 4
carbons, alkoxy of 1 to 4 carbons, alkylsulfonyl,
phenyl, substituted phenyl, 2-furfurylideneamino
. ~ , . . . . .
~ ~ ' ,-. .
-: -
~5~5~
_5_ GC227
O~r_CH=N-
( ~ ), benzylideneimino and substituted
alkyl groups (wherein the alkyl group has 1 to 4
carbons). One type of "4,5,6 or 7-membered
S heterocycle" is the "heteroaryl`' group. The term
"heteroaryl" refers to those 4,5,6 or 7-membered
heterocycles which are aromatic. Exemplary
heteroaryl groups are substituted and unsubstituted
pyridinyl, furanyl, pyrrolyl, thienyl,
1,2,3,-triazolyl, 1,2,4-triazolyl, imidazolyl,
thiazolyl, thiadiazolyl, pyrimidinyl, oxazolyl,
triazinyl, and tetrazolyl. Exemplary nonaromatic
heterocycles (i.e., fully or partially saturated
heterocyclic groups) are substituted and
unsubstituted azetinyl, oxetanyl, thietanyl,
piperidinyl, piperazinyl, imidazolidinyl,
oxazolidinyl, pyrrolidinyl, tetrahydropyrimidinyl,
dihyrothiazolyl and hexahydroazepinyl. Exemplary
of the substituted 4,5,6 or 7-membered heterocycles
are 1-alkyl-3-azetinyl, 2-oxo-1-imidazolidinyl,
3-alkylsulfonyl-2-oxo-l-imidazolidinyl,
3-benzylimino-2-oxo-l-imidazolidinyl,
3-alkyl-2-oxo-1-imidazolidinyl, 3-phenyl (or
substituted phenyl)-2-oxo-1-imidazolidinyl,
3-benzyl-2-oxo-1-imidazolidinyl, 3-(2-aminoethyl)-
2-oxo-1-imidazolidinyl, 3-amino-2-oxo-1-
imidazolidinyl, 3-[(alkoxycarbonyl)amino]-
2-oxo-1-imidazolidinyl, 3-[2-[(alkoxycarbonyl)-
amino]ethyl]-2-oxo-1-imidazolidinyl, 2-oxo-1-
pyrrolidinyl, 2-oxo-3-oxazolidinyl, 4-hydroxy-6-
methyl-2-pyrimidinyl, 2-oxo-1-hexahydroazepinyl,
2-oxo-3-pyrrolidinyl, 2-oxo-3-tetrahydrofuranyl,
2,3-dioxo-1-piperazinyl, 2,5-dioxo-1-piperazinyl,
4-alkyl-2,3-dioxo-1-piperazinyl, and 4-phenyl-2,3-
dioxo-1-piperazinyl.
~ '
~, ! , . . .
128595.~
-6- GC227
The term "substituted amino" refers to a
group having the formula -NZlZ2 wherein Zl is
hydrogen, alkyl, phenyl, substituted phenyl,
phenylalkyl or (substituted phenyl)alkyl, and Z2
5 is alkyl, phenyl, substituted phenyl, phenylalkyl,
(substituted phenyl)alkyl, hydroxy, cyano, alkoxy,
phenylalkoxy, or amino ~-NH2).
The terms "salt" and "salts`', when used to
describe the ~-lactams of this invention, refer to
basic salts formed with inorganic and organic
bases. Such salts include ammonium salts, alkali
metal salts like sodium and potassium salts,
alkaline earth metal salts like the calcium and
magnesium salts, salts with organic bases, e.g.,
dicyclohexylamine salt, benzathine, N-methyl-
D-glucamine, hydrabamine salts, salts with amino
acids like arginine, lysine and the like.
Pharmaceutically acceptable salts are
preferred.
Salts of an azetidinone-l-sulfonic acid are
formed by reacting the free acid form of the
sulfonate with one or more eguivalents of an
appropriate base providing the desired cation in
water or in a solvent mixture containing water.
The salt is isolated by removal of solvent in
vacuo or, in the case of water, by lyophilization.
The free acid of the sulfonate is formed by
treating an azetidinone-l-sulfonic acid salt with
an insoluble sulfonic acid such as a cation
exchange resin in the hydrogen form (e.g. a poly
styrene sulfonic acid resin like Dowex 50).
Alternatively, salts may be formed by cation
interchange. A salt of a ~-lactam compound soluble
in an organic solvent is combined with a salt
containing the desired cation, also soluble in the
:
~.. . ... . . . .
. . , ' ` ~ , ' ` ' -
~ ~ .
3595~
GC227
--7--
same solvent system. The solvent system is chosen
so that the formed salt is much less soluble
than either of the added salts and thus
precipitates from the medium and is collected.
The ~-lactams of formula I, and
pharmaceutically acceptable salts thereof, have
activity against gram-negative organisms. The
compounds of this invention can be used as agents
to combat bacterial infections (including urinary
tract infections and respiratory infections) in
mammalian species, such as domesticated animals
(e.q., dogs, cats, cows, horses, and the like) and
humans.
For combating bacterial infections in
mammals, a compound of this invention can be
administered to a mammal in need thereof in an
amount of about 1.4 mg/kg/day to about
350 mg/kg/day, preferably about 14 mg/kg/day to
about 100 mg/kg/day. All modes of administration
which have been used in the past to deliver
penicillins and cephalosporins to the site of the
infection are also contemplated for use with
~-lactams of this invention. Such methods of
administration include oral, intravenous, intra-
muscular, and as a suppository.
The compounds of this invention can be
prepared using a variety of procedures. One method
utilizes as a starting material the known mono-
~ ~ cyclic ~-lactam antibiotics having the formula
::
,...... . . ...... . . . .
, . . .... . ........ .... . .
. . . . , - . . . . ~ . . ... ., . : , ~.
~X~S95~
GC227
--8--
II1~ ,,2
~ -C-~-OH
/~ ~S~ ~ F 6_ -~7
and salts thereof. Compounds of formula II are
described in the literature; see, for example,
United Kingdom patent application 2071650,
published September 23, 1981. Reaction of a
compound of formula II with a hydrazide having the
formula
III l3 l4
EN-N -R5,
or a salt thereof, in the presence of a coupling
agent, yields the desired products of formula I.
If the starting material of formula II is an inner
salt (-SO3H in the l-position), it is preferable to
first treat the compound with one equivalent of a
base (e.a., tributylamine or trioctylamine) to
form the salt of the sulfonic acid. Preferably,
the reaction is run in the presence of a substance
capable of forming a reactive intermediate in
situ, such as N-hydroxybenzotriazole and/or a
catalyst such as dimethylaminopyridine, using a
coupling agent such as dicyclohexylcarbodiimide.
Exemplary solvents which can be used for the
reaction are dimethylformamide, tetrahydrofuran,
dichloromethane or mixtures thereof.
Alternatively, the compounds of this
invention can be prepared by acylating a compound
having the formula
:~:
., , ., - : ~ . : , .
: , . , - ~ : : -
~2~5~5~
GC227
_g_
IV 2 R6, ~R7
~ N-S03H
or a salt thereof, with a carboxylic acid having
the formula
V R1 R2 ~3 14
/O~ N-N-R5
N O
~ o
H2N
Well-known acylation procedures can be used for
the reaction. Exemplary techniques include the
use of a carboxylic acid of formula V or a
corresponding carboxylic acid halide or carboxylic
acid anhydride. The reaction with a carboxylic
acid proceeds most readily in the presence of a
carbodiimide such as dicyclohexylcarbodiimide and
a substance capable of forming a reactive inter-
mediate in situ such as N-hydroxybenzotriazole or
N-hydroxysuccinimide.
Compounds of formula V are novel compounds,
and as such, form an integral part of this
invention. They can be prepared by reacting
~2-amino-4-thiazolyl)glyoxylic acid, which has
the formula
VI 0 O
Nl ~ C-C-O~
/ S
; H2N
with a compound having the formula
,.~ ...... . . . . . . . . .... . . . . .
,,:
: : - . : . . .
12~5~5~
GC227
--10--
VII Rl R2 13 R4
H2N-O-~C-N- ~-R5,
or a salt thereof. The reaction proceeds best in
water and in mixtures of water and organic
solvents, such as methanol, ethanol, tetrahydro-
furan or dioxane.
