Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~2~
~XPR~S~ B 62531271
--1--
APPARATUS AND METHOD FOR MEASURING
NATIVE MAMMALIAN BLOOD VISCOSITY
This invention is related to a co-pendiny
Canadian application serial ~umb~r 538,318 fil2d May
28, 1987.
Field of the Invention
This invention relates to the meas~1rement of
the full range of shear rate dependent viscosities of
mammalian blood and of other pseudoplastic fluids,
i.e., it generates a profile of vlscosity vs. shear
rate for non-Newtonian fluids.
Description of the Prior Art
U.S. Patent No. 3,720,097 discloses a diag-
nostic method and apparatus by which a sample of fresh
mammalian blood is taken by puncturing a blood vessel
and introduced into a system including (1) a capillary
generating a linear relationship between pressure and
flow of Newtonian ~luids, and which is calibrated to
dellver 20 ml. of normal saline control solutlon ln
one minute at -50 mm. Hg. at 37C, 12) a programmed
vacuum pump whi~h draws the blood sample through the
caplllary at a variety o~ pressures in th~, range o~ o
to 200 mm. Hg., (3) an electromagnetic ~lowMeter which
senses the flow rate of the blood through the
capillary, and (4) X-Y recording means to instantane-
ously plot the resultant of the blood flow with
respect to pressure variations from which information
on the blood viscosity at various shear rates may be
determined. U.S. Patent Nos. 3,999,538 and 4,083,363
disclose methods and apparatus by which a sample of
fresh ma~malian blood is taken by puncturing a blood
--2--
vessel while the sample is maintained under a constant
predetermined pressure, and the sample is wlthdrawn
through a volume measuring device connected directly
to a pressure measuring device. sy correlatiny the
time required to withdraw a specified amount of blood
with constant pressure maintained during withdrawal,
an indication of blood viscosity is obtained. While
these methods and apparatus may function as described,
their use has been heretofore limited to research
projects, and in certain cases the application of the
apparatus has necessarily involved the introduction of
fresh blood into equipment requiring elaborate clean-
lng procedures to decontaminate the instrument between
experiments. These complexities mitigate against the
general, convenient and practical use of this
viscometric approach in clinical practice. Later
developments to overcome these problems related to
U.S. Patent No. 3,720,097 led to a completely liquid-
filled pressure measuring system, in which pressure
taps filled with sallne solution are used to access
the pressure at each end of a caplllary containlng the
blood sample to be measured. The pressure taps are
connected to opposite sides of a ~luid-filled high
compliance differential pressure transducer. Ater
settlng up an inltial flow in the capil.1.ary with a
syringe or other extornal pressur~ source, the exter-
nal ~low i~ stopped after the compliant transducer
diaphraym has been deflected by an lnitial amounk of
differential pressure between the pressure taps. The
stored elastic energy of the compliant transducer
diaphragm then causes relaxation of the diaphragm and
accompanying liquid flow from the high pressure to
low pressure side of the transducer with the flow path
including the capillary containing the blood as the
ma;or resistance to flow. sy properly chooslng the
--3--
dimensions and conflguratlons of th0 elements of the
system, the system functions as a first order dynamic
system exhibiting an exponential decay and time con-
stant proportional to viscosity for a N~wtonian fluid.
Viscosity is determined from relationship of shear
rate to shear stress. The shear stress is proportional
to the pressure in the capillary, while shear rate is
calculated from the time rate of pressure change
across the capillary, which is proportional to the
flow. Moreover, a non-Newtonian fluid will have
instantaneous viscosities which vary as a function of
the instantaneous shear rates. Therefore, the multi-
ple shear rate dependent viscosities (i.e.~ the vis-
cosity profile) of fresh blood, a non~ Newtonian fluid,
can be measured in a single experiment over a 50 - 100
X shear rate range; also by appropriate choice of
capillary and diaphragm the experiment may be designed
to last only a short period (typically 20 - 60
seconds). The shear rate range and experimental
duration are selected to provide information of clini~
cal interest (e.g., red cell aggregation effects,
particularly at low shear ~ate) while the overall
experimental time ls short enough so that any blood
sample remains well mixed, and fresh blood s~mples can
be tested without the necessity for anttcoagulant
On the other hand, the time for the test ls long
enough so that very short term relaxation phenome~a
~less than a few seconds) in the blood do not influ-
ence the results. The techniqu~ and some of the stud-
ies carried out on and results obtained with it are
described in the following publications:
A. Downs, A., Litt, M., and Kron, R.~.: Low
Shear Rate Viscosity o~ Fresh Blood,
Biorheology, Vol. 17, 25-35, Pergam~on Press
Ltd., 1980.
