Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1~9(~SS3
- 1 - O.Z. 0050/3?490
Removal of C0? and/or H S from gases
The present invention relates to a process for
removing C02 and/or HzS from gases by means of an
aqueous absorpt;on liquid contain;ng methyldiethanolamine.
It is disclosed, for example in German Patent
2,551,717, that C02 and/or H2S can be removed from
gases by means of aqueous solutions of alkanolamines.
Although the known process is very cost-effective, it
is not satisfactory in every case.
It is an object of the present invention to pro-
vide a process for removing C02 and/or HzS from ~ases
containing C02 and/or H2S by means of a-n aqueous alkanol-
am;ne-containing absorption liquid, the said process being
capable of being operated at lower energy costs and with
greater cost-effectiveness than the known processes.
We have found that this and other objects and
advantages are achieved, in accordance with the invention,
by a process for removing C02 and/or H2S from gases
containing C02 and/or H2S by means of an aqueous alkanol-
amine-containing absorption liquid, wherein the gas
containing C02 and/or H2S is treated in a first
absorption stage at from 40 to 100C with an aqueous
absorption liquid containing from 20 to 7ûX by weight
of methyldiethanolamine, the gas obtained at the top of
the first absorption stage is fed to a second absorption
stage in which, in order to remove further C02 and/or
H2S, it is treated at from 30 to 90C with an aqueous
absorption liquid which contains from 20 to 7ûX by ~eight
of methyldiethanolamine and has a lower content of C02
and or H2S than the absorption liquid fed to the f;rst
absorption stage, the treated gas ;s taken off at the
top of the second absorption stage, the aqueous absorption
liquid laden with C02 and/or H2S and obtained a~ the
bottom of the second absorption stage is fed to the top of
the first absorption stage, the aqueous absorption liquid
laden ~ith C~2 and/or HzS and obtained in the lower
part of the first absorption stage is regenerated
~290SS~
- 2 - o.z. no50/37490
by being let down in two or more flash stages, the final
flash stage being operated under reduced pressure which
is generated by means of a mechanical vacuum-producing
apparatus and a steam-jet ejector, which are connected
S in series, a stream of absorption liquid obtained at the
bottom of the final flash stage is recycled to the first
absorption stage, a further stream of absorpt~ion liquid
obtained at the bottom of the final and/or penult;mate
flash stage or stages ;s fed to a str;pp;ng zone for
further regeneration, and the regnerated absorption
l;qu;d obtained at the bottom of the str;pp;ng zone ;s
recycled to the second absorpt;on stage.
In an advantageous embod;ment of the process,
the water losses result;ng from removal of water ;n the
gas streams taken off at the top of the second absorpt;on
stage and/or from the flash stages and/or from the
str;pping zone are compensated by feed;ng ;n, at the
bottom of the penultimate flash stage, an amount of steam
corresponding to the water loss.
In another preferred embodiment of the process,
the steam-jet ejector is connected downstream of the
mechanical vacuum-producing apparatus. It may be advan-
tageous for the gas taken off at the top of the f;nal
flash stage to be fed ;n, together with the steam used
to operate the steam-jet ejector at the bottom of the
penult;mate flash stage.
The procedure according to the ;nvention gives
a regenerated absorption l;qu;d hav;ng a lower content
of C2 and/or H2S, so that ;t ;s possible to c;rculate
smaller amounts of absorpt;on l;qu;d. This results in
a corresponding saving ;n the energy requ;red for trans-
port;ng the absorption liquid. Th;s procedure perm;ts
the use of less compl;cated apparatus, thereby reducing
the capital costs. Another advantage of the process is
that the ~ater balance of the gas washer can be controlled
in a simple manner. Using th;s procedure, it is possible
to regulate not only the ~ater balance of the gas ~asher
1~0553
- 3 - O.Z. 0050/37490
but also its heat balance, so that any heat exchanger
present in the gas washer for regulating the heat balance
can be smaller or, if appropriate, can be omitted.
Examples of gases which can be treated us;ng the
novel process are coal gasification gases, coke oven
gases, natural gases and synthesis gases.