Reactants of formula VII can be prepared by
reacting a compound having the formula
VIII R
~ -O-~-C-OH
lS or
~I-O~-halo
with a hydrazide having the formula
III IR3 R14
HN-N-R5
to yield the corresponding compound having the
formula
X [~N-O-C/-~;-N-N-R5 .
: 30
If an acid reactant of formula VIII is used, a
suitable coupling agent, such as dicyclohexylcarbo-
diimide, should be present. Alternatively, an
acid of formula VIII can be activated by formation
of a mixed anhydride. If an acid halide
, . . ,,, , ,: ~
, . . . ~ . . ' . , :
' ', ''. '., , , ~. , .
~2~5~3~
GC2~7
derivative of formula IX is used, a suitable base
should be present. The hydrazides of formula X
can be deprotected using standard methodology to
yield the desired reactants of formula VII.
Exemplary deprotecting agents are hydrazine and
methylhydrazine.
Hydrazine derivatives of formula III, and
methods for their preparation, are well known in
the literature. Reviews of their synthesis can be
found in Smith, "The Chemistry of Open-Chain
organic Nitrogen Compounds", Vols. I ~ II,
Benjamin, Inc., New York, Amsterdam, 1966; Muller,
"Methoden der Organischen Chemie" (Houben-Weyl),
Vol. 10/2, Georg Thieme Verlag Stuttgart, 1967;
Sandler and Karo, "Organic Functional Group
Preparations", Vol. 1, Academic Press, New York,
1968; and Timberlake and Stowell, "The Chemistry
of ~ydrazo, Azo, and Azoxy Groups", ed. S.Patai,
part 1, Interscience, New York, 1975.
Reactants of formula IV are described in
United Kingdom patent application 2071650,
published September 23, 1981.
The compounds of this invention (both the
pharmaceutical products of formula I and the
intermediates of formula V) contain an imino group
(-~-) and can exist as syn or anti isomers or as
N-
mixtures of both. All of these isomeric forms are
within the scope of this invention. The syn
isomers are preferred, however, because that
isomeric form has superior activity.
Whether the pharmaceutical products of
formula I are prepared from a starting compound of
formula II or from a starting compound of formula
~5 V, the isomerism of the starting material will
determine the isomerism of the product. In
lX~5~5~
-12- GC227
preparing a compound of formula V, the ratio of
syn/anti will depend on the reaction conditions.
If the reaction of compounds of formula VI and VII
is run at room temperature, the ratio of syn/anti
will be favorable to the obtaining of the syn
isomer. Lowering the reaction temperature
increases the ratio of syn/anti, but slows the
reaction. Raising the reaction temperature
decreases the ratio of syn/anti, but speeds the
reaction. Separation of the syn and anti isomers
can be accomplished using fractional crystalliza-
tion.
Alternative methodology for preparing the
compounds of this invention will be apparent to
the practitioner of this invention. For example,
those compounds of formula I wherein R4 and R5 are
=C~-Y7 can be prepared by reacting the corres-
ponding compound of formula I wherein R4 and R5 are
each hydrogen with the appropriate benzaldehyde.
The following examples are specific
embodiments of this invention.
.~. ~ . . ~ . -
. . : - , - . , ,
. : . -
.. . . .
~28595~i
GC227
-13-
Example 1
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[l,1-
dimethyl-2-[2-[(1,l-dimethylethoxy)carbonyl]-
hydrazino]-2-oxoethoxy]imino]acetyl3amino]-4-
S methyl-2-oxo-1-azetidinesulfonic acid, mono-
potassium salt
~3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl}amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide, and
1.06 g of N,N-dicyclohexylcarbodiimide was added.
After stirring for 30 minutes, a solution of
lS 0.66 g of [(1,1-dimethylethoxy)carbonyl~hydrazine
in S ml of dimethylformamide was added. Stirring
overnight at room ~emperature, filtering off the
formed dicyclohexylurea and distilling off the
solvent yielded an oily residue which was
dissolved in acetone (30 ml) an~ filtered. To the
filtrate were added 30 ml of ether and a solution
of 1.7 g of potassium perfluorobutanesulfonate in
10 ml of acetone. Crude product (1.8 g) was
obtained a`s a precipitate. Purification was by
column chromatography on ~P-20*, using water and
water/tetrahydrofuran (9:1) as eluents, and yielded
1.3 g of the title compound, melting point 258C.
IR (KBr): 1765 cm 1 (~-lactam carbonyl)
H-NMR (200 M~z, DMSO-d6**): ~ = 1.38 (m, l5H); 3.68
~m,l~); 4.5 (dd, lH); 6.78 (s, lH); 7.28 (s,
broad, 2H); 8.75 (s, broad lH); 9.13 (s, broad,
lH); 9.28 (d, broad, lH) ppm
------____
*HP-20: macroreticular styrene-divinylbenzene
copolymer, Mitsubishi Chemical Industries, Ltd.
:
**DMS0-d6: deuterated dimethylsulfoxide
:
i~85~S ~
GC227
-14-
Exam~le 2
[3S- [3a ( Z ), 4~ ] ] -3- [ ~ [ [2-[2-(Aminocarbonyl)hydrazino]-
1,1-dimethyl-2-oxoethyl]imino](2-amino-4-thiazolyl)-
acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, monopotassium salt
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-1-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-l-azetidinesulfonic acid (2.18 g)
and 0.93 g of tributylamine in 20 ml of dimethyl-
formamide were stirred at room temperature andO.1 g of N-hydroxybenzotriazole and 0.05 g of
dimethylaminopyridine were added followed by
1.06 g of N,N-dicyclohexylcarbodiimide in 5 ml of
dimethylformamide. After stirring for 30 minutes,
a solution of 0.6 g of (aminocarbonyl~hydrazine,
hydrochloride and 0.93 g of tributylamine in 15 ml
of dimethylformamide wcre added. Stirring at room
temperature overnight, filtering off the formed
dicyclohexylurea and evaporating the dimethyl-
formamide of the filtrate yielded a residue whichwas dissolved in 30 ml of acetone/tetrahydrofuran
(1:1) and fi}tered. After adding 3.4 g of
potassium perfluorobutanesulfonate in 15 ml of
acetone, the filtrate formcd a precipitate of
2.7 g crude product. This was purified by column
chromatography on HP-20, using water as eluent,
and yielding 1.3 g of the title compound, melting
point 245C, after freeze drying.
IR (XBr): 1763 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.38 (d, 6H); 1.40
(d, 3H); 3.65 (m, lH); 4.53 (dd, lH); 5.98 (s,
broad, 2H); 6.80 (s, lH); 7.23 (s, broad, 2H)
7.75 (s, lH); 9.25 (d, 2H); 9.35 (s, lH) ppm
~:
.
~285~35~
GC227
-15-
Example 3
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-
[2-(aminothioxomethyl)hydrazino-1,1-dimethyl-2-
oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
S azetidinesulfonic acid, monoPotassium salt
[3S- [ 3a ( Z ), 4~ ] ~ -3- [ [ ( 2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]a~-etyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxy-
benzotriazole and 0.05 g of dimethylaminopyridine
were dissolved in 30 ml of dimethylformamide and
1.06 g of N,N-dicyclohexylcarbodiimide in 5 ml of
dimethylformamide was added. After stirring for
15 minutes at room temperature, 0.46 g of (amino-
thioxomethyl)hydrazine in 10 ml of dimethylform-
amide was added and stirring was continued for 6
hours. ~ormed dicyclohexylurea (0.98 g) was
filtered off and the dimethylformamide of the
filtrate was distilled off in vacuo. The oily
residue was dissolved in 50 ml of acetone,
filtered and after adding a solution of 1.7 g of
potassium perfluorobutanesulfonate in 10 ml of
acetone, crude product separated from the
solution. It was washed with ether and purified
by column chromatography on HP-20 using water as
eluent and yielding 1.3 g of the title compound,
melting point 215C (dec).
IR (KBr): 1765 cm (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.35 (s, 6H)i 1.40
(d, 3H); 3.63 (m, lH); 4.52 (dd, lH); 6.80 (s,
lH); 6.95 (s, lH); 7.30 (s, broad, 2H); 8.00 (s,
lH); 9.18 (d, lH); 9.38 (s, lH); 9.68 (s, lH) ppm
. .: : . . . : , . . .