--4~
s. Yepse~, G., Boutin, D., Litt, M t ~nd Kxon,
R.E.: Rheological Modelling o~ Fresh ~lood
from Transient Pressure Measurements,
Biorheology, Vol. 18, g75-484, Pergamon
Press Ltd., 1981.
C. Seybert J., Kron, S., Litt, M. and Kron,
R.E.: A portable Computerized Clinical Whole
Blood viscometer. Proceedings of the VI
International Conferece of the
Cardiovascular System Dynamics Society,
332-335, November 1984.
Despite the above described improvements of
U.S. Patent No. 3,720,097 there still remain
deficiencies which complicate their application in the
general clinial setting, including complexity, cost
and safety in blood handling, an important factor with
the inrreased risks from blood borne diseases such as
AIDS and hepatitis. Moreover, undiluted fresh blood
samples cannot easily be recovered from the
instrumentation for additional laboratory testing, the
system must be disassembled to be cleaned if blood is
inadvertently permitt~d to clot in ~he fluid~carrying
elements and care mus~ be taken. to avold contaminatlon
from spilIing of blood or blood contact with the
operator. Also, because lt must be completely li~uid
filled, the system is sensitive to errors due to the
presence of air withln any of the fluld enclosures.
If anticoagulated (rather than fresh) blood is used,
the same deficiencies apply ~xcept for the clotting
problem, but an additional complication of
inhomogeneity due to RBC settling is added. Most
importantly, these prior developments are not amenable
to the design of a cost effective, disposable device.
With the introduction af F~A-approved drugs
to reduce blood viscosity in human beings, the need
--5--
has arisen for a practical clinical method and appara-
tus for monitoring hemorheological drug therapy and to
determine whole blood viscosity. The method must be
inexpensive, safe, easy to use and accurate.
Heretofore, "blood viscosity determinants" such as
blood hematocrit and flbrinogen content have been used
to estimate high shear rate blood viscosity but they
suffer from wide lndividual variability. Also, these
indirect determinants are time consuming and expensive
to obtain and do not accoun~ for many factors such as
red blood cell interactions and red blood cell
flexibility that contribute heavily to low shear rate
blood viscosity. Therefore, accurate, direct, immedi-
ate and clinically useful blood viscosity measures
cannot be determined in an individual patient from
knowledge of hematocrit and fibrinogen parameters aloneO
Summary of the Invention
We have now invented a method and apparatus
that provides from a single small sample of blood ~or
other non-Newtonian liquid) a complete proile of
viscosity versus shear rate over the full range of
clinlcal lnterest ln a sa~e and le~s comple~ manner
than prior instrumentation and methods. In summary,
the method lnvolves acquirlng a sample of blood and
(1) establlshing a body o~ the sample wlthin a
constricted space (such as a capillary), sald hody in
one direction communicating with the atmosphere (or
other body of fluid of known pressure), and said body
in the other direction communicating either with (a
an enclosed body of blood (or any other llquid~
retained by a compliant diaphra~m, or (b) with a body
of blood having a free surface in contact with a body
of air (or other compressible fluid), (2) establishing
a body of air of known mass communicating on one side
-6-
with either (a) the compliant diaphragm or ~b) the
free surface of said body o~ blood and on the o~her
slde wlth means for sensing the change ln ~he pressure
with time of the known mass of air (31 establishing an
initial viscous flow of blood in the constricted
space, thereby changing the pressure of the body of
air (and deflecting any compliant diaphragm), (~)
allowing said compressed body of air ~and any
deflected compliant diaphragm) to relax, thereby
establishing a transient viscous flow in the
constricted space, (5) sensing the changing pressure
with time in the enclosed body o~ air during said
transient viscous flow with said se~sing means, and
(6) processing the output of said pressure sensing
means to determine the time varying shear stress and
shear rate of said sample so as to calculate the
changing viscosity of said sample as a ~unction of
shear rate during the relaxation of said compliant
diaphragm and/or said body of air, ther~by providing a
continuous profile of viscosity versus shear rate of
said sample plus other rheological parameters of
interest.