The gases contain in general from 1 to 90, prefer- -
ably from 2 to 90, and in particular from 5 to 60, mol%
of C02~ They can also contain H2S as a further acid
gas, or can contain H2S alone, for example in an amount
of from a few mol ppm, for example 1 mol ppm, to 50 mol%,
preferably from 10 mol ppm to 40mol%.
The solvent used for the novel process is an
aqueous absorption liquid containing from 20 to 70, pre-
ferably from 3û to 65, in particular from 40 to 60, Z byweight of 0ethyldiethanolamine. Advantageously, an
aqueous methyldiethanolam;ne solution is used, for example
an aqueous solution of technical grade methyldiethanol-
am;ne. In an advantageous embodiment of the process,
an aqueous methyLdiethanolamine solution is used which
additionally contains from 0.05 to 1, in particular from
û.1 to 0.8, in particular from 0.1 to 0.6, mole/l of
primary amine or alkanolamine, such as monoethanolamine,
or preferably secondary amine or alkanolamine, advantage-
ously methylmonoethanolam;ne, very particularly advant-
ageously piperazine.
The aqueous absorption liquid containing from
20 to 70X by weight of methyldiethanolamine may addi-
t;onally contain a physical solvent. Examples of suitable
phys;cal solvents are N-methylpyrrolidone, tetramethylene
sulfone, methanol and oligoethylene glycol dialkyl ethers,
such as oligoethylene glycol methyl isopropyl ether
(SEPASOLV MPE) or oligoethylene glycol dimethyl ether
(SELEXOL). The physc;al solvent is present in the absorp-
tion liquid in general in an amount of from 1 to 60,preferably from 10 to 50, in particular from ZO to 40,
X by weight.
* trademarks
.~ .
~29(~5~3
- 4 - O.Z. OOS0/37490
The novel process is carried out as follows: the
gas containing C02 and~or H2S is first treated with
the methyldiethanolamine-containing absorption liquid in
the first absorption stage, where the temperature is main-
tained at from 40 to 100C, preferably from S0 to
90C, in particular from 60 to 90C. Advantageously,
the gas being treated is fed to the lower part of the
first absorption stage, preferably to the lower third,
countercurrent to the absorption liquid, ~hich is advan-
tageously fed to the upper part of the first absorp-
tion stage, preferably to the upper third~ The gas ob-
tained at the top of the first absorption stage is fed
to a second absorption zone in which, in order to remove
further C02 andtor H2S, it is treated at from 30 to
90C, preferably from 40 to 80C, in particular from
50 to 80C, with the methyldiethanolamine-containing
absorption Liquid, which has a lower content of C02
and/or H2S than the absorption liquid fed to the first
absorption stage. Regarding the second absorption stage
too, the gas being treated is advantageously fed to the
lower part, preferably to the lower third, of this absorp-
t;on stage, countercurrent to the absorption liquid, which
is advantageously fed to the upper part, preferabl~ to the
upper third, of the second absorption zone. The product
gas is taken off at the top of the second absorption zone.
The aqueous absorption liquid laden with C02 and/or
H2S and obtained at the bottom of the second absorp-
tion stage is fed to the top of the first absorption stage.
The pressures employed in the first and second absorp-
tion stages are in general from 5 to 110, preferably from10 to 100, in particular from 20 to 90, bar, and it is pos-
sible to use different pressures in the first and second
stages. In general, however, the first and second absorp-
tion stages are operated under the same, or substantially
the same, pressure, the pressure d;fferences being due to,
for example, the pressure loss in the absorption stages.
Advantageously used absorption stages are absorption
~9~5S3
- 5 - O.Z. 0050/374~0
columns, in general packed columns or columns equipped
with trays. The absorption liquid laden with the acidic
gases C02 and/or H2S is taken off in the lo~er part
of the first absorption zone, preferably in the lo~er
third, in pa^ticuLar at the bottom of this zone.
