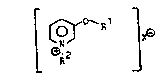Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
12894~9
Oxypyridinium salts and a~ents for_ combating microbes
.
The present invention relates to a microbicidal
composition containing 0-substituted 3-oxypyridinium salts
for combating microbes, and a method of combatin~ mi-
crobes.
More particularly, the present invention pro-
poses a microbicidal composition for combating algae,
bacteria or fungi of the non-phytopathogenic type, out-
side the crop protection sector. This composition which
contains a toxic amount of an 0-substituted 3-oxypyridinium
salt of the formula:
[ ~~Rl ~
where R1 is an alkyl, alkenyl or alkynyl radical which is
unsubstituted or substituted by at least one alkoxy,
cycloalkoxy, phenylalkoxy or halogen, and has a total of 8
to 20 carbon atoms,
R2 i8 unsubstituted or halogen-substituted alkyl,
alkenyl or alkynyl of up to 8 carbon atoms, or one of the
groups
-(CH2)1 2~Ar, -(CH2)2 50CH21CHCH2Ar or -(CH2)2 6-0-Ar
R
where Ar is l-naphthyl, 2-naphthyl, biphenyl or phenyl,
phenyl being unsubstituted or substituted by F, Cl, Br,
NO2~ 3CF3, CN~ Cl-13~alkYl~ C2_4-alkenyl or Cl 2-alkoxy
and R is H, Cl-C2-alkyl, C3-C5-alkenyl or Cl-C5-alkoxy
and
X~ is F0, C10, B~0 or I0 or an anion which is the
equivalent of an acid, have proved to have a good
microbicidal action.
,'~i ~1
,
,
~289469
- la -
Examples of meanings for R are branched or
straight-chain octyl, decyl, undecyl, dodecyl, tridecyl,
tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl,
P 2 10alkYl-O-cs lgalkyl~ -0-cyclohexyl or
-0-C3-C5-alkylphenyl with a total of 10 to 20 carbon at-
oms. 2
Examples of meanings for R are Cl-C4-alkyl,
methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl,
allyl, 2-chloropropenyl, 2-bromopropenyl, 3-chloro-
propenyl, but-2-en-1-yl, 2-methylpropenyl, 3-chlorobut-
2-en-1-yl, propargyl or the radicals -(CH2)1 2-Ar or
-(CH2)2 6-OAr, Ar denoting unsubstituted phenyl or phenyl
containing one to three halogen substituents, such as
4-fluorophenyl, 3- or 4-chlorophenyl,4-bromophenyl,
3,4-dichlorophenyl, 2,4-dichlorophenyl, 2,6-dichloro-
phenyl, and 2,4,6-dichlorophenyl.
/
..
. ;.
~289~69
~ASF Aktiengesellschaft 2 O.Z. 0050/38~12
Examples of meanings for ~ are Cl~, ~r~ or an anion which is one
equivalent of an acid, such as phenylsulfonic acid, p-methylphenyl-
sulfonic acid or one equivalent of the sulfuric acid anion.
5 The 0-substituted 3-oxypyridinium salts of the formula I are obtained by
reacting a compound of the formula
R2X II,
1O where R2 and X have the above meanings, With an 0-substituted
3-oxypyridine derivative of the formula
~0--R1
~NJ III,
where R1 has the above meanings.
The reaction is carried out in bulk or if desired in the presence of an15 inert solvent or diluont at from 20 to 150, pref0rably 50 to 150, CC. The
starting material of the formula III is advantageously reacted with up to
10 times the molar amount Ibased on III) of the alkylating agent of the
40rmula II.
20 Examples of preferred inert solvents or diluents are aliphatic or aromatic
hydrocarbons or halohydrocarbons, such as pentane, cyclohexane, heptane,
benzene, toluene, chlorobenzene and dichloro-benzenes; aliphatic ketones
such as acetonQ, methyl ethyl ketone, diethyl ketone and cyclopentanone;
ethers such as diethyl ether, methyl tert-butyl ether, dimethoxyethane,
25 tetrahydrofuran and dioxane; esters such as ethyl acetate; nitriles SUCh
as acetonitrile; amides such as dimethylformamide, dimethylacetamide and
N-methyl-pyrrolidone; and mixtures of these solvents.
