Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1~9~ 87
--1--
The present invention is concerned with a procesq
for the detection of an analyte in a ~ample, a~ well as
with an agent suitable for this proces~.
For many year~, immunological detection processe~
have played an important part in clinical diagnosis.
They are characterised in that they are highly ~pecific
and extremely sensitive. Thes~e detection procesQes are
based upon the immunological exchange action between the
analyte to be detected and its binding partner or
partners. By labelling one of the binding partners, the
degree of reaction and thus the concentration o~ the
analyte to be mea~ured can be determined. The immuno-
logical determination proces~es differ, depending upon
the selected labelling, for example radio-immunoa3say
(radioactive labelling), enzyme immunoa~say (enzyme
labelling), fluorescence immunoa~say (fluore~cence
label) and the like.
A disadvantage of the3e methods is that, in some
cases, very long incubation times are necessary and the
detection proces~e~ are very labour-intensive. In the
case of the radio-immunoa~say~, there are also the
difficulties which are involved with the handling of
radio-active substances.
Therefore, attempts have not been lacking to
improve these tests. Thus, for example, a measurement
process,which is eqpecially preferred for many purpoqes,
takas place in quch a manner that the analyte to be
;LZ~3~7
~2--
measured is first incubated with an excess of the
labelled binding partner. The labelled binding partner
not bound by the analyte is fixed with the help of
carrier-bound analyte After a usual ~eparation of the
bound from the non-bound part, the original analyte
content of the sample can be determined on the basi3 of
the labelling.
Like all other immunological proces~es, this
measurement principle display!3 a very high ~ensitivity
but, in addition, has the advantage that it can be
carried out more quickly than the previou~ly u3ual test
procedures. However, an important disadvantage of this
variant is that the labelled binding partner of the
analyte to be determined must be used in excess, i.e.
relatively large amounts of labelled i~munological
binding partners are needed. The detection of highly
concentrated analytes in undiluted blood, ~erum or
plasma is, because of the necessarily high concentration
of antibody and the costs involved therewith, scarcely
capable o~ being carried out.
Therefore, the present invention
seeks to provide a process in which not so high
concentrations of labelled binding partners are
necessary and which, therefore, can be carried out more
coYt-favourably than the previously known processes.
Thus, according to the present invention, there
is provided a process for the detection of an analyte,
~Z931~7
wherein a sample containing the analyte i3 incubated
either with a labelled binding partner and the
immobilised analyte or an immobilised analyte analogue
or with the labelled analyte or analyte analogue and
an immobilised binding partner, the binding partner
displaying towards the immobilised or labelled analyte
or analyte analogue a higher affinity than towards the
free analyte, and the labelled~ binding partner or the
labelled analyte or the labelled analyte analogue is
present in insufficiency compared with the concentration
of the analyte to be detected.
By an analyte, there is understood, quite
generally, a substance which can enter into a specific
exchange action with a suitable binding partner. Here-
under is to be understood, for example, every kind ofexchange action, for example an antigen-antibody,
enzyme-coenzyme or also any other ligand-receptor
exchange action. The analyte ig preferably a hapten,
for example theophylline, thyroxin (T4), phenobarbital
or the like, an antigen, for example a nucleic acid or
a protein, such as human choriogonadotropin (hCG),
human serum albumin (HSA), carcinoembrionic antigen
(CEA), a-foetoprotein (AFP), glycosilated haemoglobin
(HbAl), imrnunoglobulins or the like, or also an anti-
body. The binding partner is determined by the partic-
ular analyte. If the analyte is, for example, a hapt~n
or antigen, then, as binding partner, there is needed
3~7
an antibody directed against this hapten or antigen,
if an antibody is to be determined, then, a~ binding
partner, a related antigen or hapten or anti-antibody
is necessary.
