Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-1 -
3;~L
LIQI~ID MI~NG E~qPLOYING EXPANDING, T~ G LIQUID SXEETS
ckground - Field OI th0 Invention
This invention relatss to very rapid and complete mixing of fluids in a
continuous manner by a process that mixes very thin liquid sheets of the different
fluids together.
Bac~ound -- ~escription of P~orAl t
The prior art has been concerned for many years with the rapid and complete
mix;ng of liquids so as to reduce segregation R components within the mixture.
Less than very rapid and complete dispersion is particularly deleterious in
processes utilizing very fast reactions. The term fast reaction implies a reaction
that has a time scale that is more rapid or on the same order of the time scale of
mixing of the reactants. If the fast reactions are complex, i.e., they involve
reactions tllat are multistep, then product distribution can be adversely affected (see
J.Y. Oldshue, Fluid Mixing Technology, ~cGraw-Hill Publications Co., New
York, N.Y., 1983, pp. 222-229). Segregation that occurs due to inhomogeneities
within the mixture on the molecular scale can change the product distribution from
that calculated assuming perfect and complete mixing before the reactions begin.Particularly with multiple reactions, failure to pay attention to segregation within
the mixture can cause wastage of raw materials in producing undesired
substances, difficulties in scale-up, and an increased load on the separation plant
(see J.R. Bourne, F. Kozicki, and P. Rys, "Mixing and Fast Chemical Reaction",
Chem. Eng. Sci., 36(10), pp.1643-1663,1981).
In general, liquid-phase reactions occuring in viscous media, such as
polymerization and biochemical reactions, are particularly subject to the in-fluence
of segregation. A recent symposium entitled "Rapid Mixing and Sampling
Techniques in Biochemistry" examined the problems of characterizing
biochemical reactions that proceeded more rapidly than the time scale of the initial
mixing of the reactants (see Chance, B., et. al. (eds.), Rapid Mixing and Sampling
Techniques in Biochemistry, Academic Press, New York, N.Y., 1964).
~l2~632~
Prior art for rapid mixing generally uses jets of liquid th~t impinge against one
another or tangentially mounted feed tubes that mix the fluids in a swirl cup. An
example of such a mixing device is given in U.S. patent 4,239,732, granted Dec. 16,
1980 to F.W. Schneider. These types of mixers can give fairly complete mixing ofvery small amounts of ]iquids in times as low as a few milliseconds for low
viscosity fluids. However, these streams are relatively thick and this limits the
speed vith which solutions can be mixed. It has long been known that if two or more
liquids can be made as thin as possible before they are mixed, then rapid and
complete mixing is virtually assured (see pp. 49-~3 in Chance, B. et.al., supra).
To this end a Russian scientist, Yu B. Kletenick in the Russian Journal of
Physical Chemistry, Vol. 37(5), p. 638 (May, 1963) has devised a mixing device that
mixes thin liquid layers together. It does this by flowing two liquids between very
nnrrow parallel plates similar to a triple decker sandwich. Between the first two
plates the first fluid flows and between the second and third plate the second iluid
flows. The liquids are accelerated to high velocities (two meters/second or higher),
so that they flow separately through the parallel plates, and are mixed once they
flow beyond the end of the plates and into free space. With this system Kletenick
claims to have obtained mixing times on the order of 90 to 100 microseconds for low
viscosity liquid sheets of 200 microns thickness.
Although Kletenick claims that his device provides fast mixing it suffers from anumber of drawbacks.
1) Since the device requires flow between two parallel flat plates with a very
narrow gap and of significant length, the pressure drop is r01atively high. Any
attempt to further decrease the size of the thin film produced further increases the
pressure drop. This is particularly severe if the reactants are viscous.
2) The width of the plates themselves must also be very thin (100 microns),
otherwise the fluids will completely miss each other once they flow out the end of the
narrow gaps. Such a device is not only difficult to construct but is also very
delicate, rendering it unsuitable for industrial use. Applications where the fluids
must be injected at only moderately high pressures are not feasible.