Most of the starting materials of the formulae II and III are known, and
30 some are commercially available; tho~e that are not may be produced
analogously to examples given in the literature Ifor III, for example, see
Chem. ~er., 116, 239~, 1993).
When R3 is not hydrogen, the novel 0-substituted 3-oxypyridinium salts may
35 pOS5QSS a chiral carbon atom at R1 and/or R2. The optically pure
enantiomers and diastereomers may be obtained by conventional methods. The
present invention encompasses both these compounds in pure form and
mixtur0s thereof. Not only the pure enantiomers and homogeneous
diastereomers, but also the mixtures thereof which are usually obtained on
40 synth~sis are effective.
1289469
BASF Aktiengesellschaft 3 O.Z. 0050/3~l2
The example which follows illustrates the preparation of the novel
compounds:
PreDaration examDle
~OC 14H29
CH2-C6H5
a) Starting material
~ ~ C14N29Br ~ ~ C14N29
100 9 11.U~ moles~ of 3-hydroxypyridine in 500 ml of dimethylsulfoxide
lDMSO) is stirred at room temperature (20C) with 87.~ 9 ll.56 moles) of
KOH powder under a nitrog-n blanket. After about 30 minutes, 360 9 tl.3
30 moles~ of tetradecyl bromide is added dropwise and the mixture is stirred
for a further ~ hours at room temperature. The reaction mixture is then
poured into 1 liter of water and extracted three times, each time with 500
ml of methylene chloride. The combined extracts are washed twice with 1
liter of water, dried with Na2SO~ and concentrated. Filtration over silica
35 gel with n-pentane and methyl chloride gives 1~ 9 of product A tm.p.
36C).
b~ Pyridinium salt formation
BrCH2-~ > ~4H29
A a
12~39469
OASF Aktiengesellschaft ~ O.Z. 0050/38~12
11.5 9 (0.0~ mole) of 3-tetradecyloxypyridine A is stirred with 5 ml (0.0
mole) of benzyl bromide for 20 minutes at 100 to 120C. After the mixture
has cooled to room temperature it is suspended in pentane and the
suspension is suction filtered. The filtrate is washed with pentane and
5 dried: 16.8 9 of pyridinium salt 0, m.p. 129C ~compound no. 23).
The compounds listed in the table below may be obtained analogously:
1289469
. Z. 0050/38412 - 5 - B~SF ~tiengesellschaft
c~
_. ,~ ~o
I cr- I ~ ~ ~ ~o u~
~D X Cr~
_
:~ `D `D
X
~ ~ Y T~ :~
C~l y y y
o o
~: 3 ~ Y 'i' ~, Y, ~ ~ ~ Y
YYY~YYY~ YY
- lo o - o ~ ~ ~ ~ ~ ~ ~ ~ - - - -
l yyy l yyyyyyy l yy
1~ ~ Cl C ~ C: ~ Ct R 1: C ~
,. Z_ _ _ _ __
'
,
~ ,.
.
128~469
~ 7. 0050/~8412 - 6 - B~SF ~ktiengesellschaft
c~ u~
C~` ~`3 u~
~ J ~ x
_ _ _
_~
~: ~
~ ~ ~ c~
Y Y Y l
I q y ~ y :~ x ~ ~
~ I I I o o o Sl) ~
Y~ 3~ , rr 7~S'S'S'Y
~ y ~
~ a I I I ~ I ~ c ~
.
.~
~O 1~ X ~ o -- ~ ~ ~ U~ o -- ~ ~ ~r
.