By an analyte analogue there is to be understood,
according to the present invention, a compound which
displays a structural relationship with the anaLyte and
thus also related binding properties. Such analyte/
analyte analogue pairs include, for example, T3/T4
theophylline/theobromine, human serum albumin/monkey
serum albumin. As an analyte analogue, there can also
be used anti-idiotypical antibodies which are directed
against the antigen binding points. Such antibodies
automatically have a structural relationship with the
analyte to be determined.
By antibodies in the meaning of the present
invention are to be understood not only monoclonal but
also polyclonal antibodies. They can be used as
complete antibodies or also in the form of antibody
fragments, for example Fab fragments. According to the
present invention, there are used antibodies which
recognise and more firmly bind the carrier-bound or
labelled analytes or the carrier-bound or labelled
analyte analogue more specifically than the free
analytes.
Such antibodies include, for example, antibodies
which co-recognise the bridge member with ~hich the
~31~37
--5--
analyte or the analyte analogue is bound to the carrier
matrix or to the labelling material. Such antibodies
can be obtained according to known methods, whereby,
for the immunisation, a substance is used which consists
o~ the analyte or the analyte analogue and the bridge
member, as well as, if the analyte or the analyte
analogue is itself not immunogenically active, an
immunogenic protein, for example albumin, ede~tin or
the like.
Antibodies can also be used which racognise the
analyte analogue specifically and cross-react with the
actual analyte only with low affinity. ~hus, for
example, according to the process of the present
invention, T4 can be determined when T3 is bound to
the carrier matrix and an antibody is used which has a
high affinity towards T3 but only little affinity
towards T4. Proteins can also be determined in an
analogous way. Thus, in the manner according to the
present invention, human serum albumin (~SA) can be
detected in that monkey serum albumin is bound to the
solid pha~e and the antibody is directed against the
monkey serum albumin with less cross-reactivity to
human serum albumin. The analyte can also be determined
when, as analyte analogue, a particular part structure
or part sequence is bound to the carrier matrix and an
antibody is used which recognises this immobilised part
structure or part sequence more speci~ically than the
- ~Z93~
--6--
analyte to be detected. This variant can be advantage-
ously utilised, for example, for the detection of
glycosilated haemoglobin in that, on the solid pha~e,
there is bound a synthetic peptide with the glycosilated
place of the glycosilated haemoglobin and an antibody
against this peptide is used.
With the help of such antibodies, it is possible,
in the process according to the present invention, to
use the enzyme-labelled binding partner or the labelled
analyte or the labelled analyte analogue in an
insufficiency with regard to the analyte to be determined.
How different the concentrations of analyte to be
determined and labelled binding partner or labelled
analyte or analyte analogue can be depends decisively
upon how great is the affinity difference of the binding
partner towards the free analyte and the immobilised or
labelled analyte or analyte analogue~ The lower is the
affinity of the binding partner towards the free analyte
in comparison with the bound or labelled analyte or
analyte analogue, the lower can be the concentration of
the labelled binding partner or of the labelled analyte
or analyte analogue in comparison to the concentration
of the analyte to be determined~ We have found that
the process according to the present invention can be
carried out with advantage at a concentration ratio of
labelled binding partner or labelled analyte or analyte
analogue to analyte to be determined of up to 1:10000
~L~93~7
--7--
when the binding partner used displays an affinity
difference of the same order of magnitude. A concent-
ration ratio of 1:2 to 1:3000 haq been shown to be
especially advantageous.
In the case of the incubation of the sample
which contains the analyte to be determined with the
labelled binding partner and the carrier bound analyte
or analyte analogue, the following reactions compete
essentially with one another:
(1) A -~ B-M = A-B-M
(2) A-B-M ~ AfiX = Afi -B-M I A
In these equations, A is the analyte to be
measured, B is the binding partner directed against
the analyte, M is the labelling agent and AfiX is the
analyte or analyte analogue bound to the carrier.