~2~5954
GC227
-16-
Example 4
[3S-[3a(Z),4~]]-3-[[[[2-(2-Acetylhydrazino)-l,l-
dimethyl-2-oxoethoxy]imino](2-amino-4-thiazolyl)-
acetyl]amino)-4-methyl-2-oxo-1-azetidinesulfonic
acid, monopotassium salt
~ 3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methy}ethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 q)
and 0.93 g of tributylamine were dissolved in 20 ml of
dimethylformamide and stirred at room temperature.
N-Hydroxybenzotriazole (0.1 g) and 0.05 g of
dimethylaminopyridine were added followed by a
solution of 1.06 g of N,N-dicyclohexylcarbodiimide
in 10 ml of dimethylformamide. After 20 minutes
stirring, a solution of 0.3 g of acetylhydrazine in
S ml of dimethylformamide was added. After 5
hours, the formed dicyclohexylurea was filtered off
and the dimethylformamide of the filtrate was
distilled off in vacuo. The oily residue was
dissolved in 30 ml of acetone, filtered and a
solution of 1.7 g of potassium perfluorobutane-
sulfonate in 10 ml of acetone was added. Crude
product (1.7 g) was obtained as a precipitate,
washed with ether, dried and purified by column
chromatography on HP-20 using water as an eluent
and yielding 0.8 g of the title compound, melting
point 241C (dec).
IR ~KBr): 1760 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.38 (s, 6H); 1.40
(d, 3H); 1.83 (s, 3R); 3.68 (m, lH); 4.53 (dd,
lH); 6.80 (s, lH)i 7.28 (s, 2H); 9.15 (s, broad,
lH); 9.28 (d, lH); 9.73 (s, broad, lH) ppm
: '
~ :
::::
- - - , .. . . . .
~.2~595~ -
-17- GC227
ExamPle 5
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-[2-
(2-furanylcarbonyl)hydrazino]-1,1-dimethyl-2-oxo-
ethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidineculfonic acid, monoPotassium salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(1-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
O.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide. After
adding 1.06 g of N,N-dicyclohexylcarbodiimide and
stirring at room temperature for 20 minutes, a
solution of 0.75 g of (2-furanylcarbonyl)hydrazine
in 5 ml of dimethylformamide was added. Overnight
stirring followed by filtering off the formed
dicyclohexylurea and distilling off the dimethyl-
formamide of the filtrate yielded an oily
residue. It was dissolved in 50 ml of acetone,
filtered and to the solution was added 1.7 g of
potassium perfluorobutanesulfonate and 20 ml of
ether yielding a precipitate of the crude product
(2.4 g). Purification by HP-20 column chromato-
graphy using water as eluent yielded 1.5 g of the
title compound, melting point 255C (dec).
IR (KBr): 1763 cm 1 (~-lactam carbonyl)
H-NMR (200 MNH, DMSO-d6): ~ = 1.45 (dd, 9H); 3.68
(m, lH); 4.51 (dd, lH); 6.60 (m, lH); 6.85 (s,
lH); 7.23 (d, lH); 7.28 (s, lH); 7.85 (s, lH);
30 9.31 (d, broad, lH); 9.41 (s, broad, lH); 10.25
(s, broad, lH) ppm
.-. . , .:
. .
128~954
GC227
-18-
Example 6
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[(2-
(formylhydrazino)-l,l-dimethyl-2-oxoethoxy]-
imino]acetyl]amino-4-methyl-2-oxo-1-azetidine-
sulfonic acid, monoPotassium salt
[3S-t3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-l-azetidinesulfonic acid (1.09 g),
0.88 g of trioctylamine, 0.05 g of N-hydroxy-
benzotriazole and 0.025 g of dimethylaminopyridine
were dissolved in 50 ml of tetrahydrofuran. After
adding a solution of 0.55 g of N,N-dicyclohexyl-
carbodiimide in lO ml of tetrahydrofuran and
stirring for 30 minutes at room temperature, a
solution of 0.15 g of formylhydrazine in 10 ml of
tetrahydrofuran was added. Overnight stirring was
followed by filtering.off the formed dicyclohexylurea and
the addition of O.85 g of potassium perfluoro-
butanesulfonate to the filtrate to give crude
product as a precipitate. This was recrystallized
from methanol/isopropanol to give 0.9 g of the
title compound, melting point 254C (dec).
IR (KBr): 1762 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.40 (dd, 9H); 3.68
(m, lH); 4.52 (dd, lH); 6.58 (s, lH); 7.30 (s,
2H); 8.03 (s, 1~); 9.25 (s, broad, lH); 9.28 (d,
broad, lH); 9.90 (s, broad, lH) ppm
' ~ ~
~285~5~
GC227
--19--
Example 7
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-[2-
[[(aminocarbonyl)amino]acetyl]hydrazino]-l,1-
dimethyl-2-oxoethoxy]imino]acetyl]amino]-4-methyl-
2-oxo-1-azetidinesulfonic acid, monoPotassium salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-1-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.47 g of tributylamine, 0.05 g of N-hydroxybenzo-
triazole and 0.025 g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide. N,N-Di-
cyclohexylcarbodiimide (0.55 g) was added and,
after stirring for 20 minutes, a solution of
0.35 g of [[(aminocarbonyl)amino]acetyl]hydrazine
in 20 ml of dimethylformamide was added. Stirring
overnight, filtering off the formed dicyclohexylurea and
distilling off the dimethylformamide of the
filtrate yielded a residue which was dissolved in
30 ml of tetrahydrofuran/methanol (2:1) and
filtered. To the filtrate was added a solution of
0.85 g of potassium perfluorobutanesulfonate in
10 ml of acetone. Crude product was obtained as a
precipitate (1.3 g). Purification by column
chromatography on H~-20 using water as an eluent
yielded the title compound, melting point 205C
(dec)-
IR (KBr): 1760 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6) ~ = 1.40 (dd, 9H); 3.65
(m, 3H); 4.52 (dd, lH); 5.63 (s, 2H); 6.28 (t,
lH); 6.80 (s, lH); 7.80 (s, 2H); 9.25 (s, lH);
9.30 (d, lH); 9.80 (s, lH) ppm
. , .
.. . . . . .
1;~859s~
GC227
-20-
Exam~le 8
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[1,1-
dimethyl-2-[2-(methylsulfonyl)hydrazino]-2-oxo-
ethoxy]imino)acetyl]amino~-4-methyl-2-oxo-1-
azetidinesulfonic acid, mono~otassium saltt3S-~3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine weredissolved in 30 ml of dimethylformamide. A
solution of 1.06 g of N,N-dicyclohexylcarbodiimide
in 5 ml of dimethylformamide was added, and after
20 minutes stirring at room temperature, a
solution of 0.60 g of (methylsulfonyl~hydrazine in
8 ml of dimethylformamide was dropped in. After
stirring for 5 hours, the formed dicyclohexylurea
was filtered off, the dimethylformamide of the
filtrate was distilled off and the residue was
dissolved in 20 ml of acetone and filtered. A
solution of 1.7 g of potassium perfluorobutane-
sulfonate in 15 ml of acetone was added. A
precipitate of 1.8 g of crude product was obtained
and purified by column chromatography on HP-20
using water as eluent and yielding 1.2 g of the
title compound, melting point 235C (dec).
IR (KBr): 1760 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.40 ~dd, 9H); 2.90
(s, 3H); 3.66 (m, lH); 4.5 (dd, lH); 6.78 (s, lH);
7.26 (s, 2H); 9.06 (d, lH); 9.43 (s, broad, lH);
9.78-(s, broad, lH); ppm
.
::
,
- -
S95~
GC227
-21-
Exam~le 9
[3S-[3a(Z),4~]]-3-[[[[(2-(2-Phenylhydrazino)-l,l-
dimethyl-2-oxoethoxy]imino](2-amino-4-thiazolyl)-
acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, mono~otassium salt __
[3S-[3~(Z)`,4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and O.OS g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide and a
solution of 1.06 g of N,N-dicyclohexy}carbodiimide
was added. After stirring for 20 minutes at
room temperature, a solution of 0.54 g of phenyl-
hydrazine in 5 ml of dimethylformamide was droppedin. Stirring for 4 hours, filtering off the formed
dicyclohexylurea and distilling off the dimethyl-
formamide of the filtrate yielded a residue which
was dissolved in 20 ml of tetrahydrofuran and
filtered. To the filtrate was added 1.7 g of
potassium perfluorobutanesulfonate and 20 ml of
ether yielding 1.8 g of the title compound as a
precipitate, melting ~oint 223C (dec).