The laden absorption liquid obtained from the
first absorption stage is then regenerated by being let
do~n in 2 or more, advantageously from 2 to 5, prefer-
ably 2 or 3, flash stages, the f;nal flash stage being oper-
ated under reduced pressure, which is generated by meansof a mechan;cal vacuum-producing apparatus and a steam-
jet ejector $onnected in series, and, if necessary, the
water losses of the system which result from the removal
of water in the gas streams taken off at the top of the
second absorption stage and from the flash stages and the
stripping zone are compensated at the same time by feeding
in, at the bottom of the penultimate flash stage, an amount
of steam corresponding to the water loss. Preferably, the-
pressure maintained in the final flash stage is from û.3
to about 1, preferably from 0.4 to about 1, in particular
from û.6 to about 0.9, bar. Examp~es of mechanical vacuum-
producing apparatuses are vacuum pumps and, preferably, com-
pressors, eg. screw compressors or centrifugal compressors.
The steam-jet ejector is preferably connected downstream
of the mechanical vacuum-producing apparatus. In general,
the flash stages are operated at from 35 to 100C, pre-
ferabiy from 45 to 9ûC, in particular from 55 to 85C.
To compensate for water losses which arise in the
process as a result of water being removed in the gas
streams taken off at the top of the second absorption stage
and from the flash s~ages and the stripping zone, an amount
of steam corresponding to the water loss is advantageously
fed in at the bottom of the penultimate flash stage. As a
rule, the water present in the gas streams taken off is
removed substantially in ~he form of steam. Low-pressure,
medium-pressure or high-pressure steam, eg. steam
under 1.5 to 100 bar, can be fed to the bottom of the
1~90~S3
- 6 - O.Z. 0050/37490
penultimate flash stage. P~efer3bly, lo~-p~essure steam,
eg. steam under 1.5 - 10, advantageously 1.5 - 5, bar,
is used, since this steam is in general cheapLy available.
S The gas removed at tne too of the final flash
stage can be released into the atmosphere or mixed with
the gas stream taken off from the penultimate flash
stage, and fed for further treatment. In an advantageous
embodiment of the process, the steam-jet ejector is con-
nected downstream of the mechanical vacuum-producing
apparatus and, advantageously, the gas removed at the
top of the final flash stage, togetner with the steam
used to operate the steam-jet ejector, is fed to the
bottom of the penultimate flash stage.
Where steam from the steam-jet ejector is fed
to the bottom of the penultimate flash stage, the steam-
jet ejector is advantageously operated with the amount
of steam required to compensate the water losses of the
process. Ho~ever, it is also possible to operate the
steam-jet ejector with an amount of steam smaller than
that required to compensate the water losses, and in
addition to feed in the lacking amount of steam at the
bottom of the penultimate flash stage. The steam-jet
ejector can be operated using medium-pressure or hi!gh-
pressure steam. Medium-pressure steam, eg. under 5-Z0,
preferably 5-10, bar, is preferably used.
The penultimate flash stage is advantageously
operated under about 1 - 30, preferably about 1 - 25,
and in particular about 1 - 20, bar.
Flashing is advantageously carried out using flash
chambers which can, for example, also be in the form of
columns. These flash chambers need not contain special
baffles, although columns equipped with baffles, eg.
packed columns, may also be used.
The gas stream obtained at the top of the final
flash stage essentially contains the acidic gases C02
and/or H2S and is advantageously either combined with
the gas removed from the top of the penultimate flash
~29(~S53
- 7 - O.Z. 0050/37490
stage or, preferabLy, fed to the bottom of the penultimate
flash stage together ~ith the stsam for operating the
steam-jet ejector.