:
1289469
7. 0050/3841~ - 7 - B~SF Alctiengesell8chaft
o o o
._ ~
I ~ ~
o
~o ,~
:~: o~ U~
x ~ ~ ~ ~ ~ ~ ~ ~ m c~ a~
~ Y
C~ o o C~
C~ Y Y Y Y Ci~ Y Y ~ S~
v v 3
~ _ _ X _ N C~
~ ~v ~
- ~ y y y ~ y y y y y y y y y
~ T ~ ~ ~ T ~ ,
c ~ c c ~ a c a ~ ~ ~ ~ :C ~ ~ ~ ~ ~ ~ 5 ~
' ' r ' r ' Y l l l
u~ ~ t~ X O~ O ~ o ~
Z .
1289~6~
7~ 0050/3841~ - 8 - B~SF A~tiengesellschaft
c~
O
,~ u~
o _,
~:
., ~ c~
~ u~
c~ T
C~ O ~ U~ C~ O ~ C~ C`J ~ C~
~ ~ ~ 3 c~ c~ 3 ~ a a Y Y Y Y a a
C~ C~l ~ ~ C~l ~
Y ~ y y ~ y3
U~
:r ~ ~ c~
~o
~ v c~
~, ~, v v ~ a ~
c~ Y T c~ 3 a v ~ a 3
x 3:
T I I T I 1 ~ ~ c~l ~ ~ ~ ~ c~ ~ ~ ~ c~
^ ^ ^ ^ c~ v v -c~ u v
~ a a v a ~a a e~ ~
V ~ ~ V ~ _ _ ~ ~ ~ _ _ _ _ _ _ ~ C`~ ~ ~ e~
^ ^ ^ ^ c~ v c~ c~ v v c~ v c~ v v c~ c~ v v v c`
v v v v v v v v v r
3~ v v v v v v ^ ^ ^ ~ ^ ^
T ~ v
O O O O O O
I I I I I I O O
~ c~ v v c~ c~ 8 ~ v ~7 c~ ~ ~ ~ ~ ~ ~ ~ ~ ~
_ _ _, _ _ _ _ _ _ _ _ _ _ _ _ _ ~ ,~, ~, c~ ~ ~ ~ ,.~,
c~ v v v c~ c~ v v
l l l l l l l l
`D r~ O -- C~ X ~ O _ C~l ~ ~ u~ ~O t~ CO O~
:,
, , .
1289~69
. 0050/38412 - 9 - B~SF ~tiengesellschaft
~D ~ ~ O
o~ ~ o
æ _ _
.. .
C~l
q
~3 ~ Y ~ 2
~ C`l ~ ~ ~ ~ ~ ~ C~
2 2 2 2 ~ 3~ :~ 2 :~
Y ~ Y ~ Y Y
~ o~
2 :~ ^
. . 2
~ t~ ~ ~D `D `O `O
Y Y t~ ~ ~ ~ 2 :~
~ ~ ~ V V t~
~ ~ y y y y y Y ~
Y, C~
u~ 2 ~ 2 --'
~ ~ .......... 2 ~ 2 2
:C 2 ~
_ ~ ' r ' rr~
IY I I ~ ~t ~ ~ ~ 2 ~ 2 2
rr ' Y YY ~Y
o o 7 , , ~ , o, 7 o, ol ~
C~ U ~ ~ ~ 2 2 5~ , 2 ~
ll llllllll ll
O -- ~ ~ ~ U~ `D 1~ 0~ O~ O --
z x c~ oD X co X X o~ a~
12~39469
-- 10 --
Characteristic lH-NMR-data
Compound Solvent Chemical shift in ppm
no.
46 d6-DMSO s 5.9 [24~; broad dd 8.15 [lH~;
broad d 8.30 ~14~, d 8.90 ~1HJ;
s 9.25 L1H~
69 d6-DMSO s 5.92 ~2H~, dd 8.10 [lH~;
broad d 8.28 ~lHl; d 8.92 [lH~;
s 9-28 rlH
87 d6-DMSO s 4.35 ~3HJ; dd 8.05 [lH¦;
broad d 8.20 L1HJ; d 8.62 ~lH¦;
s 8.92 [lH~
The compounds of formula I and their use as
fungicides in the crop protector sector exclusively are
claimed per se in copending Canadian patent application
no. 512,548 filed on June 26, 1986.
