Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
12~299i
4,7-DIHYDROTHIENO[2,3-b]PYRIDINE DERIVATIVES, PROCESS
THEREOF, AND AGENTS FOR CARDIOVASCULAR DISEASES
BACKGROUND OF THE INVENTION
Field of the Invention
The compounds provided by the present invention are
classified into a group of calcium channel blockers
(hereinafter referred to as Ca-blockers), and have potent
antihypertensive and coronary vasodilating actions which last
for hours. These compounds are useful in the treatment of
cardiovascular diseases such as angina pectoris, hypertention,
cerebrovascular dysfunction, arrhythmia or the like, and have
the advantage that they have no systole inhibitory action ~g
2n adver~e reaction usually seen in thé use of the analogous
known-compounts.
Prior Art
Compounds having Ca-blocking effect have commonly been
used in the treatment of cardiovascular diseases such as
angina pectoris, hypertention, cerebrovascular dysfunction,
arrhythmia or the like, and have become well-known because of
their high efficacy In particular, a series of 1,4-dihydro-
pyridine derivatives have been extensively researched and
developed as Ca-blocker. Examples of useful Ca-blockers are
Nifedipine (U S. Patent Nos 3,485,847 and 3,644,627),
Nisoldipine (Japanese Patent Publication No. 56-47185~,
2-amino-1,4-dihydropyridine derivatives (JPN Pat. Pub No 57-
20306), Nicardipine (JPN Unexam. Pat Pub No. 49-109384), and
the like. ~ome examples of pyrazolodihydropyridine
derivatives, the production thereof and their Ca-blocking
r
lZ9Z99l
action are disclosed in JPN Unexam. Pat. Pub. No. 59-118786
and in JPN Unexam. Pat. Pub. No. 59-65089 and JPN Pat. Appln.
No~. 58-166258 and 59-53118 by the present inventors.
SUMMARY
The present invention relates to 4,7-dihydrothieno-
[2,3-b]pyridine derivatives, the process for production
thereof, and agents for cardiovascular diseases. In more
particular, it provides 4,7-dihydrothieno[2,3-b]pyridine
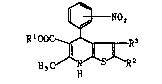
derivatives of the formula:
~}NO2
RIOOC ~ R3 ( I )
H3 H
wherein R~ is straight or branched chain Cl-C, alkyl, alkoxy-
alkyl, or arylalkyl; R2 is hydrogen straight or branched chain
Cl-C, alkyl, or alkoxycarbonyl; R3 is hytrogen, straight or
branched chain Cl-C, alkyl, phenyl which may be substituted by
one or more halogens or alkoxy groups, optionally substituted
cycloalkyl, or optionally substituted cycloalkylalkyl, or R2
ant R3 taken together may form an alkylene;
a process for production of 4,7-dihytrothieno[2,3-b]pyridine
derivatives of the formula:
lZ9Z99~
¢~NO 2
RIOOC ~ R3 ( I )
H3 H
wherein R~, R2, R3 esch is the same as above,
comprising reacting a compound of the formula:
~NO2
~ (~)
CH=C~COcH3
--COOR
wherein Rl i~ the same as above,
with a compound of the formula:
H2NJQ~R2 ( m )
wherein Rl and R2 each is the same as above; and
agents for cardiovascular diseases comprising containing at
least one of the 4,7-dihydrothieno[2,3-b~pyridine derivatives
of the formula:
129Z99l
~NO2
RIOOC ~ R3 ( I )
H3C--N~R2
wherein Rl, R2, and R3 each is the same as above.
DESCRIPTION OF PREFERRED EMBODIMENTS
The objective compounds(I) of this invention are
prepared through the Michael addition between heterocyclic
amines and a . ~ -unsaturated ketones sccompanied by
cyclization reaction.
Problems to be Resolved
As mentioned sbove1 many Cs-blocking sgent~ are widely
u~ed in the trestment of cartiovascular disease~ but highly
safe and long-acting ones have not been launched in m~rket
Such a compound, therefore, has long been desired
[Preferred Embodiment of the Invention]
_eans to Resolve the Problems
In this invention, the straight or branched chain Cl-C,
alkyl includes, for example, methyl, ethyl, n-propyl,
i-propyl, n-butyl, i-butyl, s-butyl, and t-butyl; the
alkoxyalkyl means C,-C, alkyl substituted by lower alkyloxy
and includes, for example, methoxymethyl, ethoxymethyl,
methoxyethyl, ethoxyethyl, propoxyethyl, i-propoxyethyl,
butoxyethyl, t-butoxyethyl, methoxypro W 1, ethoxypropyl,
propoxypropyl, t-butoxypropyl, methoxybutyl, i-butoxybutyl,
snd the like.
lZ9Z99l
Arylalkyl means C,-C~ alkyl substituted by phenyl which
may have one or two substituents wherein the substituent means
halogen or lower alkoxy and Cl-C~ alkyl means straight alkyl.
Arylalkyl includes, for example, benzyl, phenethyl, phenyl-
propyl, phenylbutyl, phenylpentyl, and the like.
Alkoxycarbonyl means carbonyl substituted by a lower
alkoxy and includes, for example, methoxycarbonyl, ethoxy-
carbonyl, propoxycarbonyl, i-propoxycarbonyl, butoxycarbonyl,
t-butoxycarbonyl, and the like.
Phenyl which may be substituted by one or more halogen
or alkoxy group means phenyl which may be substituted by one
or two halogens or lower alkoxys wherein halogen includes
fluorine, chlorine, and bromine and lower alkoxy includes
methoxy, ethoxy, and the like: they may be the ~ame or
different esch other.
Optionally substituted cycloalkyl means Ca-C7 cycloalkyl
substituted or unsubstituted by, for example, lower alkyl such
as methyl, ethyl, propyl, butyl, and pentyl. It includes
cyclobutyl, cyclopentyl, cycohexyl, 2-i-propyl-4-methylcyclo-
hexyl, and the like.
In the word "optionally substituted cycloalkylalkyl",
cycloalkylalkyl means C,-C, alkyl substituted by C~-C7
cycloalkyl wherein Ca-C7 cycloalkyl includes cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptyl; and Cl-
C, alkyl includes methyl, ethyl, propyl, butyl, and the like.
