Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
7~9
--1--
MEMBRANE UNIT FOR ELECTROLYTIC CELL
This invention relates to membranes for use
in electrolytic cells and, more particularly, to a
membrane unit which will resist tearing upon appli-
cation of a compressive force to a gasket bearing
surface of the membrane.
There are many well-known membranes for use
in electrolytic cells. For example, typical membranes
include the perfluorinated carboxylic or sulfonic
cation exchange membranes such as the Nafion~ membranes
manufactured by E. I. duPont de Nemours and Company or
the Flemion~ membranes manu~actured by Asahi Glass
Company, Ltd. These membranes are typically available
in sheet form and employed in filter press-type or flat
plate-type electrolytic cells having monopolar or
bipolar electrodes. Examples of bipolar, filter
press-type cells are described in U.S. Patent Nos.
4,111,779 and 4,103,742. These cells are used, for
example, to carry out electrolysis of an a~ueous alkali
metal halide to produce a halogen, for example chlorine,
and an alkali me-tal hydroxide such as sodium hydroxide.
Generally, the bipolar, filter press-type electrolytic
cell is composed of several bipolar unit cells aFranged
..
~,
31,365-F
ô'S~39
--2--
in series. One bipolar unit cell has an anode and
cathode compartment separated by a partition wall.
Typically, the anode and cathode are attached to
opposite sides of the parti-tion wall. The membrane is
usually interposed between two adjacent unit cells to
separate the anode compartment from the cathode com-
~ partment. A~plurality of anode and cathode frames areinstalled in a parallel fashion and a longitudinal
compressive clamping means is applied to the anode and
cathole frames with the membrane interposed between the
frames to form-the electrolytic cell in toto.
It is common practice to interpose a gasket
between the membrane and the anode or cathode frame to
provide the electrolytic cell with fluid-tight, i.e., a
liquid- and gas-tight seal to prevent leakage o~ elec-
trolyte between anode and cathode compartments or to
the atmosphere. It is important to have a complete
liguid- and gas-tight seal in electrolytic cells
because these cells typically operate under corrosive
environments. Generally, one side of the gasket is in
contact with the lateral face of an electrode frame and
the other side of the gasket is in contact with one
side of the membrane's peripheral surface.
Typical gasket materials include resilient
material such as rubber or an elastomer. Commercial
bipolar membrane electrolyzers generally use ethylene-
propylene (EPM) or ethylene-propylene-diene (EPDM) as
gasket material between the membrane and electrode
frames. These materials tend to deform and expand
outwardly as pressure is applied to the frames via the
frame members. As the gaskets deform outwardly, cer-
tain membranes which are in contact with the gaskets
31,365-F -2-
.
- . ~
:
' ' ' .
.
~8~5~9
--3--
tend to stretch when they are pulled under the pressure
of the outwardly deforming gaskets. This stretching of
the membrane beneath the gaskets employed on adjacent
electrode frames can cause the membranes to break or
tear when attempting to compress the frames into a
fluid-tight cell. In addition, resilient yaskets
require a high compressive force to effect a seal which
increases the risk of breaking or tearing the membrane.
Any tears or breaks in the membranes may reduce
current efficiency during operation~ yreatly increasing
electrical current usage while reducing the electro-
lytic operating efficiency and/or electrolytic operating
efficiency can require costly shutdown of the entire
cell while the damaged membrane or membranes are
replaced.
The invention resides in an ion exchange
membrane unit comprising at least one layer of a ion
exchange membrane and at least one layer of a membrane
reinforcing material secured to at least one side of the
membrane around a gasket bearing peripheral surface of
the membrane, wherein said membrane has at least one
opening and the reinforcing material is heat sealed to
the membrane through the opening.
The invention also resides in an electrolytic
cell comprising an ion exchange membrane unit separating
- 30 at least two electrode compartments, said membrane unit
comprising at least one layer of a ion exchange membrane
and at least one layer of a membrane reinforcing
material secured to at least one side of the membrane
around a gasket-bearing peripheral surface of the
membrane, wherein said membrane has at least one ope~ing
31,365-F -3-
,, , ::,
. .
,
59~
--4--
and the reinEorcing material is heat sealed to the
membrane through the opening.