In accordance with the invention, it has how-
ever been found and proved that the compounds of formula
I are also suitable for combating algae, bacteria and
fungi outside the crop protection sector, for example
for disinfecting water in air-humidifying units, cooling-
water circulation systems, water injection systems and
for oilfields, swimming baths, laundries, and in prepara-
tions used for disinfection in the food sector, dairies,
breweries, hospitals, etc. The treatment of raw hides
and leather is also worthy of mention.
- ~, ' ' -
1289469
-- 10~ --
The microbicidal agents generally contain from
0.1 to 95, and preferably from 0.5 to 90, wt~ of active
ingredie t.,
/
':'''~'
..........
~X894~9
BASF Aktiengesellschaft ~ . 0~5C 3
The compounds according to the invention may be con-
verted into the usual formulations, e.g. solutions, emul-
sions, suspensions, dusts, powders, pastes and granules.
The application forms depend entlrely on the purpose for
05 which the agents are belng used; at all events they must
ensure a fine and uniform distributlon of the active
ingredients. The formulations are prepared in conventional
manner, e.g., by extending the active ingredlent with
solvents and/or carriers, if desired using emulsifiers and
dispersants. Where water is used as diluent, other organlc
solvents may also be employed as auxlliary solvents. Sult-
able compounds for preparlng such formulatlons are sol-
vents such as aromatlcs (e.g., xylene, benzene),
chlorlnated aromatlcs (e.g., chlorobenzenes), paraffins
(e.g., petroleum fractlons), alcohols (e.g., methanol,
butanol), amlnes (e.g., ethanolamlne, dlmethylformamlde),
and water; carrlers such as natural rock flours (e.g.,
kaollns, dlatomaceous earth, talc, chalk) and synthetic
rock flours (e.g., hlghly dlsperse slllclc acld, 8111-
cates); emulslflers such as nonlonlc and anionlc emulsify-
lng agents (e.g. polyoxyethylene-fatty alcohol ethers,
alkyl sulfonates and aryl sulfonates); and dlspersants
such as llgnln, sulflte waste llquors and methyl
cellulose.
12 ~9 ~ 9
BAS~ AXtiengesellscha~ 12 - J-Z` 0050 3Q! _
The active lngredients per se are low-foam biocides. A
significant increase in the effectiveness of biocidal
preparations containing these compounds is achieved by
adding tri-C6_12-alkylmethylammonium salts, preferably in
05 amounts of from 20 to 40 wt%, based on the weight of
compounds of the general formula I.
The compounds according to the invention whlch are
contalned in such preparatlons are effectlve agalnst a
large number o~ mlcroorganlsms, for example:
Staphylococcus aureus, Escherichia coll, Klebsielle
pneumoniae, Citrobacter freundli, Proteus vulgaris,
Pseudomonas aeruglnosa, Desulfovibrlo desulfuricans,
Streptovertlcllllum rubrlretlculi, Asperglllus nlger,
Asperglllus verslcolor, Penlcllllum funlculosum,
Penlcllllum expansum, Penicllllum glaucum, Paecllomyces
varlotll, Trlchoderma vlrlde, Chaeto~lum globosum, Candlda
alblcans, Trlchophyton mentagrophytes, Geotrlchum
candldans, Monllla sltophlla, Scenedesmus quadrlcauda,
Chlorella vulgarls, Nostoc muscorlum, Osclllatorla llmosa
und Anabaena constrlcta.
Usual use concentrations are 0.01 to 2% of active
lngredlent, based on the welght of the material to be
protected; when the actlve ingredlents are used for
treatlng water or in the productlon of oil, ln swlmmlng
baths, coollng towers, alr humldlfylng unlts or ln the
paper lndustry, amounts o~ 5 to 250 ppm are sufflclent.