According to equation ~1), the immunological
complex A-B-M is formed from the analyte and the labelled
binding partner B-M. Because of the great excess of the
analyte, this equilibrium is substantially displaced to
the side of the labelled immunological complex so that,
in the reaction mixture, there is present almost
exclusively complex and excess free analyte. Because
of the higher affinity of the binding partner towards
the bound analyte AfiX, a displacement reaction accord-
ing to equation (2) takes place. The hlgher is theconcentration of the analyte to be detected in the
lZ93~ 7
sample, the greater is the displac~ment of the analyte A
to be detected from the immunological complex A-B-M by
the carrier-bound analyte or analyte analogue A~iX
countered. Consequently, the concentration of tha
analyte in the sample is proportional to the amount of
the labelling in the soluble part o the reaction
mixture or, vice versa, inve~se proportional to the amount of
the labelling which is bound to the carrier via A~iX.
By meaqurement of the labelling bound to the carrier or
al~o of the labelling present in the solution, there can
be deduced the concentration of the anaLyte in the
sample.
Analogous equa~ions can be formulated and
analogous considerations can be made for the case in
which the sample is incubated with the labelled analyte
or a labelled analyte analogue and an immobilised binding
partner.
The process according to the present invention can
be carried out in advantageous way in that the sample to
be investigated containing the analyte to be detected is
first pre-incubated with the labelled binding partner.
This incubation mixture is subsequently brought into
contact with the solid phase to which is bound the
analyte or an analyte analogue~ It is followed by a
usual separation of the bound from the non-bound part.
As the last step, the labelling is determined in the
free and/or bound part and, from this~ a conclusion can
12~31f~7
g
be made about the analyte content of the sample
The proc~3s can also be carried out in 5uch a
manner that the sample to be investigated, the labelled
binding partner and the analyte or analyte analogue
bound to a carrier is simultaneously incubated. After
the incubation step, the bound and non-bound part is
again separated, the labelling is determined in the
bound and/or in the non-bound part and, from thisD a
conclusion is made about the concentration of the
analyte in the sample.
Such a one-step test process can be carried out,
for example, in the form of a usual microtitre test.
For this purpose, the analyte i3 first immobilised on
the microtitre plate. Thereafter, it is incubated with
a mixture of labelled binding partner and the sample
with the analyte to be determined. After a definite
incubation time, the solution is removed, washing is
carried out and subsequently the amount of labelling
agent bound to the microtitre plate is measured.
The above-described process variant can also be
readily carried out when, in3tead of the labelled bind-
ing partner, there is used the labelled analyte or a
labelled analyte analogue and, instead of the immobilised
analyte or analyte analogue, there is used an immobilised
binding partner.
The binding partner or the analyte or the analyte
analogue can be labelled in the usual way. A whole
~Z5~3~8~
--10--
series of known labelling agents are available, for
example labelling with a radio-active isotope, with
an enzyme, with a fluorescing substance or with some
other substance which can be detected photometrically.
In the scope of the present invention, labellin~ with
enzymes is especially preferred. As labelling enzyme,
there can be used, for example, peroxidase, aLkaline
phosphatase, glucose oxidase or, especially preferably,
~-galactosidase.
The binding of the analyte or of the analyte
analogue or also of the binding partner to a carrier
also takes place in a conventional manner. For this
purpose, there are utilised reactive groups of the
analyte, of the analyte analogue or of the binding
partner. If these substances do not possess suitable
reactive groups, such groups can be introduced into
the molecule according to known methods. Via these
reactive groups there takes place the attachment with
reactive groups of the carrier material, usually with
the insertion of a bifunctional bridge member. The
binding partner can also be applied to the carrier
adsorptively. As carrier materials, there can be used
all materials normally employed for the immobilisation
of immunologically-active, hiochemical compounds, for
example nitrocellulose, papers, synthetic resin
particles of polystyrene and the like.