Alkylene means alkylene of the formula:
-(CH2)n-
wherein n is an integer of 3 to 6,
formed by combination o R2 with R3. Especially, butylene
(n=4) is most preferably employed.
lZ92991
The compound (I) of the present invention can readily be
produced by the reaction of an a . ~ -unsaturated ketone
reagent (~ ) with a S-aminothiophene ( m ) ~ as shown in the
following scheme:
C~ C1l3 H~J~R~ >R~ooc~
. ( I )
wherein Rl, R2, and R3 each is the same as defined above.
This reaction may be carried out in the presence or
absence of any solvent. As a solvent used in the reaction,
the followings are exemplified: alcohols such as methanol,
ethanol, isopropanol, tert-butanol, ethylene glycol, ant the
like; hydrocarbons such as benzene, toluene, xylene, and the
like; ethers such as diethyl ether, tetrahydrofuran, dioxane,
glyme, diglyme, and the like; halogenated hydrocarbons such as
dichloromethane, chloroform, dichloroethane, carbon
tetrachloride, and the like; esters such as ethyl acetate and
the like; acetic acid; dimethylformamide; pyridine; and
the like solvent An acid or organic base may be employed if
necessary The acid includes, for example, inorganic acids
such as sulfuric acid, hydrochloric acid, and phosphoric acid;
organic acids such as paratoluenesulfonic acid, acetic acid,
and formic acid; and Lewis acids such as boron trifluoride,
zinc chloride, aluminium chloride, magnesium chloride, ant tin
--6
~ZgZ991 '
chloride. The organic base includes, for example,
triethylamine, pyridine, pyrrolidinP, piperidine, and the
~ . The reaction is performed for a period of several hours
to several days at around room temperature (1-30C) or under
heating (30-100C)-
One of the starting materials, an a, ~ -unsaturated
ketone reagent (~ ) employed in the reaction is known and can
be prepared according to the manner disclosed in the JPN
Unexam. Pat. Pub. No. 59-65089. The other 5-aminothiophenes
( m ) can be prepared according to the reaction sequence as
shown below.
-
~z9z99~
I" N ('~ N
\
~ , F~. E3
_~ ~
~ Z Z;
O N N
O
ra --I
T ~=
ZO / ~ ~ ~ ~
2 1 ~\ ~ 22
Z o :Z o ~ ~
~ ~ ~ / ~ ~ N
O Vl
O c ~ O N~11
O
N N ~I
~ ~; 3 ~)
~;~
Each step in the reaction sequence may be performed
under the conditions of the following disclosures:
K. Gewald et al., Chemishe Berichte 98, 3571 (1965); ib~d, 99
94 (1966); i6id, 99 2712 (1966); K. Gewald et al., Journal fur
Pra~tishe Chemie, 315, 539 (1973); O. Yonemitsu et al.,
Journal of Organic Chemistry, 43, 2087 (1978); Masaki Ohta,
Journal of the Pharmaceutical Society of Japan, 70, 709
(1950); and K. Kariyone et al., ibid, 79, 711 (1959).
Typical compounds of this invention which are prepared
from the starting materials, a . ~ -unsaturated ketone reagents
(~ ) and 5-aminothiophenes ( m ) in the sforementioned manner
are shown below.
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-phenyl-
thieno[2,3-b]pyridine-5-carboxylate,
Phenethyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
phenylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-(4-
chlorophenyl)thieno[2,3-b]pyridine-5-carboxylate,
Phenethyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-(4-
chlorophenyl)thieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-(3,4-
dimetoxyphenyl)thieno[2,3-b]pyridine-S-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-methyl-
thieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
n-butylthieno[2,3-blpyridine-5-carboxylate,
Isopropyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
n-butylthieno[2,3-b]pyridine-5-carboxylate,
Methoxyethyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
n-butylthieno[2,3-b]pyridine-5-carboxylate,
lZ~Z991
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2,3-b]pyridine-5-carboxylate,
Isopropyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2,3-b]pyridine-5-carboxylate,
Methoxyethyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2,3-b]pyridine-5-carboxylate,
Phenetyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-(cyclo-
pentylmethyl)thieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-(cyclo-
hexylmethyl)thieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-cyclo-
hexylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-tihydro-6-methyl-4-(3-nitrophenyl)-3-phenyl-
2-methylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2-methyl-
thieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2,3-
dimethylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-cyclo-
pentylmethyl-2-methylthieno[2,3-b]pyridine-S-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2-
isopropylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
n-butyl-2-n-propylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2-ethoxy-
c~rbonyl-3-methylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2,3-
tetramethylenethieno[2,3-b]pyridine-5-carboxylate,
--I O--
129Z991
Methyl 4,7-dihydro-6-methyl-4-(2-nitrophenyl)-2-
isopropylthieno[2,3-b]pyridine-5-carboxylate,
Methyl 4,7-dihydro-6-methyl-4-(2-nitrophenyl)-3-
n-bwtyl-2-n-propylthieno[2,3-b]pyridine-5-carboxylate, and
Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isopropylthieno[2,3-b]pyridine-5-carboxylate.
[Effect of the Invention]
~ he compounds of the present invention are characteri-
zed by showing excellent antihypertensive and coronary
vasodilating effects dependent on their Ca-blocking ection
with no systole inhibitory action which is one of the adverse
reactions regarded as defect of the conventional Ca-blockers.
The biological tests on the following compounds were performed
in the manner 85 explsined below. Numbers by which the
compounds are identified correspond to Ex~mple numbers.
(Compounds Te~ted)
Reference Compound (R): Nifedipine
Compounds of the Invention:
1. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-phenyl-
thieno[2,3-b]pyridine-5-carboxylate,
7. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
n-butylthienol2,3-b]pyridine-5-carboxylate,
10. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2l3-b]pyridine-5-csrboxylate,
12. Methoxyethyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-
isobutylthieno[2,3-b]pyridine-5-carboxylate,
16. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-3-cyclo-
hexylthieno[2,3-b]pyridine-5-carboxylate,
19. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2,3-
dimethylthienol2,3-b]pyridine-5-carboxylate, and
l~9Z99l
23. Methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-2-ethoxy-
carbonyl-3-methylthieno[2,3-b]pyridine-5~carboxylate.
(Test Method)
(1) Antihypertensive Action:
Female Spontaneously Hypertensive Rats(hereinafter
referred to as SHR) with 160 mmHg of systolic pressure were
employed without anaesthetization. The systolic pressure
after SHRs were warmed at 50c for 2 - 3 minutes was blood-
lessly measured by the tail-cuff method [Japan J. Pharmaeol.,
28, 617 (1978)] using a Physiograph and an Electrosphygmo-
manometer (DMP-4B, PE-300, Narco Biosystems, Inc., Houston).