IR (RBr): 1762 cm 1 (~-lactam carbonyl)
lH-NMR ~200 MHz, DMSO-d6): 8 = 1.43 (m, 9H); 3.73
(m, 1~); 4.53 (dd, lH); 6.63 (t, lH); 6.75 (d,
2H); 6.88 (s, lH); 7.30 (s, lH); 7.73 (s, lH);
9.25 (s, broad, lH); 9.45 (d, lH); ppm
3595~
GC227
-22-
Exam~le 10
[3S-[3a(Z),4~] ]-3-[ [ [ [2-[2-(Cyanoacetyl)hydrazino]-
1,1-dimethyl-2-oxoethoxy]imino](2-amino-4-thiazolyl)-
acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
S acid, monoPotassium salt
[3S-[3~(Z),4~]]-3-~[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide. A
solution of 1.06 g of N,N-dicyclohexylcarbodiimide
in 5 ml of dimethylformamide was added and after
stirring for 20 minutes, a solution of 0.5 g of
(cyanoacetyl)hydrazine in 50 ml of dimethylform-
amide was added. Stirring overnight at room
temperature, filtration and distilling off the
dimethylformamide of the filtrate yielded an oily
residue. It was dissolved in 20 ml of methanol,
filtered and to the filtrate a solution of 1.7 g
of potassium perfluorobutanesulfonate in 10 ml of
acetone was added. Crude product was obtained as
a precipitate. Purification by column chromato-
graphy on HP-20 using water as an eluent yielded
the title compound, melting point 223C (dec).
IR (KBr): 2235 cm 1 ~cyano); 1765 cm 1 (~-lactam
carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.41 (m, 9H); 3.70
(m, lH); 3.73 (s, 2H); 4.51 (dd, 1~); 6.83 (s,
; 30 lH); 7.30 (s, 2H); 9.28 (d, lH); 9.33 (s, broad,
lH); 9.73 (d, broad, A~); 10.30 (s, broad, lH); ppm
.
.. , ;,-, ~ ,, . - - . ,, . , . :
;'35 ~
GC227
-23-
Example 11
[ 3S- [3a ( Z ), 4~ ] ] -3- [ [ [ 12- [2- (Aminooxyacetyl)-
hydrazino]-l,l-dimethyl-2-oxoethoxy]imino]( 2-
amino-4-thiazolyl)acetyl]amino]-4-methyl-2-oxo-
l-azetidinesulfonic acid, monoPotassium salt
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
~(l-carboxy-l-methylethoxy)imino]acetyl~amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine were
stirred at room temperature in 30 ml of dimethyl-
formamide. A solution of 1.06 g of N,N-dicyclo-
hexylcarbodiimide in 5 ml of dimethylformamide was
added and, after stirring for 20 minutes, a
solution of 0.5 g of (aminooxoacetyl)hydrazine in
20 ml of dimethylformamide was added. Stirring
overnight, filtering off the formed dicyclohexyl-
urea and distilling off the dimethylformamide of
the filtrate yielded a residue which was dissolved
in 30 ml of tetrahydrofuran/methanol (3:1). After
filtration, a solution of 1.7 g of potassium
perfluorobutanesulfonate in 20 ml of acetone was
added. Crude product was obtained as a precipi-
tate. Purification by column chromatography on
HP-20 u~ing water as eluent yielded 1.6 g of the
title compound, melting point 148C (dec).
IR (RBr): 1768 1 (~-lactam carbonyl)
H-NMR (200 MHz DMSO-d6): ~ = 1.45 (m, 9H); 3.68
(m, lH); 4.52 (dd, lH); 6.83 (s, lH); 7.28 (s,
2H~ 8.00 (d, broad, 2~); 9.30 ~d, broad, 2~; ppm
:
.
~: :, . ... .. . -.
, . - . . . . . .: .
.. ~ . . ... . :
. ,-, . . . . .
lZ~35~35~
GC227
-24-
Exam~le 12
[3S-[3a(Z),4~]]-3-[[~2-Amino-s-thiazolyl)[[2-
hydrazino-l,l-dimethyl-2-oxoethoxy]imino]acetyl]-
amino]-4-methyl-2-oxo-1-azetidinesulfonic acid,
and the trifluoroacetate salt thereof
Method I
A) [35-~3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-
[2-(triphenylmethyl)hydrazino]-1,1-dimethyl-2-oxo-
ethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, monoDotassium salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
t(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.1 g of N-hydroxybenzotriazole, 0.05 g of dimethyl-
aminopyridine and 0.93 g of tributylamine were
dissolved in 20 ml of dimethylformamide. With
stirring, a solution of 1.1 g of N,N-dicyclohexyl-
carbodiimide in 10 ml of dimethylformamide was
added. After stirring at room temperature for 15
minutes, a solution of 1.55 g of (triphenylmethyl)-
hydrazine, hydrochloride and 0.93 g of tributyl-
amine in 20 ml of dimethylformamide were added.
Stirring overnight, filtering off the formed
dicyclohexylurea and distilling off the dimethyl-
formamide of the filtrate yielded a residue whichwas dissolved in 20 ml of acetone and filtered.
To the filtrate was added 150 ml of ether and a
solution of 3.40 g of potassium perfluorobutane-
sulfonate in 30 ml of aceonte. Crude product was
obtained as a precipitate (2.2 g). Purification
by column chromatography on HP-20 using water and
water/tetrahydrofuran (9:1) as eluents yielded the
:
.
. . ~ ,. . .
. .
1.2~5'~
GC227
-25-
title compound, melting point 182-184C (dec).
IR (KBr): 1760 cm~l (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.10 (d, 6H); 1.38
(d, 3H); 3.63 (m, lH); 4.38 (dd, lH); 5.78 (d,
lH); 6.58 (s, lH); 7.33 (m, 17H); 8.00 (d, lH);
9.25 (d, lH)i ppm
B) [3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)~[2-
hydrazino-l,l-dimethyl-2-oxoethoxy]imino]acetyl]-
aminol-4-methyl-2-oxo-1-azetidinesulfonic acid
[3S-[3a(Z),4~]]-3-~[(2-Amino-4-thiazolyl)-
[[2-t2-(triphenylmethyl)hydrazino]-1,1-dimethyl-
2-oxo-ethoxy]imino~acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, monopotassium salt (0.8 g)
was suspended in 20 ml of dichloromethane. At
0C, with stirring, 10 ml of formic acid (98%) was
added. After continuous stirring for 20 minutes,
the clear solution was transferred into 100 ml of
ether. A precipitate of the formic acid salt of
the title compound and potassium formiate were
obtained. The precipitate was stirred in 30 ml
of acetonitrile and 5 ml of N-Methyl-N-(trimethyl-
; silyl)trifluoroacetamide was added. Stirring for
10 minutes, filtration and the addition of 5 ml of
methanol to the filtrate yielded the title compound
as a crystalline precipitate.
IR (KBr): 1762 cm 1 (~-lactam carbonyl)
H-NMR (200 M~z, DMSO-d6): ~ = 1.38 (m, 9H); 3.68
(m, lH); 4.5 (dd, lH); 6.80 (s, lH); 7.30 (m,
broad, 4H); 9.1S (s, broad, lH); 9.38 (d, broad,
lH); ppm
si~
GC227
-26-
Method II
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-
hydrazino-1,1-dimethyl-2-oxoethoxy]imino]acetyl]-
amino]-4-methyl-2-oxo-1-azetidinesulfonic acid,
trifluoroacetate salt (1:2)
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[[1,1-dimethylethoxy)carbonyl]hydrazino~-2-oxo-
ethoxy]imino]acetyl]amino]-~-methyl-2-oxo-l-
azetidinesulfonic acid, monopotassium salt (10 g)
was dissolved in 30 ml of dichloromethane and
added dropwise to 60 ml of trifluoroacetic acid
with stirring at -10C. After 20 minutes of
stirring, the reaction was complete. The addition
of 200 ml of ether to the solution precipitated
7.8 g of the title compound, melting point 220C,
(dec)-
Exam~le 13
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[1,1-
dimethyl-2-[2-(2-pyridinyl)hydrazino]-2-oxoethoxy]-
imino]acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, mono~otassium salt
[3S-[3a(Z),4~]]-3-[[~2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.04 g),
0.05 g of N-hydroxybenzotriazole, 0.025 g of
dimethylaminopyridine and 0. as g of trioctylamine
were dissolved in 20 ml of dimethylformamide.