The present invention also provides an agent for
~9~ 87
carrying out the process according to the present
invention. This agent contains either the immobilised
analyte or an immobili~ed analyte analogue and a
labelled binding partner or the labelled analyte or a
labelLed analyte analogue and an immobilised binding
partner, as well as possibly an appropriate buffer
system and, in addition, usually employed adjuvant
materials, the binding partner displaying towards the
immobilised or labelled analyte or analyte analogue a
higher affinity than towards the free analyte.
Further additional, usually employed adjuvant
materials include, for example, wetting agents,
stabilising agents, galenical adjuvant agents, structure
formers and the like.
If, for the detection of the labelling agent,
further substances are necessary, then these can also
be present in the reagent. If, as labelling agent, for
example an enzyme is used, then the substrate and other
adjuvant materials necessary for the detection of this
enzyme are advantageously added to the agent.
The agent can be produced in the most varied ways.
It can consist, for example, of different solutions
which contain the labelled binding partner or the
labelled analyte or the labelled analyte analogue, an
appropriate buffer system and detection materials for
the determination of the labelling agent, as well as
possibly further adjuvant materials, and of a solid
:
3187
phase to which i9 bound the analyte or the analyte
analogue or the binding partner, possibly via a bridge
member. As solvent, there can be used water or a
mixture of water with a water-soluble organic solvent,
for example methanol, ethanolj acetone or dimethyl-
formamide. For reasons of storage stability, it can
be advantageous to divide the reagents needed for the
test into two or more solutions which are first brought
together when carrying out the actual investigation.
For certain embodiments of the agent according
to the present invention, it can be expedient when the
components of the agent are present in part or also
completely in lyop'nilised form. For this purpose,
solutions of the component materials in question are
first prepared in the usual way and these are then
freeze-dried in known manner. Before use, the lyophil-
isates are reconstituted in the usual way with an
appropriate solvent, for example water.
It can also be expedient to produce individual
components or all of the components of the agent accord-
ing to the present invention in the form of powder
mixtures or reagent tablets. For this purpose, the
components of the agent are mixed with conventionaL
galenical additive materials and granulated. Such
additive materials include, for example, carbohydrates,
such as mono-, oligo- and polysaccharides, sugar
alcohols, such as mannitol, sorbitol and xylitol, and
3~ 7
- 13 -
other inert compounds, such as polyethylene glycols
and polyvinylpyrrolidone.
Furthermore, it is possible to apply individual
components or also all of the components of the agent
to a carrier and especially to an absorbent carrier,
or to incorporate them into a carrier. It can also
be advantageous to apply or to incorporate the reagent
component~ to various carriers or absorbent carriers
and to combine these different carriers with one
another in appropriate manner. mus, an especially
pre~erred embodiment of the reagent according to the
present invention is constructed in such a manner that
the labelled binding partner is contained on a first
carrier, the analyte or analyte analogue is applled
in immobilised form to a further carrier material and
possibly a third carrier contains the reagent con~onents
necessary for the detection of the labelling agent~
These three layers are so bound with one another that
the sample is first applied to the first carrier with
the labelled binding partner. The reaction between the
analyte to be determined and the labelled binding
partner here takes place. The reaction mixture is then
brought into contact with the second layer which contains
the bound analyte or the bound analyte analogue, the
displacement reaction thereby taking placeO The portion
of the labelled binding partner bound by the analyte or
analyte analogue ~ixed on the carrier remains adhering
~2~ 37
to this carrier matrix. The so-called "free" portion
of the labelled binding partner bound with the analyte
to be detenmined is then transferred to the third
layer on which the detection of the labelling agent
can be determined, for example on the basis of a colour
reaction or photometrically. The transport of the
reaction mixture through the individual layers can take
place mechanically, for example by applying pressure,
or also by diffusion or capillary force.