Each compound was intraperitoneally administrated to SHR at a
dose of 3 mg per kilogramm.
(2) Coronary Vasodilating Action and Systole Inhibitory
Aetion:
Guinea pigs with 400-800g of body weight were strongly
hit on their heads and the eartoid artery of eaeh Guinea pigs
was eut down in order to make them blootless. The heart was
isolated and perfused with at a eonstant pressure (50 em H20)
by the Lsngendorff method [Basie Pharmaeology & Therapeuties,
9(4J, 181 (1981)]. Krebs-Ringer bicarbonate solution (~7C)
containing 0.5~ defibrinated blood was employed as a
perfusate, into which a mixture of 95% oxygen with 5X carbon
dioxide was continuously introduced. The flowing perfusste
was led into a drop counter, and the ehsnges of the flow i,e.
increase and decrease are regarded as the respeetive
indications for coronary vasodilation and vasoconstrietion.
The isomeric eontraetion of apex and the number of drops of
the eoronary perfusate were reeorded on a Reetieorder (RJG
3006, Nihon Koden) by way of an F-D Pick-up (SB-lT, Nihon
-12-
1292991
Koden). Each of the test compounds at a dose of 0.1 ~ g was
administrated through a short rubber tube connected to the
aorta and the cannula.
(Re~ults)
Antihypertensive action is shown in a maximal decrease
of blood pressure, i.e., a maximum difference between systolic
pressures after and before the administration of the test
compound.
Coronary vasodilating action is shown in changes of the
quantity of the perfusate, and the duration of the action is
shown in the time during which the increase (over 20%) in the
quantity of the perfusate was observet.
~able 1. Antihypertensive action and Coronsry vasodilating
action ant the duration of effect.
Maximum Change in Perfusate
CompoundsDecrease
of BP (mmHg) (%)Duration (min )
1 14 42.6 10
7 54 45.2 > 60
34 53.1 > 60
12 39 49.8 > 60
~ _
Since the compounds of this invention, as clearly seen
from the above-listed results, show the high antihypertensive
-13-
lZ9Z991
and coronary vasodilating actions, they can be used in humsn
or animals as cardiovascular agents with lesser adverse
reactions.
The compounds of this invention can be orally or
pareterally administered to human or animals and formed into
various type of formulations in compliance with the usage.
They, for instance, can be tablets, capsules, pills, granules,
fine granules, aqueous solutions, emulsions or the like. In
the course of the formulation, conventional carriers or
diluents such as lactose, sucrose, starch, cellulose, talc,
magnesium stearate, magnesium oxide, calcium sulfate, powdered
gum arabic, gelatin, sodium alginate, sodium benzoate, stearic
acid and the like are employed. Injections may be used in a
form of a solution in distilled water, saline, Ringer's
solution or the like, or a suspension in ~esame oil.
The compounds o this invention may be administered to
an adult orally at a dose of about 1-50 mg a day, or intrave-
nously at about 0.5-20 mg a day.
-14-
lZ9Z~9l
EXAMPLE 1
Preparation of methyl 4,7-dihydro-6-methyl-4-(3-nitrophenyl)-
3-phenylthieno[2,3-b]pyridine-5-carboxylate (1)
(Method A)
~NO2
~NO2 11 J
YH=C~COOOCH H2N~1~ Cl3300C~O
(2) (3) H ( )
In 10 ml of t-butanol are dissolved 1.42 g (5.71 mmol)
of methyl 3-nitrobenzylideneacetoacetate (2) and 1.0 g (5.71
mmol) of 5-amino-3-phenylthiophene (3) and the solution i~
stirred at 80-C ~or 4 hours. The reaction mixture is then
evaporated to give a residue, which is chromatographed on a
silica gel column with benzene/ethyl acetate (9/1) as an
eluent to give 0.254 g (yield 10.9%) of the titled compound
(1). This is recrystallized from methanol to give yellow
plates, mp. 213-215C.
Elementary Analysis: Calcd. (%) for C22HI8N20,S: C, 65.01; H,
4.46; N, 6.89, Found (%) C, 64.83; Hl 4.34; N, 6.93.
IR(Nujol) ~ max: 3300, 1632, 1350cm~'.
NMR(CDCl3) ~ ppm: 2.45, 3.58(s, 3HX2), 5.35(s, lH), 6.48(s,
lH), 6.48(NH), 6.85-8.00(m, 9H).
-15-
lZ9Z99i
(Method B)
NO2 ~ tBuOH
+ H2N u
~ (3)'
(2)
CH300C ~ Bu ~ Bu
CH3 H NO2 (1)"
(1) '
~NO2
CF3COOH
CH300C
CH3 H (1)
In the sequence, tBu represents tertiary butyl.
-16 -
129~991
A solution of 0.18 g (0.73 mmol) of methyl 3-
nitrobenzylideneacetoacetate (2) and 0.20 g (0.73 mmol) of t-
butyl 5-amino-3-phenylthiophene-2-carboxylate (3)' dissolved
in 2 ml of t-butanol is degassed and stirred at 95C for 95
hours under nitrogen atmosphere, then evaporated to give a
residue. The residu~ is chromatographed on a silica gel
column to give 33 mg (yield ll.lX) of the Schiff s base (1)"
as an ethylene chloride fraction and subsequently 0.268 g
(yield 73.4%, yellow amorphous) of 2-t-butoxycarbonyl deriva-
tive (1) of the objective compound (1) as a methylene
chloride/acetonitrile (9/1) fraction.
NMR(CDCl~) ~ ppm: 1 81(s, 9H), 2.38, 3.53(s, 3H X2), 5.00(s,
lH), 6.82~7.95(m, 9H), 8.07(NH).
A solution of 0.268 g (0.53 mmol) of the derivative(l)'
dissolved in 2 ml of trifluoroacetic acid is stirred at 20C
for about an hour and evapora~ed in vacuo to give a residue,
to which ice and water are added. The mixture is alkalized
with aqueous sodium hydrogen carbonate and extracted with
methylene chloride. The organic layer is dried over magnesium
sulfate, filtered, and evaporated to give a residue, which is
chromatographed on a silica gel column (with methylene
chloride as an eluent) to give 0.174 g (yield 80.2X) of the
titled compound (1).
129Z99l
EXAMPLE 2-27
Method A
C}~ OcH3 + l~2y,)~R2 , R, OOC~I2
(I )
In the sequence, Rl, R2, and K3 each is the same as
above.