The invention further resides in a method of
sealing an electrolytic cell comprising the steps of
(a) interposing at least one gasket between at
least one electrode frame and an ion
exchange membrane in an electrolytic cell,
securing at least one layer of a
reinforcing material to at least one side
of the membrane around a gasket bearing
peripheral surface of the membrane,
wherein said membrane has at least one
opening and the reinforcing material is
heat sealed to the membrane through the
opening, and
(b) applying a compressive force to the cell.
~5
.
31,365-F -4-
',
. . .
,:
~Z~7~ 64693-370~
Although alternative embodiments of the present
invention are shown in the following Figures, the same reference
numbers are used in the drawings to describe identical elements.
Figure 1 is a perspective view of the membrane unit of
the present invention showing a membrane sheet haviny a
reinforcement material along the periphery of the sheet.
Figure 2 is a perspective view of another embodiment of
the present invention showing a membrane having a plurality of
openings and a reinforcing material along the periphery of the
sheet.
Figure 3 is a cross-sectional view taken along line 3-3
of Figure 1.
Figure 4 is a cross-sectional view of an alternate
embodiment of the present invention showing a membrane having a
reinforcement material on one planar peripheral surface of the
membrane sheet.
Figure 5 is a cross-sectional view taken along line 5-5
of Figure 2.
``:
` - 4a -
', ' , ' ~ ~ ' ' ' ' ' '
.
75~9
--5--
Figure 6 is a sectional view showing a por
tion of an electrolytic cell series assembly including
the membrane unit of Figure 1.
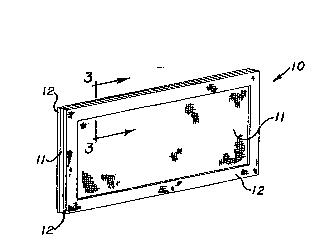
With reference to Figure 1, a rectangular
sheet ll made of a membrane material is shown with a
layer of a reinf-orcing material 12 attached, bonded or
otherwise adhered to a peripheral portion of the mem-
` brane on opposite planar surfac`es thereof.~ Figure 3
more clearly illustrates the reinforcing material 12 as
a strip secured to both sides of the membrane 11 andonly along a gasket-bearing surface of the membrane.
Figure 4 shows the reinforcing material 12 applied to
only one planar sur~ace of the membrane and only along
the peripheral, gasket-bearing surface of the membrane.
"Gasket-bearing surface" is defined as that portion of
the periphery of the membrane sheet which is subject to
compression forces in order to effect a seal at the
periphery of an electrode frame of an electrolyzer. In
Figure 1, the reinforcing material 12 has a picture-frame
shape. It is to be understood, however, that the
membrane unit or structure of this invention is not
limited to a rectangular sheet but can be circular or
of some other desired shape.
The membrane 11 is made of a material having
ion exchange properties. Such membrane is substantially
impervious to the hydrodynamic flow of the electrolyte
and the passage of gas products produced during el~c-
trolysis. Suitable are cation exchange membranes such
as those composed of fluorocarbon polymers having a
plurality of pendant sulfonic acid groups or carboxylic
acid groups or mixtures of sulfonic and carboxylic-acid
gr~ups. The terms "sulfonic acid groups" and "car-
boxylic acld groups" are meant to include salts of
31,365-F -5-
..
. .
. . . .
.
.~2~
--6--
sulfonic acid or salts of carboxylic acid which are
suitably converted to or from the acid group by pro-
cesses such as hydrolysis. An example of a carboxylic
acid type cation exchange membrane is commercially
available from Asahi Glass Company under the trademark
Flemion~. Another example of a suitable membrane
having cation exchange properties is a perfluoro-
sulfonic acid membrane sold commercially by E. I.
duPont de Nemours and Company under the trademark
Nafion~.
The reinforcing material 12 can be made of
any material suitable for strengthening the gasket
bearing surface area of the membrane 11. The rein-
forcing material 12 can be of the same or different
material as the membrane. Preferably the reinforcing
material 12 should have a heavier scrim than that of
the membrane material. Both the membrane and the
reinforcing material should be made of a corrosion-
resistant, non-contaminating material which is stable
upon contact with electrolyte media present in an
electrolytic cell. Suitable ma~erials which can be
employed in accordance with this in~ention include, but
are not limited ~o, the following: fluorine~containing
polymers such as polytetrafluoroethylene (PTFE), fluo-
rinated ethylene propylene copolymer (FEP) and per-
fluoroalkoxy resin (PFA); polysulfide polymers, poly-
vinyl chloride, fluoroelastomers such as Viton~, a
trademark of E. I. duPont de Nemours and Company, and
chlorosulfonated polyethylenes such as Hypalon~, a
trademark of E. I. duPont de Nemours and Company.