N,N-Dicyclohexylcarbodiimide (0.55 g) in lO ml of
tetrahydrofuran was added. Stirring for 15
minutes at room temperature, followed by the
addition of 0.3 g of (2-pyridinyl)hydrazine in
5 ml of dimethylformamide and overnight stirring
completed the reaction. Dicyclohexylurea was
filtered off, the solvents of the filtrate were
distilled off and the residue was dissolved in
:
:
,. , ., . ~. ,~ . , , . , . : . :,
1~5~35~
-27- GC227
20 ml of tetrahydrofuran and filtered.
Potassium perfluorobutanesulfonate (0.85 g) was
added, stirring for 5 minutes followed by the
addition of 10 ml of ether formed crude product as
a precipitate (0.9 g). Purification by column
chromatography on HP-20 yielded the title
compound, melting point 205-207C ~dec).
IR (KBr): 1762 cm 1 (~-lactam carbonyl)
lH-NMR (200 MHz, DMSO-d6): ~ = 1.40 (dd, 9H); 3.65
(m, lH); 4.53 (dd, lH); 6.65 (m, 2~); 6.84 (s,
1~); 7.30 (s, 2H); 7.43 (t, 1~); 8.00 (d, 1~);
8.18 (s, lH); 9.35 (s, lH); 9.40 (d, lH); ppm
ExamDle 14
~3S-[3a(Z),4~]]-3-[[[[2-[2-(3-Pyridinylcarbonyl)-
hydrazino]-l,l-dimethyl-2-oxoethoxy]imino](2-amino-
4-thiazolyl)acetyl]amino]-4-methyl-2-oxo-1-azetidine-
sulfonic acid,, mono~otassium salt
~3S-[3a(Z),4~]]-3-t[(2-Amino-4-thiazolyl)-
[(1-carboxy-1-methylethoxy~imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine in
30 ml of dimethylformamide were stirred and a
solution of 1.06 q of N,N-dicyclohexylcarbodiimide
in 10 ml of dimethylformamide was dropped in.
After 30 minutes, a solution of 0.68 g of (3-pyridinyl-
carbonyl)hydrazine in 10 ml of dimethylformamide
was added. Stirring overnight, filtering off the
dicyclohexylurea, and stripping off the dimethyl-
formamide of the filtrate yielded a residue which
was ~issolved in 30 ml of acetone. After adding
1.7 g of potassium perfluorobutanesulfonate in
20 ml of acetone, the title compound was obtained
. : ~ . . . :
~2~5~ t
GC227
-28-
as a precipitate (1.8 g), melting point 243C
(dec).
IR (KBr): 1760 cm 1 (~-lactam carbonyl)
lH-NMR (200 MHz, DMSO-d6): ~ = 1.44 (dd, 9H); 3.70
(m, lH); 4.53 (dd, lH); 6.85 (s, lH); 7.28 (s,
2~); 7.51 (m, lH); 8.20 (m, lH); 8.73 (~, lH);
9.01 (d, lH); 9.30 (d, lH); 9.S0 (s, lH); 10.63
(s, broad, lH); ppm
Exam~le 15
[3S-t3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-[2-
(aminocarbonyl)-2-methylhydrazino]-1,1-dimethyl-
2-oxoethoxy]imino]acetyl~amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, mono~otassium salt
t3S- [3a (Z),4~]]-3-t[(2-Amino-4-thiazolyl)-
t(1-carboxy-1-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.47 g of tributylamine, 0.05 g of N-hydroxybenzo-
triazole and 0.025 g of dimethylaminopyridine were
dissolved in 15 ml of dimethylformamide. After
adding a solution of O.S5 g of N,N-dicyclohexyl-
carbodiimide in 5 ml of dimethylformamide and
stirring at room temperature for 15 minutes, a
solution of 0.4 g of 2-(aminocarbonyl)-2-methyl-
hydrazine and 0.47 g of tributylamine in 10 ml of
tetrahydrofuran was added. Overnight stirring,
filtering off the dicyclohexylurea and distilling
off the æolvents of the filtrate yielded a residue
which was dissolved in 20 ml of tetrahydrofuran
and filtered. To the filtrate was added a
solution of 0.85 g of potassium perfluorobutane-
sulfonate in 20 ml of tetrahydrofuran and S0 ml of
ether. Crude product ~1.1 g) was obtained as a
precipitate. Purification by column chromatography
'
- . , -.~ , . - . .
.. . .
~28595~
GC227
-29-
on HP-20 yielded the title compound, melting point
203-205C (dec).
IR (KBr): 1762 cm 1 (~-lactam carbonyl)
lH-NMR (200 MHz, DMSO-d6): ~ = 1.36 (s, 6H); 1.40
(d, 3H); 2.93 (s, 3H); 3.65 (m, lH); 4.53 (dd,
lH); 6.08 (s, broad, 2H); 6.78 (s, lH); 7.23 (s,
2H); 9.25 (d, lH); 9.78 (s, lH); ppm
Example 16
t3S-~3a(Z),4,~]~-3-[[(2-Amino-4-thiazolyl)[[2-[2-
[(methylamino)carbonyl]hydrazino]-1,1-dimethyl-2-
oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, mono~otassium salt
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.47 g of tributylamine, 0.05 g of N-hydroxybenzo-
triazole and 0.025 g of dimethylaminopyridine were
dissolved in 20 ml of dimethylformamide. N,N-Di-
cyclohexylcarbodiimide (0.55 g) was added, andafter 20 minutes stirring at room temperature
a solution of 0.28 g of [(methylamino)carbonyl]-
hydrazine, hydrochloride and 0.4 g of tributylamine
in 20 ml of dimethylformamide was also added.
Overnight stirring, filtering off the dicyclo-
hexylurea and distilling off the dimethylformamide
of the filtrate yielded a residue, which was
dissolved in 30 ml of dimethylformamide and
filtered. To the filtrate was added a solution of
1.7 g of potassium perfluorobutanesulfonate in
20 ml of acetone. Crude product (1.1 g~ was
obtained as a precipitate. Purification by column
chromatography on HP-20 using water as an eluent
yielded the title compound, melting-point 218C
3s (dec)-
, .:. .
-: . - , , - . , -
i.~8595~
GC227
-30-
IR (KBr): 1760 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.36 (d, 6H); 1.39
(d, 3H); 2.51 (d, 2H); 3.66 (m, lH); 4.52 (dd,
lH); 5.65 (d, broad, lH); 6.80 (s, lH); 7.28 (s,
2H); 7.86 (s, lH); 9.23 (s, lH); 9.33 (d, lH); ppm
Exam~le 17
[3S-~3~( æ ), 4~]]-3-~(2-Amino-4-thiazolyl)[[2-[2-
[(methylamino)thiozomethyl]hydrazino]-l,l-dimethyl-
2-oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, monoDotassium salt
[3S-t3atZ),4~]]-3-t~(2-Amino-4-thiazolyl)-
[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.05 g of N-hydroxybenzotriazole, 0.025 g of
dimethylaminopyridine and 0.88 g of trioctylamine were
dissolved in 50 ml of of tetrahydrofuran and
0.55 g of N,N-dicyclohexylcarbodiimide was added.
After stirring for 30 minutes at room temperature,
0.27 g of [(methylamino)thioxomethyl]hydrazine in
20 ml of tetrahydrofuran was added. Overnight
stirring, filtering off the formed dicyclohexyl-
urea and adding 0.85 g of potassium perfluoro-
butanesulfonate in 15 ml of acetone to the
filtrate formed crude product as a precipitate.
Adding ether yielded a second crop. (Total
1.1 g). Purification by column chromatography on
HP-20 using water as an eluent yielded the title
compound, melting point 206-208C, (dec).