The invention is further described in the follow-
ing Examples with reference to the accompanying drawings
in which:
FIGURE 1 illustrates graphically extinction curves
produced in Example l;
FIGURE 2 illustrates graphically a competition
curve produced in Example 2;
FIGURE 3 illustrates graphically the calibration
curve of Example 3, and
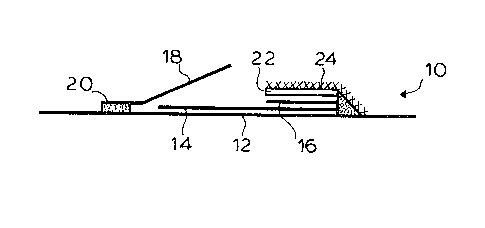
FIGURE 4 shows a test strip in accoraance with
the invention.
With further reference to Figure 4 a test strip 10
comprises a support layer 12 having a carrier layer 14
containing an immobilized analyte component, a carrier
layer 16 containing labelled binding partner and a
flap layer 18 hinged at 20 to support layer 12. Flap
layer 18 contains reagent components for detection of
the labelling agent. A glass fibre fleece 22 overlies
layer 16 and is secured to layer 12. Layer 24 is a cover layer.
~Z93~
- 15 -
The following Examples are given for the purpose
of illustrating the present invention:
Example 1.
Detection of theophylline.
A) Produc_ion of a ~olyclonal antibody aqainst
theo ~ ledestin
Sheep are immuniqed in known manner with
theophylline-9-carboxypentyledestin. An antibody
solution is isolated in known manner from the antisera
taken from the sheep and the antibody solution is
purified over theophylline-9-pentyl-Sepharose*, elution
taking place with graduated theophylline gradients.
The fraction which is obtained with a high theophylline
concentration is, after dialysis, further used.
B) ~
Theophylline-9-carboxypentylhydroxysuccinimide
ester is coupled to rabbit IgG (non-specific). The so-
obtained polyhaptens (PH) are mixed with bicarbonate
trade mark
~3~ 7
- 16 -
buffer (200 mmolar, pH 9.4) (10 ~g. polyhapten/ml. of
carbonate buffer). A microtiter plate iq coated with
this polyhapten solution.
The antibodie~ described in A) are diluted with
incubation buffer (PBS, 1% bovine serum albumin, 0.1%
TWEEN*20) so that a 10 8 solution is obtained. One park
by volume of this solution is mixed with the same volume
of a serological dilution seriles (factor 1:2n) of theo-
phylline, theophylline-9-carboxypentyl-N-t-BOC-lysine
or caffeine and incubated for one hour at ambient
temperature. (N-t-B~C is an abbre~iation for the tert.-
butyloxycarbonyl radical which is a conventionally used
nitrogen protective group).
These incubation batches are applied to the
microtitre plates coated with polyhapten, followed by
incubation for a further hour at ambient temperature~
Thereafter, the solution is removed and washed several
times. Finally, into the microtitre plate is applied
rabbit anti-sheep antibody labelled with peroxidase
~100 mU/ml.), followed by incubation for one hour at
ambient temperature and subsequent washing. Into each
hollow of the microtitre plate is introduced loo ~i.
ABTS substrate solution (ABTS = 2,2'-azino-d-(3~ethyl
benzthiazoline-6-sulphonic acid). After one hour, the
extinction is determined with a micro-ELISA reader.
Typical mea urement curves are shown in Fig. 1 of the
accompanying drawings. In this Figure:
* trade mark
,
~Z~3~l~7
(1) is the curve for theophylline
(2) is the curve for theophylline-9-carboxypentyl-
N-t-BOC-lysine
(3) i~ the curve for caffeine
It can be read off therefrom that an affinity
difference of a factor 200 to 400 exist~ in the case
of an acceptable cross-reaction of 2.5% with caffeine~
C) Detection of theophylline
The detection of theophylline takes place
completely analogously to the proce~s described above
in B).