Brief Description of the Drawin~
Flg. 1 is a schematlc diagram of the appara
tus of an embodiment of thls invenkion;
Flg. 2 is a schematic diagrarn of the record-
ing and data analysis instrumentation of an embodiment
of this invention;
Fig. 3 is a r~productiQn of a semi~log
record of the viscosity measurements obtained using a
sample of normal saline solution in accordance with
the process and using the apparatus of an embodiment
of this invention;
Fig. 4 is a reproduction of a semi-log
record of the viscosity measurements obtained using
one sample of fresh whole blood in accordance Wi th the
process and using the apparatus of an embodiment of
this invention;
Fig. 5 is a schematic diagram of the appara-
tus of another embodiment of this inve~tion; and
Fig. 6 is a schematic diagram of the appara-
tus of another embodiment of this invention.
Description of the Preferred Embod1ments
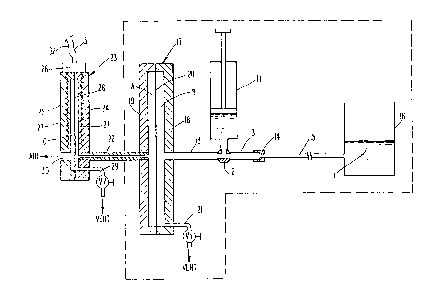
Referriny now to Fi~. 1, a conventional
hypodermic syringe 11 is shown containing a blood
sample. The syringe 11 is adapted for connection to
and is shown connected to a thr~e-way valve 12
positioned medially of a conduit 13. One end of the
conduit 12 is connected through a connector 14 to a
capillary 15, which is in turn connected into an open
reservoir 16, shown containing a body of liquid L.
The opposite end of the conduit 13 is connected into
an assembly 17 consistlng of a recessed shell 18, a
mating recessed shell 19, a first compliant diaphragm
20 and a venting system to air 21 containin~ valve Vl.
As constructed, the first compllant diaphragm 20 is
sealed between the recessed shell 18 and th2 mating
rec2ssed shell 19. Moreover, conduit 13 i5 connected
i~to assembly 17 through recessed shell. 18, and a
conduit 22 is connected lnto assembly 17 through
mating rec~ssed sheLl 19 at one end thereof, while at
the opposlte end lt is connected into the measuring
assembly 23 consisting of a recessed shell 24, a
mating recessed shell 25, a second compliant diaphragm
26, embedded coils 27, coil output means 28, and
venting system to air 2g containing valve V2. ~ating
recess0d shell 25 has an openlng 30 formed therein
open to the air, and the second compliant diaphragm
26 is sealed between recessed shell 24 and mating
recessed shell 25.
Referring now to Figs. 1 and 2, electrical
leads 31, 32 connect the coil output means 28 to a
transducing modulator-demodulator means 33. The out-
put from the modulator-demodulator 33 is a voltage
proportional to the deflection of the compliant
diaphragm 26 sensed by coils 27 and is proportional to
the pressure of the air or fluid in space A and is fed
to a record~r 34 adapted to produce logarithmic pres-
sure versus time cuxves in semi-log strip chart form,
and also to differentiator and filter 35 adapted to
produce a voltage proportional to the instantaneous
sensed pressure in space A, and a voltage proportional
to the instantaneous ~ime rate of pressure change
(pressure-time derivative) in space A, These voltages
are fed to the inputs of an analog to digital
converter 36, which feeds digitized data to a
programmed computer 37, which ls served by a disk
drive 38, a monitor 39, and a printer 40.
In order to carry out the method of this
invention, the apparatus is first calibrated with nor-
mal saline solution at 37C. With reference to Flgure
lA, the syringe 11 and three~way valve 12 are used to
fill the space B which is the portlon of assembly 17
bounded by the fir~t compli~nt diaphragm 20 and the
recessed shell 18, through condult 13. The ven~ing
system 21 is used to eliminate air from space B.