In a solvent are dissolved compounds (~ ) and ( m ) and
the solution is, if required under nitrogen atmosphere,
allowed to react at room temperature or unter heating The
reaction mixture is evaporated in vacuo to give a residue,
which is either recrystallized from methanol or
tetrahydrofuran (THF)/methanol or chromatographed on a silica
gel column to give an objective compound (I ) This may be
further refined by recrystallization
1292991.
Method B
( ~ ) + H2N)~OOtBu
(m)~ ,
¢~NO2
'~ CF3COOH
~ 3 3( I )
CH3 OOtBu
H ( I )
In the sequence, Rl, R2, and R3 each is the same 8S
above.
In a solvent are tiscolved compounts (~ ) and ( m ) and
the solution is, under nitrogen atmosphere, allowet to react
at room temperature or under heating. The reaction mixture is
evaporated in vacuo to give a residue, which is
chromatographed on a silica gel column to give an objective
compound (I )'. The compound (I ) is reacted with
trifluoroacetic acid-under cooling or ~t room temperature and
the reaction mixture evaporated in vacuo to give a residue, to
which ice and water are added. The mixture i5 alikalized with
a base then extracted with a qolvent. The organic layer is
dried and evaporated in vacuo to give a residue, which i~
chromatographed on a silica gel column to give an objective
compound ~I ). This may be further refined by
recrystallization.
--19--
lZ9Z99~
Compounds of the present invention can be prepared
according to the method A or B.
The compounds of the present invention prepared in
Examples 1-27 and details of the reaction conditions are shown
in Tables 2 and 3, respectively. Further, Table 4 shows
recrystallization solvents for the products or the acid
addition salts, and appearance (crystal form, color),
molecular formulae, and the elementary analysis data of the
compounds; and Table 5 shows IR and NMR data on each compound.
--20--
- ~292991
Table 2 (No.l)
~ NO2
RlOOC~R23
H3C H
....
Exam. Postn.
No. Rl R2 R3 -Nf 2
1 -CH~ H -C6Ht 3
2 ~ C8Hs H -
3 -CH~ H ~ C
.
4 ~ C8Hs H ~ C "
S -CH~ N ~ N3 _
6 -CH, H ~CH
7 -CH~ H-n-C4H,
8 -isoCaH7 H-n-C,H~
~ CCN3 H-n-C,H,
-CHJ H-iso-C,H
il -isoCJH7 H¦ -igo-C,N,
12 ~ CCN3 H-iso-C,H
13 ~ -C8Hs -iso-C,H
14 -CH~ N _
15 ~ H3 H
16 -CH~ H
. - 2l -
lZ92~91
T~ble 2 (No.2)
_
Exam. Postn.
Rl R2 R3 of
No . -NO2
17 -CHa -CH3 -C6H6 ~J
_
18 -CHt -CH~ H
19 -CH3 -CHt ~CH
-CH3 -CH
21 -CH3 isoCaH7 H
22 ~CHa n4aH7 n-C,H
23 ~CHa COOC2H5 CH
24 -CH3 -(CH2).- ~
-CH3 isoCaH7 M 2
26 -CHa n-CaH7 n-C,H9 ~
D -CH3 H -i~o-CJH7 3
-~2-
lZ~2~9i
O~ O~ ~ ~D _i ~ r~ _- o~ ~ oo ~ o r~
~_ o ~ o ~ ~ ~ o~ ~ ~ ~ ~ ~ ~ ~ Ll~
~ _ _ _
~ ~ a~ ~ a~ ~ ~ e~ ~ ~ ~ a~ a~ ~q ~ ~
_ ~ _
~:; E Ll _ _~ _ ~ _I ~ ~ ~ _ _I ._ _.
3 ~ _ _
~ ~ æ o o ~3 o ~ ul 2 ~n ~ æ ~ æ
. . _ _
~,_- ~ 8 ~ ~ ~ ~ _ ~ ~i ~ ~ _
a a~ o O ~ O O _ _ O _ O O O O
EO 0 ~ Ei ~ ~ ~i4 S4 ~3 ~n ~4 ~ R ~ n ~ ~
~ bD O O O O O O O O O O O O O
5 b ~ ~ ~ ~ ~ c . o o __ ~ ~3 ~3 ~3
.~ _ __ _
~ ~ ~ ~ o. ~ ~ ~ ~ oD o ~ ~, o CJ o ~
_ _ _ ~ ~ _ ~ _ _ _ _ _ _ _ ~
o C~l U~ ~ ~ C~ o o ~ ~ ~ ~ ~ ~ ~
~a -~ ~ ~ ~ --_ ~ _ _ _ _ _ _ _
V~ 1- ~ ~ ::~ :~ :: ~ ~ ~ :: :: :
_~ ~. ~ ~ ~--~. ~_ ~ û-, ô ~ ~--8 _ ~. ~. _
~0 O U~ O ~ ~ _ O O~ -I . ~ _~ ~ _ ~ _~
~ O O N `J O _ N _ _ N _ _ _ ~ O _
~D E3 _ o o o o o _ o o o o o o o
~ ~ ~ ~ ~ _ _ _ _ ~ o ~ _ _ ~î ~
~ o~t=J In -- -- -- 0~ -- N _i _ _ _ _I _
r ~ a~ ~ ~ 3 1~ ~ ~ S~ ~ b~ ~t ~ ~ ~
~a ~, ~ O O O O O t~i ~ O O O O O O O
_ __ __
E o _I s~ t~t `J Ll~ `.0 ~ O~) O~ _ _ _ _
W __ _ _ _ _
-23-
l~9Z99~
o C~ _ ,~ ~ 0 ,~ ~ ~ U~ Ul 0 ~ o
.a~ ~ ~ ~ . ~ o~ o~ . ~ ~ o~ 0 o~ I
~ n ~ _. ~ ~ ~ ~ ~ ~ r~ ~o u~ _l
~ _ _ _ _ __ _
~ A C~ a~ <~ ~ ~1: ~: ~: ~ ": '1: ~ ~ ~: 1~
'~" ~1 ~ ~
~
n _
,
_l
O
~ 0~- ~ ~0
O
_. ~ D _~ O
O
111 _ _ _ _
z .~ ~ 8 ~ c~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
,~ _ _ _ _ _ _ _
R ~ ~ ~
_ _ _--_ _ _ _ _ _ _ _ _ _ _
~ Ul ô ~ ~ o ~ ~o ~o o o ~o ,~ ~
~ I _, _ ~ _ _ ~ _ _ _ ~ _. _ _ _
,~
U~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
~ _
~ _ _ _ ~ _ _ _ _ ~ _ _ ~
_, ~ ~ ~0 ~ ~ ~ ~ ~ ~ ~ ~ ~ U~
8 o~ ~o ~ In ~ ~ u~ ~ ~ ~ o~
_ o~ ~ ~ 8 In ~ ~ ~ n ~ a~ ~ In Ln ~
b~ V~ _ O ~ O O O O O O _l O O O O
_ ~ ~ ~3 Ln R ~ ~ ~ o _ In ~ ~i
~O _ In ~ ~ Ln _ ~ ~ _~
o ~ ~ ~ ~o ~ ~ ~ ~ ~ ~ o~ ~ ~ ~
V _, o _ o o _ o o o ~, o o o o
_ . _ _ _
~ ~ __ ~ ,~ ~ o~ ~ ~ ~ ~ _ ~ ~ ~
--24--
129Z99l
~ ~ ~ ~--~ o--~ ~ ~ ~ 8 u~
z ~o u~ ~O u~ u~ co I~ I ~o ~o r~ ~ ~o
~e ~ ~ o. I~ ~ ~ ~ ~ ~ o~ ~ o _.