A stream of the absorption liquid which is obtained
at the bottom of the final flash stage and has been freed
to a great extent, advantageously to more than 50%, pre-
ferably more than 60Z, from the acidic gases C2 andlor
H2S is then recycled as wash liquid to the first absorp-
tion stage, where i~ is advantageously introduced at the
topv A further stream obtained at the bottom of the
f;nal and/or penultimate flash stage or stages is fed,
for further regeneration, to a stripping zone, in which
the acidic gases C0z and/or H2S still present in this
stream are substantially stripped off. In a preferred
embodiment of the procedure described above, all of the
absorption liquid obtained at the bottom of the final
flash stage is recycled to the first absorption stage,
and a bleed stream of the absorption liquid obtained at
the bottom of the penultimate flash stage is fed to the
stripping zone for further regeneration. In another pre-
ferred embodiment, some of the absorption liquid obtained
at the bottom of the final flash stage is ecycled as a
wash liquid to the first absorption stage, and a further
bleed stream of the absorption liquid obtained at the
bottom of the final flash stage is fed to the str;pping
zone for further regeneration. However, it is also pos-
sible for a bleed stream of the absorption liquid obtained
at the bottom of the final flash stage to be recycled
as wash liquid to the first absorption stage, and a further
bleed st eam of the absorption liquid obtained at the
bottom of the final flash stage and a bleed stream of
the absorption liquid obtained at the bottom of the pen-
ultimate flash stage to be fed to the stripping zone for
further regeneration. The ratio of the amount recycled
to the fir-st absorption stage to the amount fed to the
stripping zone is in general from 10:1 to 1:2, preferably
from 5:1 to 1:1. The gas st eam obtained at the top of
129~SS;3
_ ~ _ o.z. 0050/37490
the stripping zone and essentially conta;n;ng C02 and/or
H2S can be removed from the system. However, ;t may
also be advantageous if this gas stream, which contains
C2 and/or H2S in addition to steam, is recycled to
the lower part, preferably the lower half, in particular
the lower third, of the penultimate flash stage, in order
to reduce the ~ater losses of the system. The stripping
zone used is advantageously a stripping column, in
general a packed column or a column equipped with trays.
The stripp;ng column is generally operated at from 85
to 115C, preferably from 85 to 110C, in particular
from 90 to 110C.
The regenerated absorption liquid obtained at
the bottom of the stripping zone is recycled to the
second absorption stage, advantageously to the top of
this stage.
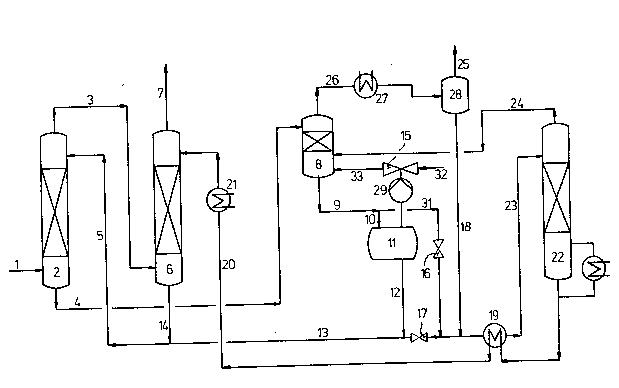
The Example which follows illustr'ates the inven-
tion in mofe detail, the course of the process being
sho~n diagrammatically in the Figure.
In the'Figure, a gas which contains C02 and/or
H2S, for example a synthesis gas containing C02 as
the acidic gas, is passed under superatmospheric Pressure~
via line 1, ;nto the bottom of the first absorption
column 2. At the same time, from 20 to 70Z strengh by
~eight aqueous methyldiethanolamine solution, as the
absorption liquid, is passed via line 5 to the top of
the first absorption column. The prewashed gas obtained
at the top of'the first absorption column is introduced
into the bottom of the second absorption column 6 via
line 3, for fine purification. At the same time, from
20 to 70X strength by weight aqueous methyldiethanolamine
solution, ~hich is obtained from the stripping column
22 and is virtually free of acidic gases, is fed, as
absorption liquid, via line 20, to the top of the second
absorption column. The washed gas is taken off at the
top of the second absorption column 6, via line 7. The
aqueous absorption liqu;d obtained at the bottom of the
1~,9(3553
- 9 - O.Z. 0050/37490
second absorption column and laden with the acidic gases
is fed via lines 14 and 5, together with the absorption
liquid obtained from the final flash stage 11 via lines
12 and 13, to the top of the first absorption column 2.