Space A consists of the remaining space in assembly
17 bounded by first compliant diaphragm 20 and mating
recessed shell 19 and is connected throuyh conduit 22
with the space ln pressure measuring assembly 23
bounded by recessed shell 24 and second compliant
diaphragm 26. Referring to ~lgure lB, the syringe 1 l
and three-way valYe 12 are now used to fill the
'7
g
rernainder of conduit 13 and capillary 15 with norrnal
saline solution. Referring to Figure lC, the thr0e-
way valve 12 is now set so that there is no flow and no
net pressure difference between the portions of the
apparatus filled with liquid L because diaphragms 20
and 26 are not deflected and the pressure in space A
is atmospheric. Space A is adjusted to atmospheric
pressure by means of the venting system 29. At this
point, the output voltage of the modulator-demodulator
33 is set at zero to indicate atmospheric pressure
throughout the system. Th~ computer is activated with
the required program and test settings. The
calibration run is begun by introducing more saline
solution under pressure from syringe 11 through three-
way valve 12 in the position shown in Fig. lA. This
increased pressure causes the first compliant
diaphragm 20 to deflect, thereby increasing the air
pressure in the space A. In response to this
increased alr pressure, the second compliant diaphragm
deflects against the atmospherlc pressure in space C
which ls between diaphragm 26 and recessed shell 25
and that amount of deflection is sensed by embedded
coils 27, generatiny a signal which is fed through the
electrical leads 31, 32 as input to the modulator-
demodulator 33. Additional syringe pressure is
appli~d until the voltage output from modulator~
demodulator 33 is ~reater than 10 volts, at which
point the recoxder 34 and computer 37 are activated.
Now the three-way valve 12 is set to close off ~yringe
11, as sAown in Fig. lC, to permit fluid flo~ under
the force due to the elastic relaxation of both
compliant diaphragms and intervening fluid in space A,
through conduit 13, capillary 15 and into reservoir
16. As this flow continues, the pressure against
first compliant diaphragm ~0 decreases with time, as
--10--
does the pressure in the space ~ contained by the
compliant diaphragms 20, 26, which in turn causes a
decreasing voltage signal from modulator-demodulator
33. When this pressure-related signal drops below 10
volts, the computer program is automatically activated
so as to acquire data representing the relationship of
fluid pressure in space A versus time. At the same
time, the recorder 34 continuously prints out a sPmi-
logarithmic plot of pressure versus time. Fig. 3 is a
reproduction of a print of the resulting viscometric
relationships generated by recorder 34 during the
calibration of the apparatus with normal saline
solution. with referenc~ to Fig. 3, Curve A repre-
sents the equilibrium base li.ne pressure before pres-
sure is applied with a syrlnge. Curve B represents
the increase in pressure in space A resulting from
compressing the syringe. Curve C is the 10 volt maxi-
mum output of modulator-demodulator 33 when pressure
is applted with the syringe~ While the modulator-
demodulator 33 output is above 10 volts, the valve 12
is turned to configuration shown in Fig. lC and the
voltage output decays until it falls below 10 volts as
shown at polnt D. Curve E shown the relaxation and
incorporates the pressure-time data requir0d to
calculate vlscosity. Curve F represents the return
of system to baseline atmospheric pressure. ~he ~act
that the plot g~norated for normal saline soluti~n in
Fig. 3 ~Curve E) is a straight llne shows that the
system senses saline solutlon as a Newtonian fluid,
whose first order decay in such a system is expected
to be negatively exponential, with a single time con-
stant which gives a falling straight line when plotted
as a logarithm against time. The fact that the curve
is completely linear also shows that no non-linear
second order effects are present.
The plotted curve shown in Fig. 4 was gener-
ated during a test run utilizing fresh whole blood.
After filling the apparatus with normal saline solution
as in the aforementioned calibratlon run, syringe 11
was filled with a sample of fresh whole bl~od, and by
utilizing three-way valve 12, the syringe 11 was
operated according to ~he configuration shown in Fig.
lB so as to displace saline from the portion of
conduit 13 betw~en connector 14 and three-way valve
12, through capillary 15 and in~o the body of liquid L
in reservoir 16. At this point, three-way valve 12
was set as in Fig. lC so as to permit flow in each
direction through conduit 13 in order to achieve
pressure and flow equalization therein. The valve
was then turned to configuration shown in Fig. lA to
deflect the diaphragms 2~ and ~6 so that output of
modulator-demodulator 33 exceeds 10 volts. Valve 12
is then turned to configuration shown in Fig. lC,
allowing pressure in space B to relax as in the
aforementioned calibration test run. It is noted from
comparing Figs. 3 and 4 that the time represented by
Curve C between pressurization and drop of the pres-
sure generated voltage below 10 volts is significantly
longer for blood than for the saline solution because
of the greater vlscosity of blood~ Also, 1~ 15 appar-
ent that curve ~, showing decay o~ the pressure in
Fig. 4, is significantly prolonged for blood compared
to sallne and ls curved concave upward. Such non-
linear behavior is characteristic o~ a pseudoplastic
(non-Newtonlan) fluid such as blood, and indicates
that the viscosity of the blood sample is increasing
as the rate of flow (shear rate) decr~ases. The
computer data from the run generating the curve of
Fig. 4 (Table I) showed a shear rate range from 1
sec-l to 46-1, which covers a significant part of the
shear rate range of clinical interest in analyzing
-12-
blood rheology. Within said range, the viscosity of
the blood sample varied from 2.5 centipoise to 46
sec~l to 8.6 centipoise at 1 sec~l. The com-puter also
fits a Power Law or Casson rheological equation of
state to the viscosity data thereby providing
parameters which have clinical utility in
characterizing the rheology of blood. For the sample
shown in Fig. 4, the value of the Power Law exponent n
is 0.68 and the value of the parameter K is 0.085.