3) The device is limited to a gap of about 0.1 mm between plates which limits the
thinness of the sheets formed to 0.2 mm or about 200 microns as per Kletenick's
analysis of how his mixing device works.
4) Because of the very narrow plate gaps plugging is a potential problem in
systems containing suspended solids.
~ 2~32~
- 3 -
5) Kletenick's device is impractical at usual industrial flowrates of liters perminute and higher.
SummaIg and Objests o~the Invention
It has now been surprisingly found that the very thin liquid sheets formed from
liquid atomizers prior to droplet ~ormation can be contacted or impinged at one
another to produce a thin liquid mixed sheet. The result is surprising because such
thin sheets ( 25 microns and less) are generally subject to disruption if they are
contncted~ However, if the sheets are contacted together in the same general
direction of flow, and if a gentle angle of approach between the thin sheets is used,
tllen the sheets will mix together and produce a new sheet of the mixture that is
highly turbulent.
It is one object of the present invention to provide a means OI contacting two or
more liquids in a sheet thinner than has heretofore been possible, yet at flowrates
suitable for industrial use. As the contacting of fluids occurs in thinner and
thinner sizes, the mixing becomes more complete as well as rapid. The thinner the
physical scale of mixing, the faster compnnents can diffuse towards each other.
It is another object of the present invention to not only contact thin liquid sheets
together, but further to mix them through turbulence within the newly-formed liquid
sheet. This enhances mixing many fold by providing bulk movement of liquids
towards one another rather than relying on molecular dif~usion alone.
It is also an object of the present invention to provide a means of mixing liquid
sheets together for fluids that are significantly viscous without unreasonable
pressure drop.
A further object of the present invention is to provide devices for mixing thin
liquid sheets together thflt are very simple and easily manufactured. The preferred
embodiments of the present invention are simple to manufacture, easy to maintain,
and require no alignment to maintain mixing of the ultra thin liquid sheets.
Readers will find further objects and advantages of the invention from a
consideration of the ensuing description and the accompanying drawings.
}~ieDescription oIDrawings
Fig. 1 illustrates the method by which a liquid atomizer forms a thin liquid sheet
prior to droplet formation.
. ' ' ' ~' . ' ~
~2~32~
Fig. 2 is a sectional view of a preferred liquid atomizing device for use in thepresent invent;on.
Fig. 3 illustrates a preferred embodiment of the present invention.
Fig 4 illustrates other atomizing devices for use in the present invention.
l~esQ~ption of a P~ed Ernbodiment
F`ig. 1 illustrates a swirl atomizer that generates droplets by the disruption of a
liquid sheet, Liquid 10 enters chamber 12 where it is swirled. The swirled liquid
tlows at high velocity (greater than 0.5 meter/sec) as a thin film 11 with an air core
16 along the walls of the atomizer 14 until finally ejected into free space as acontinuous thin liquid sheet 18. At some distance beyond the atomizer the sheet
breaks up into droplets 20. Because the liquid velocity and liquid flowrate within
the sheet are constant (therefore, the cross sectional area must remain constant),
but the sheet expands radially or in its width, the liquid sheet must thin. Thisthinning continues to occur unl;il surface tension forces exceed inertial forces,
causing the liquid to roll back on itself to form droplets 20. These sheets are very
thin (of order microns) and have a residence time on the order of milliseconds.
The sheets are stable, provided that they are not prematurely disrupted into forming
droplets. The droplets that form are typically 100 times or so thicker than the liquid
sheet.
A preferred atomizer for practicing the present invention is shown in Fig. 2.