The aqueous absorption liquid obtained at the bottom of
the first absorption column 2 and laden with C02 and/or
HzS is regenerated by being let down via line 4 into a
first flash chamber 8, for example via a valve or,
preferably, in an expansion turbine. In th;s stage, an
intermediate flash gas is liberated from the absorption
liquid and is taken off via line 26. After passing the
heat exchanger 27 and the separation vessel 28, the
combined gas streams are removed via line Z5. Liquid
which separates out in separation vessel 28 is removed
via line 18. The partially let down absorption liquid is
removed from the bottom of flash chamber 8 via line 9; in
a first version of the process, with valve 16 closed
and valve 17 open, the said partially let down absorption
liquid is let do~n completely via line 10 into a second
flash chamber 11, in whic-h reduced pressure down to, for
example, O.S bar is maintained by means of the mechanical
vacuum-producing apparatus 29, eg. a compressor, and the
steam-jet ejector 15. The steam-jet ejector 15 is advan-
tageously supplied, via line 32, with the amount of steam
required to compensate the water losses of the system.
~he gas removed at the top of flash chamber 11 is fed,
together with the steam used for operating the steam-jet
ejector 15, to the bottom of the first flash chamber 8,
via l-ne 33. Some of the absorption liquid which has
been let down and is removed from the bottom of the flash
chamber 11 via line 1Z is recycled via lines 13 and 5 to
the top of the absorption column 2, while the other part
is fed to the top of the stripping column 22 via line
23.
In a second version of the process, with valve 17
closed and valve 16 open, some of the partially let-
down absorption liquid removed via line 9 is let down
1~9(~53
- 10 - O.Z. 0050/3~490
via line 10 into ehe second flash chamber 11, whil2 the
remainder is fed to the top of the stripping column 22
via lines 31 and Z3. In the second version, all of ehe
let-down absorption liquid removed from the bottom of
the flash chamber 11 via line 12 is recycled to the top
of the absorption column 2 via lines 13 and 5.
The regenerated absorption liquid obtained at
the bottom of the stripping column 22 is recycled via
line 20 to the top of the second absorption column ~ pass-
ing through the heat exchangers 19 and 2t. The waste
gas stream obtained at the top of the stripping column
22 and containing C02 and/or H2S is fed via line 24,
advantageously to the lower part of the flash chamber 8.
However, it is also possible for the said waste gas stream
to be removed directly from the system without being fed
beforehand to the flash chamber 8.
The Example which follows illustrates the
invention.
EXAMPLE
The gas washer used is shown in the Figure and
comprises two absorption c~lumns in succession, two flash
chambers in succession, and a stripping column. In the
absorption columns, 98ûO kmol/h of a C02-containing
synthesis gas are washed with a 50X strength by weight
aqueous methyldiethanolamine solution as the absorption
liquid. The synthesis gas being treated is fed, under
28 bar, to the bottom of the first absorption column.
This gas originates from a steam reformer and has the
following composition:
C218.3X by volume
C00.4X by volume
H261.0% by volume
N22û.0% by volume
35 CH40.1% by volume
Ar0.2X by volume
The temperature of the absorption liquid in the
feed to the first absorption column is 60C, while the
1290553
~ O.Z. OOS0/37490
abso-ption l;qu;d fed to the second absorpt;on column
is at 75C. The treated synthesis gas removed at the
top of the second absorotion column has the fo~lowing
5 compos;tion:
C2 0.01% by volume
C0 0.5X by volume
H2 74.6% by volume
N2 24.5% by volume
10 CHz 0.2X by volume
Ar 0.3X by volume.
The laden absorption liquid leaving the bottom
of the first absorption column is let down to 5 bar in
the first flash chamber, from the top of which 1150 kmol/h
of a flash gas are removed. The absorption liquid taken
off at the bottom of the second flash chamber is then
let down into the second f~ash chamber, in which a pressure
of 0.7 bar is maintained by means of a steam-jet ejector
and a compressor. About 3/4 of the ~et-down absorption
liquid obtained at the bottom of the second flash chambe
is recycled to the first absorption column, the remainder
being regenerated in the st ipping column and then recycled
to the second absorption column.
The novel process permits the use of absorption
columns which have a substantially smaller diameter and
contain fewer trays, so that the capital costs for the
gas washer can be considerably reduced.
Drawing.