20 ~1. theophylline-containing serum and 20 ~1.
of the above-mentioned antibody solution are incubated
in the way described above under B) and analysed on a
microtitre plate pre-coated according to B). First,
with the help of serum samples which contain a precise,
known amount, namely 2.5, 5, 10, 20 and 40 mg./litre
theophylline, the following calibration table is
obtained:
Ctheo n~
2.5 490
392
300
224
143
L _
i
~ 7
-18-
The unknown theophylline content in sarnples i~
determined on the basis of this Table.
Exa~E~2~
Detection_of thyroxin (T4)
A) Product on of_a monoclonal antibody aqain~t
tetraiodothyronine-N-t=BOC-edestin
Female balb/c mice are immunised in known manner
with T4-N-t-BOC-edestin. Spleen cells of these immunised
mice are fusioned with the myeloma cell line Ag 8.653
according to the known method of Kohler and Mil~tein.
Clones obtained are selectioned. These clones are
selected which pos~ess a high affinity for T4-N-t-BoC-
ir~nunoglobulln.
For the selection, the antibodies are incubated
with a concentration series of T4 (factor 1:4 J (one
hour at ambient temperature) and thereater transferred
to a PH-coated microtitre plate. After a further two
hours incubation, the supernatant solution is removed,
the plates washed and, analogously to ~xample 1, the
bound antibody made visible with peroxidase-labelled
anti-mouse antibodies and ABTS. Fig. 2 of the accompany-
ing drawings shows the competition curve of such an
antibody against rnodified ~4 (curve 2~ in comparison
with a curve which has been obtained with an anti-T4
antibody which is directed directly against T4 (curve 1).
An affinity difference of a factor of 2000 can be saen.
~2~3~7
-19-
B) Conjuqation of the
~-aalactosidase
Purified monoclonal antibodies or Fab fragments
obtai'ned herefrom are reacted with maleimidohexanoyl-
hydroxyquccinimide (MHS). The MHS-modified antibodies
or antibody fragments are reacted with ~-galactosidase.
The reaction mixture is separated via gel filtration
(Sephacry~ S 400). A conjugate was used which is in
the molecular weight range of 700,000 to 20,000,000 D.
C) Detection of tetraiodothyronine
Microtitre plates are coated analogously to
section A) above with tetraiodothyronine-t-BOC-immuno-
globulin (1 ~g./ml.). Serum samples (50 ~1.) with 0,
6, 25, 50, 125 ,and 247 ~g./litre of tetraiodothyronine
(for the preparation of these serum samples, there is
used TBG-free ~tandard serum, Boehringer Mannheim) are
filled into the hollows of the microtitre plates and
mixed with 150 ~1. antibody-~-galactosidase conjugate
(20 mU/ml.). After incubation for one hour at ambient
temperature, the supernatant is removed and the micro-
titre plate is washed and each hollow filled with 200 ~1~
substrate ~1 mmole chlorophenol red galactoside, prepared
according to Federal Republic of Germany Patent Specific-
ation No. 33 45 7~8). The calibration table obtained
herefrom is given in the following:
* trade mark
1~93~
-20-
CT4 ( ~g, ji. ) ~ . . .
. . . _ .~
0 620
6 5g6
~5 519
433
125 320
247 225
Serum samples with an unknown T4 content can be
determined on the basis of this calibration table~
10 ExamPle 3.
Test str~ with 1 w-affinity antibodies for the
determination of theophylline
Monoclonal antibodies are produced in kn~ manner with th2
help o~ the ~noqen theophylline-8-carboxYProPYledestm.
;~ 15 The antibodies are selected by competition
experiments: 10 ~l. amounts of antibody solution and
10 ~~1. of a theophylline concentration series (5 x lO 4 M
o
to 6 x lO M theophylline in PBS~ are incubated for two
hours in a theophylline-8-carboxypropyl-rabbit IgG-coated
microtitre plate. The batch is then remo~ed from the
microtitre plate, the plate is washed and the antibody
bound to the polyhapten made ~isible as in Example l B~.