Ul ~ ~ ~ ~ ~ ~ ~ In ~O ~O U~ ~ ~
~) ~ N O~ ~t ~ N 0\ _I _4 ~i ~O
3 ~ o ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
~ z ~ ;~; ~ ~ 8 _ N ~ " ~ N O N
~ 11i Y~ I~ ~ CO I~ ~O ~O t~ ~) ~C)
_~ ~ ~ ~ ~ Ln ~ ~ ~ O ~ ~ ~
~ ~ ~ ~ ~ ~i ~ ~ u~ ~ ~O u~ ~ ~i
V O _ ~ ~i4 ~ N ~O 1~ ~ _ ~ ~
~3 1~ ~4 ~2 ~O ~4 ~O ~3 _ ~C) ~; _
_~ _ _ _ __ _ _ O
~ U~ V~
~ ~ u~ o o v~ ~n u~ u~ u~ v~ v~ ul
0 O" O" Z Z O" 0" O O O O O O
Z Z V V Z Z Z; ~2!; Z Z Z Z
~_o~ ~ ~,., ~ ~ ....o~ ~ ..
_IZ:~: X Z:1: Z:r: ~ Z :c5: P:
O ~~ ~ o~.~ ~. o o~ ~ oo~
r~ ~ ~7V~ ~7 ~7 ~ C_7 ~7 ~7 ~ ~7~7 ~
~:~ ~,V ~ O ~ r-~ ~ ~ ~1 _ ~1 ~ ~
v ~ _ c~ ~ ~i ~? ~l N _ _ ~; ~ C~ _
_ _ _ _
~; ~7
~ _ ~ ~ ~ ~
~ 2-~
_, ~ ~ _, _, _, __ _ _. _
z ~: a0)~ ~ ~ ~ Q. ~4
__ _ _ _ _
aJ 0 z _1 ~I ~) ~ 111 ~O I~ t~ O~ O _ N
td # _ _ _ _
E~
`- ~Z9299i
_ --r~ ~ c~--~ n ~ ~ o~ ~ ~ ~ ~ _
:z ~ ~ ~ `D ~O ) r~ ~ 1~ ~ ~ ~ I~ ~
K :~ ~ _. ~; 8 aD ~ ~ ~ ~ ~ ~ ~ o
Ui ~o ~ ~ ~ ~ ~o Ln ~o ~ Ul
.In ,~ ~ r~ Ln oo ~o ~ o~ ~ C~ ~ 1~ ~
C~ _ 00 1~ O u~ ~ ~ ~O ~ ~ ~ Ln OD O
3 ~ ~ ;~; ;~; ~ s4 ~ ;~; ~ ~!; ~n ~ ~i3 __
~ ~; 0 o~ ~i o~ ~8 ~ ~ n ~ ù~ ~ ~a ~ In
a\ ~a~ ~ ~ ~o ~ ~ 0 1~ ~ 1~ ~ ~o 1~ 1~ 1
o\ ~ ~ ~ ~ $ ~ ~ ~J ~ ~ ~ ~ ~
~ ~ ~ n ~i ~o u~ ~i ~ u~ ~ u~ ~O ~ u~ u~ In
C-~ ~; O 1~ O I~ ~a ~ ,~ C~l ~ ~ ~ ~I æ
. 0 ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~O ~O ~
~ __ 0
S~ ~
~ ~n u~ v~ v~ u~ v~ ~ u~ ~ v~ ~nO u~ u~ u~ ~
~,
z z z z z z z ~ ~0 z z z
~ O~ .. .. .. O~ ........ O~ O~ O~ .. O~ .. ..
x s s s s s~ s r sO s sO sO ~ ~i
~7 t~ ~ ~7 ~ c7 v ~7 u ~ ~ ~ ~ c~
. _ ~ ~ ~ ~ ~ u~ ~ ~ ~ ~y ~ ~ ~ uO~ ,u
o~ v ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~s~
~0 ~ ~ ~ ~ ~ C~ ~ ~ ~ ~ ~ ~ o
_ _ _ _
O ..