Referring to Fig. 2, atomizer 30 swirls fluid 32 in core 34. The swirled fluid flows
along the walls of atomizer 30 as a fairly thin liquid layer of order hundreds of
microns in thickness. The liquid sheet 42 exits through opening 36 at an angle of
about 110 to 150 degrees. The center of atomizer 30 contains a deflector plate 38 that
is screwed into the very center of the atomizer on thread 40. The liquid sheet 42
exiting the atomizer is deflected by deflector 38 to an angle of about 120 to 170
degrees, preferably 150 degrees relative to the atomizer.
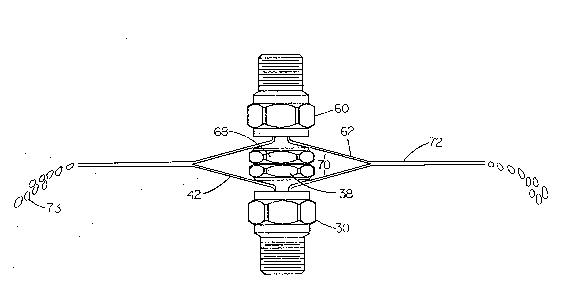
Figure 3 illustrates how two of the atomizers considered in Fig. 2 can be used to
practice the present invention. Atomizer 30 is now joined by an exact duplicate
atomizer 60 that is inverted. Liquid sheets 42 and 62 are formed at angles of 1~0
degrees relative to atomizer 30 and atomizer 60 respectively. As the atomizers are
brought physically closer to one another the liquid sheets 42 and 62 begin to contact
and mix into a new sheet 72. As shown in Fig. 3, these single sheets are conical,
resulting in a circular mixed sheet. The mixed sheet 72 is typically of equal length
as either single thin liquid sheet 42 or 62. The preferred method of operation is to
physically touch or join the deflector plates 38 and 58 together as is shown in Fig. 3.
~2~Ei3~
- 5 -
The liquid sheets 42 and 62 contact and form a new, continuous, highly-turbulent,
mixed sheet 72. The mixed sheet 72 continues to thin until surface tension forces
prevail, causing the formation of droplets 73. The contacting of the sheets is in the
same general direction of liquid flow, and the contact angle 70is usually about 30
degrees. If the liquid sheets were contacted at each ot'her while flowing in opposite
directions, annihilation of the thin liquid sheets would occur. Further, if the angle
of contact is larger than about ~0 degrees, the sheets of some liquids, particularly
low viscosity liquids such as water, have a tendency to disrupt into droplets, rather
than forming a mixed thin liquid sheet.
This preferred embodiment of the present invention has the advantage that
physical joining of deflector plates 38 and ~8 ensures proper alignment for mixed
sheet formation at all times. Of course, this preferred means of mixing thin liquid
sheets could initially be manufactured as one unit.
Single thin liquid sheets of water are generally transparent. However, a thin
mixed liquid sheet is substantially more opaque indicating turbulence within theliquid sheet. It is the combination of contacting two or more liquids as thin sheets
and producing a high degree of turbulence within the mixed sheet that provides for
e~tremely rapid mixing times. For low viscosity fluids the mixing time is of order
tenths of a millisecond, as demonstrated in Example 1, infra.
The degree of turbulence within the mixed thin liquid sheet is generally a
function of the velocity of the single liquid sheets and the angle of contact orimpingement. As the velocity and/or angle increases, so does the level of
turbulence within the mixed sheet. In general, any angle of contact up to about 60
can be used for nonviscous liquids. For viscous fluids angles greater than 30 are
preferred in order to provide a high degree of turbulence. Further the maximum
angle of contact allowable up to the point of sheet annihilation is higher for viscous
fluids because the high viscosity tends to hold the liquid sheet together.
In general, however, contact angles greater than about 60 can lead to sheet
disruption with premature droplet formation. This is not a preferred method of
operation for two major reasons. The droplets that form are typically 100 times
thicker than the thickness of the liquid sheet just prior to disruption. Further it is
usually very difficult to generate turbulent flow within droplets. In contrast, the
preferred embodiment not only contacts two thin liquid sheets, but it also produces a
high degree of turbulence within the subsequently formed mixed liquid sheet.