Antibodies are selected, the binding of which to
the polyhapten-coated plates could only be competed with
large amounts of theophylline ( ~ 2 x 10 5 M).
129~ 7
-21-
I'he complete antibodies or the antibody fragments
are linked with ~-g~lactosidase as described in Ex ~ le 2
under B).
Production of the solid carrier layers.
A) Coniuqate_carrier
Filter paper (liquid take-up about 10 ~l./cm )
is impregnated with a solution which contains
100 U/ml. anti-theophylline~8-carboxypropyledestin
antibody
lo 1% bovine serum alburnin
1 mmole magnesium chloride and
25 mmole hepes/sodium hydroxide, pH 7.2.
The filter paper is subsequently dried.
B) Polyhapt-en-matrix
The hapten is applied to a nitrocellulose membrane
~0,2 ~pore size). For this purpose, the membrane is
incubated for 12 to 1~ hours with polyhapt~n solution
(3 mg./ml. polyhaptan in PBS). ~hereafter, it is
after-loaded for two hours with 1 mg./ml. bovine serum
albumin and washed for 30 minutes with 0.05% Triton
X-100 in PBS.
C) Substrate ca rier
Filter paper (liquid take-up about 10 ~l~/cm )
is impregnated in the usual way with a solution which
contains
0.4% Tween*20
3 mM chlorophenol red galactoside
* trade mark
~;~93~7
25 mM hepes/sodium hydroxide, pH 7.2
D) Test stri~s
With the~e layers i4 produced the test strip
shown in Fig. 4 of the accompanying drawings, the plasma
separation being according to Federal Republic of
Germany Patent Specification No. 30 29 579.
E) Measurement
l'he sample (30 ~l. wholle blood, serum or the like)
i9 applied to the glass fibre fleece and diffuses to
the conjugate on carrier 1. This is thereby dissolved and
interacts with the analyte present in the
sample. The solution mixture passes to the polyhapten
membrane 2 and is chromatographed thereover. Thereafter,
thefla~ with the substrate carrier 3 ispressed on to the matrix 2. me
part of the solution mixture not retained by the hapten
matrix at the start of the matrix comes into contact
with the substrate, the enzyme-sub~trate reaction taking
place. After 20 seconds, the colour development i~
determined by reflection photometry on the end of the
polyhapten matrix (~ % R = change of the reflection in
% of the light shone in after 20 second~). On the basis
of samples with known theophylline concentrations, there
is obtained the calibration curve shown in Fig. 3 of the
accompanying drawings which covers the whole therap~utic-
ally relevant range. With the help of this calibrationcurve, there can be determined the theophylline
concentration in unknown samples.
1~31~7
-23-
Example 4.
Determination of phenytoin by means of low-affinity
antibodies.
A) Production of the low-affinity antibodies
The antibodies are produced analogously to
Example 1 A) by immunisation of sheep with diphenyl-
hydantoin-valeric acid-bovine serum albumin. Tests for
the competitiveness are carried out analogously to
Example 3 A). They show that a relatively high concen-
tration (6 x 10 6 M) of phenytoin is necessary for the
competition of the polyhapten (antibody concentration:
about 5 x 10 M, determined by absorption at 280 nm).
Therefore, the antisera are not further fractionated.
V) Production of the test element
The determination process is carried out accord-
ing to the method described in European Patent
Specification No. 0,073,513. There is used the insert
element for a centrifugal automatic analyser described
therein in Figures 1 a and 1 b. This insert element
contains seven chambers connected with one another
which, in part, contain different fleece and are dosed
successively under the influence of centrifugal force.
The fleece are impregnated with different impregnation
solutions.