The reinforcing material 12 can be attached
or otherwise secured to the membrane 11 by any well
known method in the art, for example, bonding with an
31,365-F -6~
~:
.
.
~Z~3'75j9~1
adhesive, heat sealing, or ultrasonic sealing. It is
preferred to heat seal the reinforcing material to the
membrane.
In Figures 2 and 5, the membrane 11 contains
perforations or openings 13 along its periphery or
gasket-bearing surface. The reinforcing material 1~ is
secured to the gasket bearing surface and covers the
openings 13 on both sides o~ the membrane 11. The
membrane having such openings 13 allows the reinforcing
ma~erial 12 on one side of the membrane to form a bond
through the membrane to the reinforcing material 12 on
the opposite side through the openings 13. This is
particularly useful when bonding a reinforcing material
which is di~ficult to attach to the membrane material.
Generally, when the reinforcing material and the mem-
brane material are made of dissimilar materials, open-
ing~13 should be provided in the peripheral surface of
the membrane to provide additional securement of the
reinforcing material to the membrane.
Referring to Figure 6, an electrolysis cell
assembly is shown wherein a membrane unit generally
designated by reference number lO, comprising a mem-
brane 11 and a reinforcing material 12 attached to both
sides of the membrane 11, is interposed between two
electrode frame units 14. A gasket 18 may be inter-
posed between the membrane unit lO and an electrode
frame 14. It- is also within the scope of the invention
to interpose a gasket 18 on both sides of the membrane
unit 10 and two adjacent electrode frames 14. Any
gasket used in an electrolytic cell of the filter press
type may be used. The gasket should be made of a
corrosion resistant material, shduld have a high volume
.
31,365-F -7-
. .
~2~
resistivity and ~ood sealability after it has been
compressed. Suitable materials for the gasket 14 may
be, for example, EPDM, a chlorinated polyethylene
(CPE), a polytetrafluoroethylene such as Teflon~,
manufactured by E. I. duPont de Nemours and Company,
and reinforced asbestos. An anode 15 and a cathode 16
are electrically connected with connectors 17 through
the electrode frame 14. The electrolysis assembly
abovè is typical of bipolar electrolytic cells of the - ~ -
filter press type such as described in U.S. Patent Nos.
4,111,779 and 4,108,742. Any cell of the filter pr~ss
type may be used in the present invention.
In order to effect sealing of the periphery
of the electrode frame 14, the membrane unit 10 and a
gasket 18 are interposed between two adjacent electrode
frames 14 and a compressive force is app~ied to the
cell assembly. The compressive force may be applied by
any means known to those skilled in the art, for example,
by clamping the frames together or by using a hydraulic
ram. Preferably a hydraulic ram is used to squeeze the
electrode frames, gaskets and membranes together. The
actual compressive force applied will be dictated by
the type of material used for the gasket.
The invention will be illustrated further in
~5 the examples which follow.
Example 1
A 10 cm by 10 cm test sample o Nafion~ 901
membrane, obtained from the duPont Company of Wilmington,
Delaware, was reinforced by heat sealing strips of PFA
fluoroplastic film around the edges on both sides of the
- membrane. Any heat-sealing technique known in the art
.
31,365-F -8-
, . .
: - .
.
. - . : : .
- . '.: : ~ '
.
- 9 -
may be used. In this instance, the heat sealing was
performed by the EGC Corporation of Houston, Texas,
under contract to The Dow Chemical Company. The film
thickness used on the ca-thode side of the mer~rane was
15 mils thick while the film -thickness used on the
anode side of the membrane was 5 mils thick. The
actual design used is the design shown in Figures l and
3.
A laboratory electrolytic cell was used for
testing the test samples. The cell consisted of an
anode compartment frame and a cathode compartment
frame. The anode compartment frame was made of
titanium having the titanium surfac~e located under
the gasket area coated with a ruthenium dioxide
coating to avoid possible crevice corrosion problems.
The cathode compartment frame of the cell was made of
an acrylic polymer. The anode of the cell was made of
titanium with a ruthenium dioxide coating and -the
cathode was a nickel cathode.