While Fig. 3 illustrates a preferred means for practicing the present invention,any atomizing device that forms a thin liquid sheet, and can be devised to impinge
upon another thin liquid sheet in the same flow direction at a gentle angle, can be
used for practicing the process of the present invention. As shown in Fig. 4 such
~6~2~
- 6 -
atomizing devices can be whirl (or swirl) chamber-hollow cone atomizers,
deflected fan-spray atomizers, oval-orifice f`an-spray atomizers, jet-impingement
deflected atomizers, centrifugal atomizers, rotary atomizers, rotary disc wheel or
cup atomizers. These produce a sheet 18 and drops 20 similar to Fig. 1.
The preferred embodiment illustrated in Fig. 3 provides for physical contact
between the atomizing devices. While this is highly desirable for maintaining
proper alignment and thus reliability in mixed sheet formation, it is not required
for the practice of the present invention. As an example, consider atomizers 30 and
60 of Fig. 3. If deflector plates 38 and 68 are removed, thin liquid sheets 42 and 62
will still impinge and form a mixed liquid sheet 72. This device, however, is less
reliable because alignment of the liquid sheets is not as easily maintained and
because the angle of contact or impingement approaches or exceeds 60 degrees
since the sheet is not deflected to a 150 angle but retains about a 120 angle. These
considerations are particularly important in high pressure applications where
vibration can cause misalignment.
Although the discussion has primarily focused on the mixing of two liquids, tests
have shown that three or more liquids may be mixed simultaneously to produce onesingle mixed liquid sheet by preferred embodiments of the present invention.
~Iowever, the complexity of arranging and maintaining proper alignment may
make such applications impractical. ~ better method, depending on the fluids to be
mixed, is to use the present invention in series and mix only two liquids at a time.
For example, if three liquids are to be mixed, liquids 1 and 2 can be mixed by the
process of the present invention. The mixture of fluids 1 and 2 and the single liquid
3 can be mixed by another liquid sheet mixer in series with the first.
The present invention is applicable to any mixing process of liquids that can
ordinarily be pumped through the liquid atomizing devices for the production of
liquid sheets. For viscous liquids this means that the fluid velocity exiting the
atornizer must be at least about 0.5 meter/sec; otherwise, no thin liquid sheet will
form. Preferred liquid velocities for the practice of the present invention are about
2~0 meter/sec or greater. Particulate or solid matter in the fluid streams can be
handled as long as they are smaller than the smallest opening in the atomizer sothat plugging does not occur. In regards to this point atomizers with orifices as
large as 12 cm and larger can be used to practice the present invention.
The flowrates of each liquid to be mixed can be varied over quite a wide range.
Liquid flowrate ratios of 10:1 have been tested, and it is believed that much higher
ratios can be used. The important parameter is pressure drop through the atomizer.
The pressure drop for equal flowrates through each atomizer for equally viscous
liquids should be approximately the same. If widely different flowrates are
desired, one simply uses different atomizer orifice sizes. If liquids with widely
:~2~i3~
- 7 -
di~erent viscosities are to be mixed, then the Reynolds number and velocity of each
liquid sheet are the important parameters.
The present invention is applicable to any liquid-liquid mixing process where
the liquids in question can be formed into thin liquid sheets by the applicable
atomizing devices. It is especially applicable to fast, multiple step reactions where
selectivity is a problem due to incomplete or not rapid enough mixing. An example
of such an applicable process is the coupling of 1-napthol with diazotised
sulphanilic acid. The present invention is also very applicable to the reaction
injection molding process where two somewhat viscous monomers or oligmers (100
to 1000 centipoise) are mixed together and rapidly react to form a high molecular
weight, high viscosity polymer that will harden in a mold. Note that because thepresent invention forms thin liquid sheets and a thin mixed liquid shset in freespace, clogging of the atomizers and viscous product polymer buildup are not a
problem.