IR (KBr): 1760 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6): ~ = 1.38 (d, 6H); 1 40
(d, 3H); 2.85 (d, 3H); 3.68 (m, lH); 4.52 (dd,
lH); 6.79 (d, broad, lH); 6.89 (s, lH); 7.33 (s,
2H); 9.28 (d, lH); 9.46 (s, broad, lH); 9.53 (s,
broad, lH); ppm
~ ~:
- - . . - . . .
~ - . ~ - ,. .
: : . . .. . .
~S~5~
GC227
-31-
Example 18
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-[2-
[imino(methylthio)methyl]hydrazino]-1,1-dimethyl-
2-oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, monoPotassium salt
[3s-[3~Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.-1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine were
dissolved in 30 ml of dimethylformamide. N,N-Di-
cyclohexylcarbodiimide (1.06 g) in 5 ml of dimethyl-
formamide was added and, after stirring for 20
minutes, a solution of 1.17 g of [imino(methyl-
thio)methyl]hydrazine, hydrogen iodide salt and0.93 g of tributylamine in 15 ml of dimethyl-
formamide was also added. Continuous stirring
overnight at 5C, filtering off the dicyclohexyl-
urea and distilling off the dimethylformamide of
the filtrate yielded an oily residue. The residue
was dissolved in 30 ml of tetrahydrofuran and 5 ml
of methanol, filtered and a solution of 3.4 g of
potassium perfluorobutanesulfonate in 20 ml of
acetone was added. Crude product (1.2 g) was
obtained as a precipitate. Purification by column
chromatography on ~P-20 using water as an eluent
yielded 1.4 g of the title compound, melting point
208C,(dec).
IR (KBr): 1765 cm (~-lactam carbonyl)
30 lH-NMR (200 MHz, DMSO-d6): ~ = 1.36 (d, 6H); 1.40
(d, 3H); 2,28 (s, 3H); 3.68 (m, lH); 4.51 (dd,
lH); 6.38 (s, broad, 2H); 6.81 (s, lH); 7.28 (s,
2H); 9.06 (s, broad, lH); 9.40 (d, broad, lH); ppm
. - . , . ~ . .: . - . .
~- . ~ -: . . . : . ~ . . .
~S~5'~
GC227
-32-
Exam~le 19
[3s-~3a(z)~4~]]-3-t~(2-Amino-g-thiazolyl)~2-~2
taminothioxomethyl)hydrazino]-i~l-dimethyl-2
ethoxy]imino]acetyl~amino-4-methyl-2-oxo-1-
S azetidinesulfonic acid, mono~otassium saltt3S-t3a(Z),4a]]-3- r ~ ( 2-Amino-4-thiazolyl)-
t(l-carboxy-l-methyiethoxy)imino~acetyl]a~ino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (0.66 g),
0.3 g of tributylamine, 0.26 g of N-hydroxybenzo-
triazole and 0.01 g of dimethylaminopyridine weredissolved in 10 ml of dimethylformamide and a
soiution of 0.33 g of N,N-dicyclohexylcarbodiimide
in S ml of dimethylforma~;de was added. After
stirring for 20 minutes at room temperature,
a solution of 0.28 g of (aminothio~omethyl~-
hydrazine in 10 ml of dimethylformamide was also
added. Stirring was continued for 24 hours, formed
dicyclohexylurea was filtcred off and the
dimethylformamide of the filtrate was distilled
off. The residue was dissolved in 20 ml tetra-
hydrofuran~methanol (1:1), filtered and a solution
of 0.51 g of potassium perfluorobutanesulfonate i~
S ml of methanol was added followed ~y 20 ml of
ether. The re~ultant prccipitate of crude product
was purified by column chromatography on Hæ-20
using water as an eluent and yieldins 0.8 g of the
title compound, melting point 218C, (dec).
IR (KBr): 1768 cm 1 (~-lactam carbonyl)
1H-NMR (200 M~z, DMSO-d6) ~ = 1.23 (d, 3H); 1.35 (d,
6R); 4.05 (m, l~)i S.10 (m, lH); 6.80 (s, 1~);
6.g3 (s, broad, lH, CSN~2); 7.33 (s, broad, 2H);
8.10 (s, broad, lH, CSN~2), 9.14 (d, broad, lH);
9.40 (s, broad, 1~); 9.65 (s, broad, lH); ppm
* Trade Mark
.
., ~ - , ~ , . - ~,
~3S95 ~
GC227
-33-
ExamDle 2 0
t3S- [3a ( Z ), 4~ ~ ] ~3~ t ~ ( 2-Amino-4-thiazolyl ) [ E2- [2-
[ 3, 4-bis (acetyloxy)benzoyl]hydrazino]-l,1-dimethyl-
2-oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, mono~otassium saltt3S-~3~(Z),4~]~-3-~(2-Amino-4-thiazolyl)-
~2-hydrazino-1,1-dimcthyl-2-oxoethoxy]imino]-
acet~l]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, trifluoroacetate salt (1:2) (~.36 g)
and 1.47 g of tributylamine were dissolvcd in
15 ml of dimethylformamide. At 0C, with
stirring, a solution of 0.75 g of 3,4-(diacetyl-
oxy)benzoyl chloride in S ml of dimethylform~;de
was dropped in. After stirring for 3 hours, the
dimethylformamide was distilled off and the
residue was dissolved in 20 ml of tetrahydrofuran
and filtered. To the filtrate was added 2.75 g of
potassiu~ perfluorobutanesulfonate. After
stirring for 10 minutes, 20 ml of ether was added
and crude product was obtained as a precipitate.
This was purified by liguid chromatography on
XAD-2* using water and water/tetrahydrofuan
(9.5:O.S) as eluents, yielding 1.1 g of the title
compound, melting point 221C (dec).
IR (K3r): 176~ cm 1 (~-lactam carbonyl)
~-NMR (200 MEz, DMSO-d6) ~ ~ 1.41 (d, 3~); 1.48
(d, 6E); 2.29 (s, 6~); 3.70 (m, lE); 4.06 (dd,
1~); 6.85 (s, lE); 7.26 (s, 2~); 7.40 (d, lE);
7.81 (m, 2~); 9.30 (d, 1~); 9.48 (s, 1~); 10.49
(s, 1~); ppm
_____
:~ :
*XAD-2: macroreticular styrenë-divinylbenzene
c~pclymer, Rohm and ~aas Company
* Trade tqark
' - - - ' . ' ' : : ' . .
: . . .
- . -. .. . .
- .
: .. . .
~ 2~35~35~
GC227
-34-
Example 21
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[l,l-
dimethyl-2-[2-[(2-amino-4-thiazolyl)acetyl]-
hydrazino]-2-oxoethoxy]imino]acetyl]amino]-4-methyl-
2-oxo-1-azetidinesulfonic acid, monopotassium salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
~ carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.47 g of tributylamine, 0.1 g of N-hydroxybenzo-
triazole and 0.05 g of dimethylaminopyridine weredissolved in 10 ml of dimethylformamide and 0.54 g
of N,N-dicyclohexylcarbodiimide was added. After
stirring for 20 minutes at room temperature,
O.45 g of [(2-amino-4-thiazolyl)acetyl]hydrazine
was added. Overnight stirring, filtration from
the formed dicyclohexylurea and distilling of the
dimethylformamide of the filtrate yielded a
residue. It was dissolved in 10 ml of tetrahydro-
furan, filtered again and 1.7 g of potassium
perfluorobutanesulfonate and 10 ml of ether were
added to the filtrate yielding a precipitate. The
crude product was purified by column
chromatography on ~P-20 using water as an eluent,
yielding 0.67 g of the title compound, melting
point 147C (dec).
IR (KBr): 1765 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6) ~ = 1.43 (2d, 9H); 3.30
(d, 2~); 3.71 (m, lH); 4.51 (dd, lH); 6.31 (s,
lH); 6.80 (s, lH); 6.82 (s, 2H); 7.26 (s, 2H);
30 9.28 (s, broad, 3~); 9.77 (s, broad, lH); ppm
.