The following fleece and impregnation solutions
for the fleece are employed:
l~g3~f~7
-24-
Fleece 1 filter paper
impregnation 3% wetting agent (Tweer~ 20)
solution:
Fleece 2: filter paper
impregnation 100 mmole sodium phosphate buffer,
solution pH 7.2
5 mmole EDTA
1% bovine serum albumin
O.75% wetting agent (Tween 20)
10 Fleece 3: filter paper
impregnation anti-phenytoin antibody from sheep,
solution:
prepared according to A), labelled with
~-galactosidase, 100 mU/ml. activity,
determined with o-nitrophenylgalactoside
as substrate,
1% bovine serum albumin,
4 mmole magnesium aspartate,
50 mmole hepes buffer, pH 7.2
Fleece 4: filter paper
impregnation 2 ~g./ml. rabbit immunogLobin G which
solution:
has been derivatised with diphenylhydantoin
valeric acid,
4 ~g./ml. sheep anti-rabbit Fc antibody
produced according to Federal Republic
of Germany Patent Specification No.
34 46 636
* trade mark
~Z93~tj,
-25-
Fleece 5: filter paper
impregnation 15 mmole chlorophenol red galactoside,
solution:
prepared according to Federal Republic
of Germany Patent Specification ~o.
33 45 748
The chambers of the insert element are loaded as
follows:
chamber 1: 1 fleece 1
chamber 2: empty
chamber 3: 1 fleece 2
chamber 4: 1 fleece 3
chamber 5: 1 fleece 4
chamber 6: 2 fleece 5
chamber 7: measurement cuvette.
C) Carryinq out of the measurement
The serum used as sample solution is diluted
1:100 with 0.9~/~ aqueous sodium chloride solution. 60 ~1.
of the so obtained solution are pipetted into the sample
application chamber of the insert element and then the
following centrifuging programme carried out:
25 seconds 250 rpm
dissolving off of the detergent, buffer and conjugate,
start of the first incubation
20 seconds 2000 rpm
25 300 seconds 600 rpm
incubation of sample and anti-phenytoin-antibody
conjugate
~31~7
-26-
300 seconds O rpm
incubation with carrier-fixed spaced phenytoin
15 seconds 2000 rpm
ending of the second incubation
15 seconds O rpm
5 seconds 100 rpm
transport of the liquid into cuvettes
50 seconds 720 rpm
measurement at 578 nm.
With the above-described centrifuging programme,
the wetting agent is first dissolved from fleece 1 for
the simplification of the liquid transport. ~ubsequently,
buffer and conjugate fleece are soaked and the components
present thereon dissolved. After 300 seconds incubation
in the first valve cham~er, in which phenytoin binds
with the anti-phenytoin-antibody-enzyme conjugate, the
equilibrium is ad~usted in the next step in the 300
seconds with the spaced and fixed phenytoin present in
excess with regard to the sample. The solution is then
transported to the substrate fleece, the substrate
thereby being dissolved off. The total liquid mixture
passes into the cuvette where the extinction decrease
is monitored for 50 seconds.
With the help of samples which contain a known
amount of phenytoin, a calibratlon curve is produced
according to the above-described process. On the basis
of this calibration curve, samples with unknown phenytoin
content can be measured in corresponding way.
~Z9~ '7
- 27 -
The patent specifications referred to herein are
more particularly identified hereinafter, all assigned
to soehringer Mannheim GmbH:
EPO Patent Specification 0,073,513, Sigmar Klose
et al, filed Auyust 30, 1982, open to public March 9,
1983;
Federal Republic of Germany Patent Specification
3,029,579, Peter Vogel et al, filed August 5, 1980,
open to public February 18, 1982, issued December 12,
1985;
Fede.ral Republic of Germany Patent SpeciEication
3,446,636, Helmut Jering et al, filed December 20,
1984, open to public June 26, 1986;
Federal Republic of Germany Patent Specification
3,345,748, Manfred Kuhr et al, filed December 17, 1983,
open to public August 29, 1985.