Ready-to-use dlslnfectant solutlons contaln, for example,
from 0.5 to 10 wt% actlve ingredlent. The actlve
lngredlents may also be mlxed wlth other, prlor art,
mlcroblcldes. In many cases a synerglstlc effect is
produced.
~2~39~i9
BASF .~tiengesellscha~t l` ~ o~33'~'
Examples of such active ingredients are:
2-(thiocyanomethylthio)-benzthlazole
1-[2-(2~4-dichlorophenyl)-2-(2-propenyloxy)-ethyl]-lH-imidazole
05 2,4,5,6-tetrachloroisophthalodlnitrile
methylenebisthiocyanate
tributyltin oxide, naphthenate, benzoate, salicylate
mercaptobenzthlazole
1,2-~enzisothiazolone and lts alkall metal salts
alkall metal compounds of N'-hydroxy-N-cyclohexyl-dlazenium oxlde
2-(methoxy-carbonylamlno)-benzlmidazole
2-methyl-3-oxo-5-chlorthlazolin-3-one
trihydroxymethylnitromethane
glutardialdehyde
chloroacetamide
polyhexamethylene blsguanlde
5-chloro-2-methyl-4-isothiazolin-3-one + magnesium salts
3,5-dimethyltetrahydro-1,3,5-2H-thiadiazine-2-thione
hexahydrotriazine
N,N-methylolchloroacetamide
2-n-octyl-4-lsothiazolin-3-one
oxazolidines
bisoxazolidines
2,5-dihydro-2,5-dialkoxy-2,5-dialkyl~uranes
dlethyldodecylbenzylammoniumchloride
dimethyloctadecyldlmethylbenzylammonlum chlorlde
dimethyldidecylammonium chlorlde
dimethyldidodecylammonlum chloride
trimethyltetradecylammonlum chloride
benzyldimethylalkyl-(12_C18)-ammonium chloride
dichlorobenzyldimethyldodecylammonium chloride
cetylpyridinium chloride
cetylpyrldinium bromide
12~39469
~ASF Aktien~esellscha~t - 14 - ~.Z. I~050~3
cetyltrimethylammonium chloride
laurylpyridinium chloride
laurylpyridinium bisulfate
benzyldodecyldi(beta-oxyethyl)-ammonium chloride
O5 dodecylbenzyltrimethylammonium chloride
n-alkyldimethylbenzylammonium chloride
(alkyl radical: 40% C12, 50% C14, 10% C16)
lauryldimethylethylammonium ethylsulfate,
n-alkyldimethyl-(l-naphthylmethyl)-ammonium chloride
(alkyl radical: 98% C12, 2% C14)
cetyldimethylbenzylammonium chloride
lauryldimethylbenzylammonium chloride
lX89~6~
8ASF Aktiengesellschaft 15 O.Z. OOS0/38412
Use ExamDle 1
A~q~~l action on areen alqaQ
The active ingredients were added to a phosphate-rich nutrient solution5 suitable for favoring the propagation of the unicellular Chlorella
vulgaris genus of green algae in amounts of 10, 7.5, 5, 2.5 and 1 parts by
weight per million parts of nutrient solution. 100 ml of this nutrient
solutionlactive ingredient mixture, and of nutrient solution on its own
(control~, were introduced into 300 ml Erlenmeyer flasks. The nutrient
10 solution had been inoculated with a suspension of Chlorella vulgaris prior
to the addition of the active ingredient; the cell density was adjusted to
106 cells/ml of nutrient solution. The flasks were kept for 1~ days at
room temperature and with the admission of light, after which time the
action was assessed.
The results show that the algae were well combated by compounds 6, 7, 9,
11, 25, 27, 32, ~0, ~6, 50, 56, 6~, 69 and 79 at rates of 10, 7.5, 5, 2.5
and 1 parts by weight per million parts of nutrient solution.