These values are characteristic of fresh blood having
the viscosity profile shown in Table I. In order to
maintain physiologically meaningful test conditions,
the equipment shown in Fig. 1 was enclosed in an air
bath at 37c. The temperature of the air bath may be
set at other values if it is desired to study varia-
tion of blood rheology with relation to temperature,
as in patients with cryoglobulinemia.
TABLE I
Computer Output of Viscosities of Blood Sample
Shown ln Fiouro 4 at Selected Shear Rates
S _ r Rate (sec 1) viscosity_lcentipoise
R.56
1 0
19 3.3
28 2.9~
37 2.70
46 2.~2
In an alternative embodiment of this inventlon, the
pressure sensing means consisting of elements 23-32 of
Fig. 1 and containing the second compliant diaphragm
26 and the modulator-demodulator of Flg. ~ are
replaced by any pressure transducing device with asso-
ciated power supply that generates a suitable electri-
cal signal proportional to the pressure in space A,
-13-
and which is then processed in the described manner by
the remai~ing system of Fig. 2.
It ls not essential to the operation of this
invention that the interface between the body of liq-
uid in space s and of air in space A of Fig. 1 be pro-
vided by a physical barrier such as cornpliant
diaphragm 20. A suitable configuration can be prs-
vided for maintaining the interface between the liquid
sample and the air. ~his is shown in the alternative
embodiment of this invention illustrated in Fig. 5.
Referrlng now to Fig. 5, the capillary 41 is connected
to two chambers 42 and 43, in which interfaces are
provided between the body of liquid and the air in
spaces A1 and A2. The chamber 42 is connected through
tubing means 44 to the three-way valve v3, which can
be vented to the atmosphere or connected to pressure
measuring means 45, which communicates to the system
of Fig 2 through wires 48 and ~9. Chamber 43 is simi-
larly connected through tubing means 45 to three-way
valve V4, which can be vented to the atmosphere or
connected to syringe 47.
In order to carry out the method of this
embodiment, the system is inltially empty of all
ll~uid. Valve v3 is turned to vent the space Al, and
valve V4 is turned to connect syringe 47, which con-
tains the test or calibration liquid with tubing 46
and chamber 43. The test liquid ls introduced to the
system using syringe 47, filling the cap.illary ~1 and
partially ~illing chambers 42 and 43 so as to estab-
lish air-liquid interfaces in these chambers. Valve
v3 is then turned to connect space Al with the pres-
sure measuring means ~5. At this point the output
voltage of the modulator-demodulator 33 of ~ig. 2 is
set to zero to indicate atmospheric pressure in space
Al. The sample syringe 4~ i~ then replaced with an
-14~
air filled syrlnge or other pressurization means and
pressure applied to chamber 43, khe liquid, and the
air in space A1. When the pressure output is greater
than 10 volts, the valve V4 is turned to vent space A2
to the atmosphere. The higher pressure in space Al
then causes the liquid to flow through capillary 41
from chamber 42 to 43, with reduction in pressure in
space Al. Suitable design allows correction for the
small changes in pressure head in chambers 42 and 43
during this process. The result is a pressure-time
curve comparable to that shown in Fig. 4, which may be
processed as described above to determine the
viscosity of the test fluid as a function of shear
rate. Tests of this embodiment give results compara-
ble to those found with the first embodiment described
above.
It is also not essential to the operation of
this invention that the test fluid be introduced using
a syringe. Fig. 6 shows an alternative embodlment of
this invention in which the sample of blood or other
test fluid is supplied ln a standard vacuum-type blood
collecting tube. Referring now to Fig. 6, vacuum
collecting tube 51 contains the blood sample and ls
closed by rubber stopper RS. Needle 52, a long
hypodermic typ~ needle, is connected using fitting 61
to capillary 50, which in turn is connected to chamber
54. Chamber 54 is connected by tubing means 55 to
three-way valve V6, which can either vent air space A3
to atmosphere or connect it to the pressure measuring
means 56. Means 56 is connected by wires ~7 and 58 to
the system shown in Fig. 2. Also, short needle 53 is
connected by fitting 62 to tubing means 60, which in
turn is connected to three-way valve v5, which can
either vent the air space A4 to atmosphere or connect
it to air-filled syringe 59.