1~18 ~ l~t~
t) ~ ~ :Zi P~ C-. ~ ~ ~ 4 ~ ~ ~ ~ _1 ~ ,
z ~ ~ ~ ~ ~ ~ ~ ~ ~_ ~ ~ ~ ~ ~ o
_ !I Z ~ ~ n ~o 1~ 0 o~ ~ ~ ~ ~ ~ In L ,~ _
Z
-26-
lZ9Z99l
Tsble 5 (No.l)
_
Exam IR(Nujol)~ c,,'
No. NH C0 No2 NMR(CDCl~)~
_
1 3300 1632 1350 2.45,3.58(s,3Hx2),5.35(s,1H),6.48(s,1H),6.48
(NH),6.85~8 00(m,9H)
2 3305 1628 1345 2.36(s,3H),2.82(t,2H),4.22(m,2H),5.29(s,1H),
6.47(s,1H),6.63(NH),6.90 7.90(m,14H)
3 3290 1627 1350 2.43,3.60(s,6H),5.31(s,1H),6.50(s,1H),6.57
(NH),6.93~8.00(m,8H)
4 3285 1623 1340 2.37(s,3H),2.85(t,2H),4.27(m,2H),5.22(s,1H),
6.48(s,1H),6.67(NH),6.88~7.95(m,13H)
3350 1648 1353 2.45,3.62,3.72,3.91(s,3Hx4),5.37(s,1H),6.50
_ (s,lH),6.73(NH),6.50~8.00(m,7H)
6 3290 1640 1350 1.88,2.40,3.62(s,3Hx3),5.22(s,1H),6.22(s,1H),
6.45(NH),7.17~8.13(4m,4H)
7 3305 1630 1345 0.67~2.33(m,9H),2.40,3.65(s,3Hx2),5.27(s,1H),
6.23(s,lH),6.55(NH),7.27~8 13(m,4H)
8 3300 1627 1341 0.63~2.33(m,9H),1.20(d,6H),2.40(s,3H),5.00(m,
lH),5.27(s,1H),6.23(s,1H),6.47(NH),7.27~8.17
(m,4H)
9 3280 1627 1339 0.67~2.33(m,9H),2.38,3.35(s,3Hx2),3.55(t,2H),
4.18(t,2H),5.27(s,1H),6.22(s,1H),6.53(NH),7.23
~8.13(m,4H)
3300 1636 1340 0.78(d,6H),1.60(m,1H),2.07(d,2H),2.37,3.63(s,
3Hx2),5.25(s,1H~,6.22(s,1H),6.53(NH),7.23^8.10
(m,4H)
11 3260 1620 1350 0.79(d,6H),1.20(d,6H),1.60(m,1H),2.08(d,2H),
2.38~s,3H),4.98(m,1H),5.26(s,1H~,6.20(s,1H),
6.86(NH),7.25 8.17(m,4H)
_
12 3315 1635 1350 0.78(d,6H),1.58(m,1H),2.08(d,2H),2.39,3.38(s,
3Hx2),3.60(m,2H),4.22(m,2H),5 30(s,1H),6.22(s,
lH),6 94(NH),7.28-8.17(m,4H)
.
13 3325 1640 1341 0.79(s,6H),1.57(m,1H),2.03(d,2H),2.32(s,3H),
2.93(t,2H),4.30(m,2H),5.15(s,1H),6.20(s,1H),
6.52(NH),7.00~8.03(m,9H)
--2~--
lZ9Z991~
Table 5 (No.2)
_
Exam IR(Nujol)~ c~,
No. NH C0 No2 NMR(CDCl;)~
14 3315 1633 1348 0.73~2.32(m,11H),2.38,3.66(s,3Hx2),5.27(s,1H),
. 6.24(s,1H),6.62(NH),7.25~8.13(m,4H)
3315 1630 1340 0.43~1.75(m,11H),2.09(d,2H),2.38,3.67(s,3Hx2),
5.27(s,1H),6.20(s,1H),6.77(NH),7.28 8.13(m,4H)
16 3310 1630 1340 0.73~2.57(m,11H),2.40,3.67(s,3Hx2),5.31(s,1H),
6.22(s,1H),6.70(NH),7.17~8.13(m,4H)
17 3335 1662 1338 2.10,2.41,3.50(s,3Hx3),5.07(s,1H),6.52(NH),
6.70~7.93(m,9H)
18 3275 1632 1340 2.26(d,3H),2.42,3.55(s,3Hx2),5.25(s,1H),6.13
(q,lH),6.48(NH),7.23~8.16(m,4H)
19 3280 1628 1338 1.72,2.13,2.28,3.48(s,3Hx4),5.09(s,1H),9.63
(NH),7.41~8.07(m,4H)
3345 1685 1348 0.73~2.27(m,11H),2.20,2.35,3.65(s,3Hx3),5.23
(~,lH),6.47(NH),7.23 8.10(m,4H)
21 3300 1630 1350 1.20(d,6H),2.43,3.58(s,3Hx2),2.95(m,1H),5.28
(s,lH),6.18(s,1H),6.45(NH),7.23~8.15(m,4H)
_
22 3300 1632 1345 0.62~1.78(m,12H),2.35(m,4H),2.36,3.63(s,3Hx2),
5.22(s,1H),6.55(NH),7.17~8.13(m,4H)
_
23 1.31(t,3H),2.06,2.42,3.64(s,3Hx3),4.24(q,2H),
5.19(s,1H),7.13~8.03(m,5H)
_
24 3310 1640 1350 1.43-2.73(m,8H),2.38,3.58(s,3Hx2),5.13(s,1H),
6.42(NH),7.17~8.13(m,4H)
3400 1690 1350 1.22(d,6H),2.42,3.45(s,3Hx2),2.97(m,1H),5.67
(s,lH),6.48(s,1H),6.32(NH),7.39(m,4H)
26 3430 1690 1356 0.55-l.90(m,12H),2.43(m,4H),2.28,3.50(s,3Hx2),
5.93(s,1H),6.68(NH),7.02~7.77(m,4H)
27 3285 1639 1338 0.96(d,6H),2.38,3.67(s,3Hx2),2.61(m,1H),5.33
(s,lH),6.29(s,1H),6.63(NH~,7.28~8.13(m,4H)
--2~--
lZ9Z991
Reference Preparation 1
Production of t-butyl phenyl-5-aminothiophene-2-
carboxylate (3)
OCH2COOtBu ~ OOH ~ ~ ~ H2COOtBu
(4) 1l (5)
Dr~ CHCN
s D r~
) H2N~--COOtBu
Et2NH
(3)
Wherein tBu is the same as defined above, and Et represents
ethyl.
A mixture of 3 28 g (14 9 mmo~) of t-butyl benzoyl-
acetate (4), 1 3 g (15 3 mmol) of cyanoacetic acid, and 0.3 g
of ammonium acetate with 0 1 ml of piperidine and 15 ml of t-
butanol is refluxed ùnder heating for 110 hours The rection
mixture is evaporated to leave a residue, which is dissolved
in ethyl ether, washed with dil aqueous solution of sodium
-hydrogencarbonate, and dried over magnesium sulfate. The ethyl
ether is removed to leave a residue, which is distilled in
vacuo to give 1 52 g (yield 42.0X: as a mixture of Z- and E-
forms) of 3-phenyl-4-cyano-3-butenoate (5), bp 126'C (0.1
mmHg).