: . -
~X~595~
_35 GC227
Example 22
[3S-[3~(Z),4~]]-3-[[(2-Amino-~-thiazolyl)[[2-
[2-[(2,3-dihydroxyphenyl)methylene]hydrazino]-1,1-
dimethyl-2-oxoethoxy]imino]acetyl]amino]-4-methyl-
2-oxo-1-azetidinesulfonic acid, monopotassium
salt, dimethYlformamide (1:2)
t3S- [3a (Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[[2-hydrazino-1,1-dimethyl-2-oxoethoxy~imino]-
acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, trifluoroacetate salt (1:2) (0.68 g) and
0.37 g of tributylamine were dissolved in 10 ml of
dimethylformamide. A solution of 0.14 g of
2,3-dihydroxybenzaldehyde in 10 ml of dimethylformamide
was added. Ater stirring for 12 hours, the
solution was filtered and the dimethylformamide
filtrate was distilled off. The residue was
dissolved in 30 ml of methanol and a solution of
1.1 g of potassium perfluorobutanesulfonate in
20 ml of methanol was added. The title compound
(0.45 g) was obtained as a crystalline precipitate,
melting point 199C (dec).
IR (KBr): 1770 cm 1 (~-lactam carbonyl)
H-NMR (200 MHz, DMSO-d6) ~: 1.42 (d, 3H); 1.50
(d, 6H); 3.80 (m, 1~); 4.50 (m, lH); 4.68 (s,
broad, 2H); 6.65-7.00 (m, 4H); 7.25 (s, broad,
2H); 4.49 (s, lH); 9.43 (d, 2H); 10.99 (s, lH); ppm
- . . . . ......................... . .
, -. - , : - - -
. . . . . . . .
~2~5~5~
GC227
-36-
Example 23
[3S-[3~(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-
[2-[(3,4-dihydroxyphenyl)methyiene]hydrazino]-
1,1-dimethyl-2-oxoethoxy]imino]acetyl]amino]-4-
methyl-2-oxo-1-azetidinesulfonic acid, mono-
Potassium salt _
~ 3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[[2-hydrazino-1,1-dimethyl-2-oxoethoxy]imino]-
acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic
acid, trifluoroacetate salt (1:2) (0.68 g) and
0.37 g of tributylamine were dissolved in 15 ml of
dimethylformamide. To this solution was added 1 g
of molecular sieves (3 angstroms) together with
0.14 g of 3,4-dihydroxybenzaldehyde. After
stirring for 8 hours at room temperature, the
solution was filtered and the filtrate was
distilled off. The residue was dissolved in
methanol and filtered again. To the filtrate was
added 1.1 g of potassium perfluorobutanesulfonate
and 10 ml of diethyl ether. Crude product was
obtained as a precipitate. Purification by liquid
chromatography on XAD-2 using water and water/
methanol (9.7:0.3) as eluents yielded 0.8 g of the
title compound, melting point 258C (dec).
IR (~Br): 1768 cm 1 (~-lactam carbonyl)
H-NMR (200 M~z, DMS0-d6) ~: 1.41 (d, 3H); 1.46
(s, 6H); 3.38 (s, broad, 2H); 3.78 (m, lH); 4.51
(dd, lH); 6.75 (m, lH); 6.83 (s, lH); 6.90 (m,
lH); 7.25 (s, broad, 2H); 8.13 (s, lH); 9.45 (s,
broad, lH); 10.40 (s, broad, lH); ppm
.
.. , .. . ~ ~ . ~ . .
;~, ,: ' .
~2S595~
GC227
-37-
Example 24
(Z)-2-Amino-a-[[l,1-dimethyl-2-[2-[(1,1-dimethyl-
ethoxy)carbonyl]hydrazino]-2-oxoethoxy]imino]acetic
acid
A) 1-[2-[(1,3-Dihydro-1,3-dioxo-2H-isoindol-2-yl)-
oxy]-2-methyl-1-oxopropyl]-2-[(1,1-dimethylethoxy)-
carbonyll
2-[(1,3-Dihydro-1,3-dioxo-2H-isoindol-2-yl)-
oxyJ-2-methylpropanoyl chloride (34.5 g) was
dissolved in 200 ml of dichloromethane. At 0C,
with stirring, a solution of 17 g of [(l,l-dimethyl-
ethoxy)carbonyl]hydrazine and 13 g of triethylamine
in 80 ml of dichloromethane was added. After
stirring overnight, 300 ml of ice water was added,
and after stirring for 5 minutes, the organic phase
was separated and extracted with 100 ml of 5%
sodium bicarbonate solution and then with 100 ml of
water. After drying over sodium sulfate, the
dichloromethane was evaporated. The oily residue
crystallized after several hours, yielding 42.4 g
of white crystals.
IR (XBr): 1800 cm 1 (carbonyl phthalimido)
1740 cm 1 (carbonyl ester)
lH-NMR (90 MHz, DMSO-d6): ~ = 1.34 (s, 9~); 1.50
(s, 6~); 7.93 (s, 4~); 8.81 (s, broad, lH); 9.69
(s, 1~); ppm
B) 1-[2-(Aminooxy)-2-methyl-1-oxopropyl]-2-[(t-
butYloxy)carbonyl]hvdrazide
1-~2-[(1,3-Dihydro-1,3-dioxo-2H-isoindol-
2-yl)oxy]-2-methyl-1-oxopropyl]-2-[(1,l-dimethyl-
ethoxy)carbonyl] (18.2 g) was dissolved in 200 ml
of dichloromethane and at 0C a solution of 2.3 g
of N-methylhydrazine was added dropwise in 300 ml
.
lX8595~
GC227
-38-
of dichloromethane. After 4 hours stirring, the
reaction mixture was filtered and the dichloro-
methane of the filtrate was evaporated yielding an
oily residue of the title compound (24.2 g), which
crystallized after standing im the cold.
H-NMR (90 MHz, DMSO-d6): ~ = 1.30 (s, 9H?; 1.46
(s, 6H); 5.95 (s, broad, 2H); 8.59 (s, broad, lH);
9.35 (s, broad, lH); ppm
10 C) (Z)-2-Amino-a-t[1,1-dimethyl-2-[2-[(1,1-
dimethylethoxy)carbonyl]hydrazino]-2-oxoethoxy]-
iminolacetic acid
(Z)-2-Amino-4-thiazoleglyoxylic acid
~1.72 g) was suspended in 30 ml of water/tetra-
hydrofuran (1:1) and 2.33 g of 1-[2-(aminooxy)-
2-methyl-1-oxopropyl~-2-[(t-butyloxy)carbonyl]-
hydrazine was added; the pH was adj~sted to 5.6
with sodi~m bicarbonate. Stirring overnight'
formed a clear solution. The dimethylformamide
was evaporated and the water solution was adjusted
to p~ 2 with 2 N phosphoric acid at 5~C. Crude
product was obtained as an oily precipitate which
crystallized after treat~ent with ether.
Recrystallization from dimethylformamide/iso-
propanol yielded 3.2 g of white crystals, meltingpoint 195C (dec).
IR (KBr): 1730 cm 1
H-NMR (90 MRz, DMSO-d6) ~ = 1.41 (d + s, l5H);
6.92 (s, lH); 7.27 (s, broad, 2H); 8.00 (s, broad,
30 lH); 8.71 (s, broad, lH); 9.15 (s, broad, lH)
::
~::
-. . . . . .
12~59~i~
GC227
-39-
Example 25
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)[[2-
(2,2-dimethylhydrazino)-1,1-dimethyl-2-oxo-
ethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic acid, mono~otassium salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[(l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (1.09 g),
0.47 g of tributylamine, 0.05 g of N-hydroxy-
benzotriazole and 0.005 g of 4-dimethylamino-
pyridine were dissolved in 10 ml of dimethyl-
formamide. With stirring, 0.6 g of dicyclo-
hexylcarbodiimide was added in 5 ml of dimethyl-
formamide. After 20 minutes stirring at room
temperature, a solution o 0.15 g of 1,1-dimethyl-
hydrazine in 5 ml of dimethylformamide was added.
Stirring overnight, filtering off the formed
dicyclohexylurea and distilltng off the dimethyl-
formamide of the filtrate yielded an oily
residue. It was dissolved in 25 ml of acetone and
0.85 g of potassium perfluorobutanesulfonate
was added. Crude product was obtained as a
precipitate and purified by column chromatography
on ~P-20 using water as an eluent. The product had
a melting point of 242C (dec).