-15-
In order to carry out the method of this
embodiment, the apparatus is initially empty o~
liquid. Stopper RS is pierced by needle 53 whose end
extends into air space A4, and needle 52, which
extends into the liquid sample to the bottom of tube
51. Value V6 is turned to vent air space A3 to
atmosphere. Valve v5 is turned to connect syringe 59
to needle 53 and air space A4, The pressure in air
space A4 is then raised using the syringe, pushing
liquid from the bottom of tube 51 through needle 5~ to
fill capillary 50 and partially fill chamber 54 to
establish an air-liquid interface in chamber 54.
Valve v6 is then turnPd to close off the vent and
connect the pressure measuring means 56 to air space
A3. The modulator-demodulator in Fig. 2 is then set
to zero to indicate atmospheric pressure in air space
A3. Additional pressure on the syringe pressurizes
the gas in air space A3 untll 10 volts output ls
obtained for the pressure in air space A3. Valve V5
is then turned to vent the pressure in air space A4 to
atmosphere. The higher pressure in air space A3 then
causes the liquid to flow ~rom chamber 54 through the
capillary means 50 and needle 52 back into the tube
51, reducing the pressure in air space A3 as a ~unc
tion of time. Suitable d0sign allows for the
correction in pressure du~ to the small height change~s
in charnber 54 and tube 51. The result is a pr~ssure
-time curve comparable to that obtained in the previ-
ously described embodiments which can be processed by
the system of Fig. 2 to determine viscosity as a func-
tion of shear rate and yield results comparable to
those found with the previously described
embodiments.
In alternative embodiments of this
invention, the capillary means described in the
-16
embodiments of Flgs. 1, S and 6 may be replaced by
any fluid resistlve device, linear or non-linear,
whose flow characteristics arQ known or may be deter-
mined by suitable calibration.
In another embodiment of this invention, the
analysis and recording system shswn in Fig. 2 may be
replaced by any suitable means in which the pressure-
time signal resulting from the flow is differentiated
by analog or digital means to obtain values
proportional to the lnstantaneous shear stress and
shear rate, from which values of viscosity versus
shear rate and rheological parameters may be calculated.
It should be evident to anyone familiar with
the art of constructing transient flow systems that
other embodiments of this invention are possible, in
which a time-varying flow of a liquid is used to prod-
uce a time-varying pressure in an enclosed air (or
oth~r compressible fluid), space such that the
viscosity-shear rate characteristics of the liquid can
be determlned from the dynamic behavior and time con-
stant o the system.
It will also be readily apparent that the
method and apparatus of this lnvention provide an
lnexpensive, sa~e, easy to use and accurate
determinatlon of ~resh whole blood viscoslty.
Importantly, by this means, blood samples may be
tested without introductlon into expensive and complex
laboratory equipment and the necessity of elaborate
cleaning procedures between test experim~nts. It ls
apparent that the portions of apparatus of this
invention which are enclosed in the dotted lines in
Figs. 1, 5 and 6 are either readily available as
disposable items or may be conventionally engineered
from disposable materials.
It 1s also evident that this system is espe-
~3~ 7
17-
cially applicable to measuring the viscosity of blood
because it requires small sample sizes, provides rapid
measurement with rapid data reduction, and maximum
safety in handling and cleanup since all components
t~at contact the blood may be disposed of. The
embodiments of this invention can also b~ used without
adding anticoagulants to the blood sample if desired,
since the determination is so rapid. ThiS invention
is also applicable to measuring the viscosity-shear
rate behavior of non-Newtonian fluids other than
blood, but has its greatest advantages when used with
bioloyical fluids.
It will also be readily apparent that this
invention is applicable to the analysis, diagnosis and
treatment of various hematologic disorders such as
hyperviscosity syndromes and blood clotting.
This invention also has application to meas-
urements n~cessary during the production and utiliza-
tion of polymeric fluid systems in which the time and
shear dependent flow properties of the sample are
important, such as monitoring the course of
polymPrization reactions or ln quality control of
industrial products, such as paints, plastics,
pharmaceuticals and foods.