To a suspension of 1.48 g (6.1 mmol) of the compound (5)
and 0.2 g (6.1 mmol) of sulfur in 5 ml of ethanol is dropwise
added 1 ml of diethylamine while being stirred at room
- 2~-
3 ;~9299~
temperature, and the reaction mixture is further stirred
overnight. The mixture is evaporated to leave a residue,
which is chromatographed on a silica gel column with methylene
chloride as an eluent to give 1.03 g (yield 61.3%) of the
titled compound (3) , mp. 110-116C.
IR(CHCla) ~ max: 3475, 3390, 1665 cm~l.
NMR(CDCl~) ~ ppm: 1.34 (s, 9H), 4.22 (NH2), 6.03 (s, lH),
7.37 (m, 5H).
Reference Preparations 2-9
[General Procedure]
~CN R3~ ~ H2COOtBu
R3CoCH2cootBu ~ OOH ~C
( ~ ) CHCN
NJ~co
~ H2 OtBu
Et2NH (m~
A solution of a compound (N ) and cyanoacetic ~cid
dissolved in a solvent is reacted at room temperature or under
heating to give a compound (V ). To a suspension or a solution
of the compound (V ) and sulfur in a solvent is added diethyl-
amine, and the mixture is stirred. The mixture i5 evaporated
to leave a reidue, which is either chromatographed on a silica
gel column or crystallized from a solvent to give the
objective compound ( m ) . This may be employed in the next
reaction step without purification.
According to the general procedure as above, the
-3~-
~Z9Z991
following starting materials (Reference Preparations 2-9) are
obtained (Table 6).
-31-
lZ92991
~..
rl ~ O~ O
ol-
o ^ ~ ~
~ ~ ~ r~ 8 ~
o O 1` ~ ,n ~ O O
_ ~ ~ I 1 0-ol, 0 o
+ ~ ~ _ O ~
~E~; c -~ - 8o~
2 ~ o 'n 1~ ~o o
~s ~ 1aY~y~
r ~ ~ ~ r~
--32--
lZ92991
~,~ ~ 0 o ~ ~ 0 ,` ~ ~
o~ .,, ~
o ~--
~o ~ o ~ ,.n o o O C`d
Y^ ~ ~ ~ ~ ~
_ ~ ~ o ,~ , o o
OD ~
+ ~ o o _ o _, o
3 ~ 0 ~
P5 ~ `;t O o, ~
O ~ ~ ~0~0 ~ ~
~.~
~ .
--33-
~29Z99l
^ ~ ~ E ~ E ^ ~ ^ -
~ `I O --:~ a)-- _ ~_
Q
c~ . ~r) . . ~ ~ ~ ~ ~ ^ O~
~ ~ â ~ ~ ûl _ _u~ ~U~ Ln
Co _ ~ 0
_ ~ ^u) ~ ~ -z
~1 ~ ," ~ _
52
~ ~ ~ ~ ~ '` Z _ ~~ u~
_ ^ ^ ^^ ^~0 E C') ^ ^a-- E
C X-- O
O~
o) U~ ) U3
o~ 1 o ~3 o o1
In ao
~ 0~ ~0-- 0~0-- 0
_
a) ~ I~ o o
O ~ O
~ U) U~ o 1
I~ _
aJ :~
V O O O O O
D
_
o o o ~ _.
O 00 cr
o~
ae
D~ ~
o
z
p~
lZ9Z99l
~eference Preparation 10
Production of 2-amino-5-isopropylthiophene t3-27)
~ HO + S + ~ OOE 3
EtOOC~____ 1) KOH
H2N ~ 2) HCl H2N
(3-27)
To a suspension of 17.0 g (0.15 mol) of ethyl
cyanoacetate and 4.8 g tO.15 mol) of sulfur in 17 ml dimethyl-
formamide tDMF) is dropwise added 12 ml of triethylamine while
being stirred at room temperature. 12.~ g (0.15 mol) of
isovaleroaldehyde is dropwi~e sdded to the mixture at 30-40C.
The reaction mixture i5 stirred at room temperature for 2
hours, combined with ice water, and extracted with ethyl
ether. The ether layer is washed with water, dried over
magnesium sulfate, and evaporated to leave a residue, which is
chromatographed on a silica gel column to give 26.0 g (yield
81.4%) of an oily product. A mixture of the oily product with
a solution of 14 g (0.244 mol) of potassium hydroxide in 140
ml of 50% aqueous methanol is refluxed under heating for 2
hours and then evaporated. The remaining aqueous portion is
treated with active carbon and acidified with acetic acid
under cooling to precipitate crystals, which are then
collected by filtration. To a solution of the crystals in 180
ml of methanol is dropwise added 4.5 ml of conc. hydrochloric
acid while being stirred at 60C, and the mixture is ref~uxed
~292991
under heating for 10 minutes. After cooling, water is added
thereto and the methanol is removed by evaporation ~n vacuo.
The residue is washed with ethyl ether, neutralizet with 15 ml
of 20% aqueous solution of sodium hydroxide while being cooled
under nitrogen atmosphere, and then extracted with ethyl
ether. The ether layer is washed with water, dried over
magnesium sulfate, and the ethyl ether is evaporated. The
residue is distilled in vacuo to give 6.3 g (yield 36.6%) of
the titled compound (3)' as a pale yellow oil, bp. 83C (0.9
mmHg).
IR(film) V max: 3310, 3190 cm~l.
NMR(CDCla) ~ ppm: 1.25 (d, 6H), 2.99 (m, lH), 3.4 3 (NH2),
6.00 (d, lH), 6.34(m, lH).
Reference Preparation 11
2-Amino-4-n-propylthiophene
1) ~ N EtOOC -Bu
~C=O COOEt ~
n-Bu 2) S.Et2NH H2N~ S ~ n-Pr
1) KOH ~ n-Bu
H2N~-Pr
2) HCl
(3-22)
Wherein Et and Bu each is the same as abo~e and Pr represents
propyl.
A mixture of 14.1 g (0.099 mol) of di-n-butyl ketone,
11.2 g (0.099 mol) of ethyl cyanoacetate, 1.54 g of ammonium
acetate, and 4.8 g of acetic acid with 30 ml of benzene is
-36-
~Z~91
refluxed under heating for 64 hours, then cooled. The
reaction mixture is washed with a dil. aqueous solution of
sodium hydroxide under cooling, dried over magnesium sulf~te,
and evaporated. The resulting residue is distilled in vacuo
to give 20.3 g of an yellow oil, bp. 130C (1.5 mmHg). To a
suspension of the oily product and 2.74 g (0.085 mol) of
sulfur in 20 ml of ethanol is dropwise added 5 ml of
diethylamine at room temperature, and the mixture is stirred
at 60c for 17 hours and evaporated to leave a residue, which
is chromatographed on a silica gel column (with methylene
chloride) to give 22.5 g (yield 84.5~ of the intermediate as
an yellow oil.