Exam~le 26
[3S-[3a(Z),4~]]-3-t[2-Amino-4-thiazolyl)[[2-[2-
(l-pyridinylacetyl)hydrazino]-1,1-dimethyl-2-
oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-
1-azetidinesulfonic acid, inner salt
[3S-[3a(Z),4~]]-3-[[(2-Amino-4-thiazolyl)-
[~l-carboxy-l-methylethoxy)imino]acetyl]amino]-
4-methyl-2-oxo-1-azetidinesulfonic acid (2.18 g),
0.93 g of tributylamine, 0.1 g of N-hydroxybenzo-
.
. :. ~......... . :
~Z~S'~3~
GC227
-40-
triazole and 0.05 g of 4-dimethylaminopyridine
were dissolved in 20 ml of dimethylformamide and a
solution of 1.06 g of dicyclohexylcarbodiimide in
5 ml of dimethylformamide was added. After
stirring for 15 minutes at room temperature, a
solution of 0.94 g Girard Reagent P in 20 ml of
dimethylformamide was added. After 3 hours of
stirring, formed dicyclohexylurea was filtered off
and the dimethylformamide of the filtrate was
distilled off. Addition of 50 ml of tetrahydro-
furan to the residue yielded 1.8 g of crude
product, which was purified by column chromato-
graphy on HP-20 using water as an eluent, yielding
0.63 g of product, melting point 235C.
ExamDle 27
[3S-[3a(Z),4~]]-3-1[(2-Amino-4-thiazolyl)[[2-
[2-[3,4-bis(hydroxy)benzoyl]hydrazino]-1,1-
dimethyl-2-oxoethoxy]imino]acetyl]amino]-4-
methyl-2-oxo-1-azetidinesulfonic acid,
mono~otassium salt
A) 1-13,4-bis(Acetyloxy)benzoyl]-2-[(t-butyloxy)-
carbonvl1hvdrazine
~(t-Butyloxy)carbonyl]hydrazine (1.32 g) and
1.01 g of triethylamine were dissolved in lS ml o
dichloromethane. At 0C, 2.5 g of 3,4-(diacetyl-
oxy)benzoyl chloride dissolved in 10 ml of dichloro-
methane was dropped in with stirring. After 2
hours, the dichloromethane was twice extracted
with ice water (50 ml portions). The organic
layer was dried over sodium sulfate and the
~ solvent evaporated, yielding 2.9 g of the title
-~ compound as a white crystalline material, melting
~ 35 point 78-82C.
. ... . .. . , . . : :
. . . . . . .
, . - .. . ... .. . . . .
. . . ~ ,, . . ~ . . .- , :
~3S'~35~
GC227
-41-
B) 1-[3,4-bis(Hydroxy)benzoyl]-2-[(t-butyloxy)-
carbonYl hYdrazine
1-[3,4-bis(Acetyloxy)benzoyl]-2-[(t-butyl-
oxy)carbonyl]hydrazine (2.6 g) was dissolved in
50 ml of methanol and 5 ml of water. At oC~
ammonia (gas) was bubbled into the solution for 10
minutes. After standing overnight in a
refrigerator, the solution was evaporated, and to
the remaining oil, 10 ml of dichloromethane was
added. This yielded 1.5 g of the title compound,
melting point 148C.
C) [3,4-bis(Acetyloxy)benzoyl]hydrazine,
trifluoroacetate salt
1-[3,4-bis(~ydroxy)benzoyl]-2-[(t-butyloxy)-
carbonyl hydrazine (1.4 gj was stirred in 10 ml of
trifluoroacetic acid for 30 minuteg at 5C. The
title compound was obtained as a precipitate,
which was isolated and washed three times with
10 ml of portions of diethyl ester (anhydrous);
melting point 173C.
D) [35-t3a(Z),4~]]-3-t[(2-Amino-4-thiazolyl)[t2-
t2-t3,4-bis(hydroxy)benzoyl}hydrazino]-1,1-dimethyl-
2-oxoethoxy]imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinesulfonic aci~ opotassium salt __
t3,4-bis(Acetyloxy)benzoyl]hydrazine,
trifluoroacetate salt was coupled with [3S-[3a(Z),
4~]]-3-[[(2-amino-4-thiazolyl)[(l-carboxy-1-
methylethoxy)imino]acetyl]amino]-4-methyl-2-
oxo-l-azetidinesulfonic acid using tributylamine,
N-hydroxybenzotriazole, dimethylaminopyridine,
dicyclohexylcarbodiimide, and potassium perfluoro-
butanesulfonate, using the procedure described in
12~35954
GC227
-42-
previous examples, yielding the title compound,
melting point 247C (dec).
H-NMR (200 MHz, DMSO-d6) ~ = 1.38 (d, 3H); 1.42
(d, 6H); 3.35 (s, broad, 2H (H0-)); 3.69 (m, lH);
4.05 (dd, lH); 6.75 (d, lH); 7.25 (m, 3H); 9.32
(d+s, 2H); 10.01 (s, broad, 1~); ppm.
IR(KBr): 1780cm 1 (~-lactam carbonyl)
Exam~le 28
t3S-t3a(Z),4~]]-3-tt(2-Amino-4-thiazolyl)[[2-
t2-t3,4-bis(hydroxy)benzoyl]hydrazino]-1,1-
dimethyl-2-oxoethoxy]imino]acetyl]amino]-4-
tt(aminocarbonyl)methyl]thio]-2-oxo-1-azetidine-
sulfonic acid, mono~otassium salt
Following the procedure of example 27, but
substituting [3S-t3a(Z),4~]~-3-tt(2-amino-4-
thiazolyl)[(l-carboxy-l-methylethoxy)imino]-
acetyl]amino]-4-[t(aminocarbonyl)methyl]thio-
2-oxo-1-azetidinesulfonic acid for [3S-
[3a(Z),4~]]-3-[[(2-amino-4-thiazolyl)t(l-
carboxy-l-methylethoxy)imino]acetyl]amino]-4-
methyl-2-oxo-1-azetidinesulfonic acid, yielded the
title compound.
Additional compounds falling within the
scope of this invention are set forth below.
:::
::
~: :
1285954
GC227
-43-
I Rl R2 R3 R4
0- ~ - N - N-R5
6_ _R7
-~-NH ~ N-S03H ,
2N d/
Formula I
Rl/R2 R3 R4/R5 R6 R7
H/H H R -~-NH2 H CH3
-C-NH2 CH3 H
H -~-NH2 c~3 H
~ H -q-N~2 ~ CH3
CH3/H H H -C-~-OH H CH3
CH3/~ -NH-N~2 ~ c~3
CH3/CH3 H H -~-N~-~-NH2 H CH3
CH3/C~3 H H -~-NH2 -S-CH2-~C-NH2 H
o
3/CH3 H H -~-NH-CH2- -OH H CH3
O O
CH3/CH3 H H -C-CH2-~-NH2 H CH3
O O
CH3/CH3 H H -~-CH2-C-OH H CH3
O O
Il l
CH3/CH3 H H -C-CH2-NH- ~-CH3 H CH3
:
~.. .. . . .
. . . . . . . .
,` ~ ' .- . , ~ ., . ~, '
: ' . '. ' :
~Z85~S~
_44_ GC227
Rl/R2 R3 R4/R5 6 7
o
CH3/CH3 H H -C-NH-SO -CH H CH3
Ol O
CH3/CH3 H H -C-NH-NH-C-NH2 H CH3
CH3/CH3 H H -c-NH-cH2-cH2-NH2 H CH3
OH
CH3/CH3 H H -C-CH2 ~ H H CH3
IH
H/H H H -C ~ H CH3 H
~H
CH3/CH3 H H -C-CH3-O ~ OH H CH3
0~
CH3/CH3 H H -C-NH-NH-~ ~ H H CH3
~OR
CH3/CH3 H H ~ OH H CH3
OH
CR3/C~3 H =CH ~ H H C~3
O~H
H =CH ~ OH H CH3
H
CH3/CH3 H =C ~ OH H3 H
~H
CH3/CH3 H =CH ~ OH -S-CH2-C-NH2 H
,
- , ........ . .. . , ........ :
.':'.'.: - . ' - . ' . .' - .'. .. , : ,. . -