The gasket usedj was a 6.35 mm thick gasket
made of EPDM rubber purchased from the Prince Rubber &
Plastics Co., Inc. of Buffalo, New York. The gaskets
were cut from single EPDM rubber sheets to form a
picture-frame shape with dimensions of 9.5 cm outside-
-to-outside and 7.62 cm inside-to-inside. Thus, the
width of the gasket surface was 9.5 mm. The total
gasket area was 32.65 cm2. The gasket was used on both ~ -
sides of the membrane and contact loading was distri-
buted over the reinforced surface.
Ten 9.5 mm diameter bolts were torqued to 12
ft-lbs (16.3 Joules) force to press together the anode
31,365-F -9-
:'. , ,. ,, ' .' : ' ,
, ~
.- " :: , :. , ' . . .
- : :
--10--
and cathode compartments, the mernbrane and gaskPts
resulting in a total force of 8,626 kg from the bolt
loading. The force exerted on the membrane under the
gaskets was equivalent to 25,856 kPa. The force used on
this test sample was ten times greater than the force
used on the test sample described in the Comparative
Example A, below.
~ The cell of this e~ample was operated to
produce 32 weight percent caustic while contro'ling the
anolyte salt at 200 grams per liter sodium chloride
concentration. The cell temperature was maintained at
90C with ampere loading controlled at 0.31 amp/cm2 of
projected anode area current density. The test was
conducted for 210 days and during this period the
caustic current efficiency averaged 95 percent with an
average cell voltage of 3.5. The cell operated without
leaks and performed without complications.
Visual inspection of the membrane after
dismantling the cell showed the membrane to be in
excellent condition with no tears or breaks in the
gasket contact and loading area. Thus, the reinforcing
concept of the invention protected the membrane from
damage and showed a successful improvement over the
membrane used in the Comparative E~ample A, below.
Comparative Example A
A 249 cm by 127 cm test sample of Nafion~ 324
membrane, obtained from the duPont Company of Wilmington,
Delaware was used in this test. The gaskek surfaces of
the membrane were not reinforced.
31,365 F -10-
.
`.`'"' ' ` '' ... ~. ". `' ;,`'.`- ' :
-
,
' ~ .' ~ . .
~z~
~ 64693-3700
The electrolytic cell used in this test is of a
type well known in the industry as a bipolar flat
plate-type cell having a nominal size of 1.22 met. by
3.05 met. The cell contained an anode of titanium with
a ruthenium oxide coating and a cathode of steel.
The gasket used was a 4.76 mm thick gasket made
of EPDM rubber purchased from the Prince Rubber and
Plastics Co., Inc., of Bu~falo, New York.
Speci~ications for the EPDM include "EPDM ~or Chlor-
Alkali Service, Prince #6962." The gaskets were cut
from single EPDM rubber sheets to form a picture-frame
shape with dimensions of 2.47 meter outside-to-outside
and 2.37 meter inside-to-inside in the long direction
and 1.25 meter outside-to-outside and 1.15 meter
inside-to-inside in the short direction. Thus, the
width of the gasket surface was 5.1 cm. The total
gasket area was 3,676 cm2. The gasket was used on both
sides of the membrane and contact loading was
distributed over the reinforoed surface.
A hydraulic cylinder in a filter press
arrangement was used to press together the cell units.
The total force resulting from the action o~ the
hydraulic press was 78,152 kg. The force exerted on
the membrane was equivalent to 2,080 kPa.
The cells were operated to produce from 12 to
16 weight percent caustic while controlling the anolyte
salt at 200 gram~ per liter sodium chloride
concentration. The cell temperature was maintained at
90-C with D~C. current controlled at 10.0 kiloamperes.
31,365-F
.
., ' ., , . ': ', .' :, ; ;
', .'',' . ., ~.'" '. , . , . ~,' .
., . , , : . .. . ..
. : : . ~ . " ~
.~2~5~3~
-12-
Thus, the ampere loading was 0.31 amperes per square
cm of projected anode area current density. The test
was conducted for 199 days and during this period, the
caustic current efficiency averaged 82-84 percent which
was 4 percent below the expected caus-tic current efficiency
for Nafion~ 324.
Visual inspection of the membrane after dis-
~ mantling the cell showed the me~brane to have severe
damage in the areas beneath the gaskets and in the area
next to the gaskets. The gasket loading forces had
stretched and cracked the membrane so severely to
render the overall cell performance unsatisfactory.
31,365-F -12-
,, ~. .