NMR(CDCla) ~ ppm: 0.77-1.82 (m, 15H), 2.61 (m, 4 H), 4.29
(q, 2H), 5.97(NH~).
To 0.683 g ~2.54 mmol) of the intermediate is atded a
solution of 0.33 g of potassium hydroxide in 80% methanol (2.5
ml), and the mixture is refluxed under heating for 5 hours.
The reaction mixture is evaporated to leave a residue, which
is dissolved in ice water. The solution is washed with ethyl
ether and neutralized with acetic acid to precipitate
crystals, which are collected by filtration. To a solution of
the crystals in 5 ml of n-propanol is dropwise added 0.5 ml of
conc. hydrochloric acid at 65C and then the mixture stirred
at 60C for 3 minutes. The solvent is removed and the
resulting residue is combined with ice water, washed with
ethyl ether, alkalized with 20~ aqueous solution of sodium
hydroxide, and extracted with ethyl ether. The ethyl ether
layer is washed with water, dried over magnesium sulfate, and
evaporated to give 0.289 g (yield 57.4~) of the crude titled
compound (3-22) as yellow oil.
-~7-
lZ92991
IR(film) ~ max: 3175, 3300 cm~l.
NMR(CDCla) ~ ppm: 0.70-2.72 (m, 16H), 3.50 (NH2), 5.90 (s,
lH).
Reference Preparation 12
Production of 2-amino-4-cyclopentylmethyl-5-methyl-
thiophene (3-20)
1) <
EtC ~ COOt-Bu
2) S.Et2NN
-BuOOC ~ t-BuOOC
H2 H3 H2N t
A B
CF3COOH
H2 3
(3-20)
A mixture of 1.93 g (0.0137 mol) of 1-cyclopentyl-2-
butanone, 1.94 g (0.0137 mol) of t-butyl cyanoacetate, 5 ml of
benzene, 0.2 g of ammonium acetate, and 0.6 g of acetic acid
is refluxed under heating for 20 hours while being dehydrated,
After cooled, the reaction mixture is washed witS dil. aqueous
solution of sodium hydrogencarbonate, dried over magnesium
sulfate, and evaporated. The resulting residue is distilled
in vacuo to give 17 g of a colorless oil, bp. 50-C (0.1 mmHg),
-38-
~29'~
To a mixture of the oil with 2 ml of t-butanol and 0.26
g of sulfur is dropwise added 0.5 ml of diethylamine while
being stirred at room temperature. The reaction mixture is
stir-red at 60c for 18 hours and evaporated to give a residue,
which is chromatographed on a silica gel column (with benzene)
to give 2.21 g (yield 55.2%) of the intermediates A and B (as
a mixture) as an yellow oil.
A solution of 1.11 g (3.76 mmol) of the intermediates
(a mixture of A and B) in 5 ml of trifluoroacetic acid is
stirred at room temperature for 30 minutes, then 60c for 1.5
hours, and the solvent is removed. The residue is dissolved
in ethyl ether, washed with a dil. aqueous solution of sodium
hydrogencarbonate, and extracted with lN-hydrochloric acid.
The aqueou~ layer is neutralized with an aqueous solution of
sodium hydrogencarbon~te and then extracted with ethyl ether~
The organic layer is evaporated to give a residue, which is
chromatographed on a silica gel column (with methylene
chloride) to give 415 mg (yield 56.5X) of the titled compound
(3-20) as an yellow oil.
NMR(CDClJ) ~ ppm: 1.00-2.58 (m, 9H), 2.17 (s, 3H), 2.34 (d,
2H), 3.72 (NH2), 5.92 (s, lH).
_~q _
~29Z991
Reference Preparation 13
NaH nBu~ ~CH2COOtBu
nBuCOCH2COOtBu + (EtO)2POCH2CN ) ~C~
( ~ -6) IIHCN ( V-6)
To a suspension of 0.4 g (10.6 mmol) of 60% sodium
hydride in 6 ml of dimethoxyethane (DME) is dropwise added a
solution of 1.77 g (10 mmol) of diethyl cyanomethylphosphonate
in DME (4 ml) while being stirred under ice-cooling. After the
addition, the reaction mixture is further stirred at 20c for
10 minutes. A solution of 2.0 g (10 mmol) of the starting
material (~ -6) in 2 ml of DME is added thereto and the
mixture is refluxed under heating for 4 hours, then cooled.
The reaction mixture is combined with ice water and extracted
with ethyl ether. The ether layer is wsshed with a dil.
aqueous solution of sodium hydroxide, then water, dried over
magnesium sulfate, and filtered. The mother liquor is
evaporated in vacuo to give 1.972 g (yield 88.3%) of the
objective compound (V -6) as a colorless oil, bp. 80-90-C (0.1
mmHg). This is a mixture of Z- and E-forms of the objective
compound.
NMR (C~C1J~ ~ ppm: 0.8-1.6 (m, 7H), 1.44 (s, 9H), 2.28
(t) 2.49 (t) (2H), 3.08 (d) 3.34 (s) (2H), 5.27 (d, IH).
Reference Preparation 14
NaH isoBu~ ~CH2COOtBu
isoBuCOCH2COOtBu + (EtO)2POCH2CN ) `C'
-7) CHCN ( V 7)
-40-
~29Z991
In the same manner as shown above, the reaction is
performed by using a suspension of 8.06 g (0.168 mol) of 50%
soldium hydride in 160 ml of DME, a solution of 28.0 g (0.158
mol) of diethyl cyanomethylphosphonate in 127 ml of DME, and a
solution of 31.7 g (0.158 mol) of the starting material (~ -7)
in 63 ml of DME to give 23.4 g (yield 66.2%) of the objective
compound (V -7) as a colorless oil, bp. 85C (0.25 mmHg).
This is a mixture of Z- and E-forms sf the objective compound.
NMR (CDCl~) ~ ppm: 0.89 (d) 0.96 (d) (6H), 1.46 (s, 9H),
1.95 (m, lH), 2.15 (d) 2.37 (d) (2H), 3.07 (s) 3.33 (s)
(2H), 5.26 (s) 5.35 (s) (lH).