20 Use Ex~mDle 2
Ynaicidal action on AsDeraillus nia~r
The active ingredients were added to a nutrient solution ideally suited
for promoting the growth of the fungus Aspergillus niger in amounts of
25 100, 75, 50, 25 and 10 parts by weight per million parts of nutrient
solution. 20 ml portions of the nutrient solution thus treated were placed
in 100 ml glass flasks and inoculated with 0.3 mg of Aspergillus fungus
spores. The flasks were heated for 120 hours at 36C, after which the
degree of fungus spread - predominantly on the surface of the nutrient
30 solution - was assessed.
The results show that compounds 6, 7, 9, 11, 13, 1~, 22, 23, 25, 27, 28,
30, 32 and ~0, at rates of 100, 75, 50, 25 and 10 parts per million parts
of nutrient solution, had a good fungicidal action (9OZ).
Use ExamDle 3
Action on TrichoDhvton mentag~oDhvtes and Candida albicans
The active ingredients were added to a nutrient solution suited for
40 promoting the growth of the fungi Trichophyton mentagrophytes and Candida
albicans in amounts of 100, 50, 25, 12, 6, 3 and 1.5 parts per million
parts of nutriQnt solution. 10 ml portions of this nutrient
solution/active ingredient mixture were introduced into sterile test tubes
and inoculated with one drop of a spore suspension containing 106 conidia
1~89~9
~ASF Aktiengesellschaft 16 O Z 0050/38412
or spores After 120 hours incubation, samples were taken f~om those
tubes showing no visible evidence of fungus growth, and transferred to a
fungus nutrient medium The dilution stage was ascertained at which no
more fungus growth occurred after transferral of a sample to the nutrient
5 medium
The results show that compounds ~, 5, 6, ?, 9, 11, 12, 13, 1~, 22, 23, 25,
27, 2~, 29, 30, 32, ~0, ~ 6, 50, 56, 6~, 69 and 87, when applied at
rates of 1 5 to 25 ppm, had a good fungicidal action
Use ExamDle ~
aa~tericid~L actio~ Qn Sta~h~LocoçÇUs 3l;ULLL~LiLh~ coli and
ProtevS~
15 The bacteria kill values were ascertained as follows
5 ml of a dilution of the agents in water was introduced into sterile test
tubes and 5 ml of a doubly concentrated nutrient broth w-s added and
mixed, The trial solutions contained 200, 100, 50, 25, t2, 6 and 3 parts
by weight per million parts of nutrient broth The tube contents were then
20 inoculated by dding one drop of 16-hour old broth cultures (1 10
dilution) of the bacteria Staphylococcu3 aureus, Escheria coli and Proteus
vulgaris, and the tubes were incubated for 2~ hours at 37C The dilution
stage was ascertained at which no more bacterial growth occurred after
transf~rral of a sample to the nutrient medium
The results show that compounds ~, 5, 6, 7, 11, 1~ and 23, at rates of
from 6 to 50 ppm, had a good fungicidal action
Use Ex~mDle 5
30 Action on the funai Paecilomvces variotii Aureobasidium Dullulans and
Geotrichum candidans
The active ingredients were added to a nutrient solution suited for
promoting the growth of the fungi Paecilomyces variotii, Aureobasidium
35 pullulans and Geotrichum candidans in amounts of 100, 50, 25, 12, 6, ~ and
1 5 parts by weight per million parts of nutrient solution 10 ml portions
of this nutrient solution/active ingredient mixture were introduced into
sterile test tubes and inoculated with one drop of a spore suspension
containing lo6 conidia or cells After 120 hours' incubation samples were
40 taken from those tubes showing no vi~ible evidence of fungus growth and
transferred to a fungus nutrient meJium The dilution stage was
ascertained at which no more fungus growth occurred after transferral of a
sample to the nutrient medium
12894~i~
6ASF Aktiengesellschaft 17 O.Z. 0050/38412
The results show that compounds 4, 5, 6, 7, 9, 11, 12, 13, 14. 22, 23, 25,
26, 2~, 29, 30, 32, 40, ~1, 46, 5fi and 76, when applied at rates of from
1.5 to 12 ppm, had a good fungicidal action.