The present invention is also very applicable to liquid-liquid extraction.
Liquid-liquid extraction initially requires intimate contact between the light
liquid phase and the heavy liquid phase. In many practical applications, however,
the dispersion of one liquid in the other is very difficult because of high interfacial
tension. This can lead to overall stage efficiencies of 0.1 or less leading to many
more extraction stages than theoretically required. However, because the presentinvention contacts liquids together in thin liquid sheets and then further mixesthem turbulently within a new mixed thin liquid sheet, dispersion of the liquids is
very uniform. This should increase the overall stage efficiency for liquid-liquid
extraction operations. Some other applications are fast en~yme biochemical
reactions and formation of stable emulsions.
The present invention is also useful where the mixing of two liquids is
accompanied by simultaneous absorption or desorption of a gaseous component. As
is taught in my U.S. patent application 06/818,781 entitled "Method for Carbonating
Liquids", filed Jan. 1~, 1986, liquid sheets can be used for the absorption or
desorption of gases. The thinness of the liquid sheet, coupled with turbulence
within the sheet, allows for rapid mass transfer and a relatively high approach to
equilibrium. Although a mixed sheet is typically twice as thick as a single sheet,
the extremely high turbulence generated by the mixing process of the single sheets
allows for high values of the approach to equilibrium. Ths approach to equilibrium
for the mixed sheet is as high or higher than the single sheets. Example 3 (infra)
compares the absorption of carbon dioxide in a mixed liquid sheet with a single
liquid sheet.
In short, the present invention is especially preferred in applications where the
mixing of liquids must be complete in short mixing times and/or intimate contactbetween liquids is desired. Further, absorption and/or desorption of gaseous
components can occur simultaneously with the mixing of the liquid sheets.
3~
- 8 -
While the above description contains many specificities, these should not be
construed as limitations on the scope of the invention, but rather as an
exemplification of a preferred embodiment thereof. Other variations are possible,
and these are obvious to those skilled in the art based on the principles discussed in
the description.
The following examples shall serve to illustrate the practice of the present
invention~ It should be understood that the data disclosed serve only as examples
nnd nre not intended to limit the scope of the invention.
Example 1: Demonstration of the speed and completeness with which total mixing
can be achieved by the present invention.
I`wo swirl atomizers of the type depicted in Fig. 2 producing conical liquid sheets
at a 150 degree angle were situated so that their liquid sheets produced would contact
at a point about 1.3 cm from the exiting point of each atomizer (as in Fig. 3). The
liquid sheets separately or mixed extended to a distance of about 5 to 6 cm from the
ntomizer, thus indicating no loss in sheet length as a result of mixed sheet
formation. The angle of contact between the two single liquid sheets was 30
degrees. At a distance of 1.3 cm. the single sheet thickness is about 25 microns.
The velocity in each liquid sheet was about 12 meter/sec, calculated as 60% of the
theoretical velocity head. Sixty percent is typical of swirl atomizers of the type
shown in Fig 2 and 3. Since the point at which the liquid sheets contact -forms a
mixed sheet of 1~0 degrees relative to the atomizers (as in Fig. 3), there is a
component of velocity in the direction of sheet thickness. This is true because each
single sheet before contact flowed at an angle of 150 degrees relative to the
atomizers. The component of velocity in the direction of sheet thickness is 12
meter/sec multiplied by the sine of one-half the contact angle or in this case 15
degrees. Therefore, the velocity in the direction of thickness of the mixed sheet is
about 3.1 meterlsec. Since the thickness that the components must diffuse is only 25
microns, the physical mixing time is about 8 microseconds.
An experiment was devised to estimate the time of mixing. A solution of 1.0
gmole/liter(N) NaOH containing phenolpthalein indicator was pumped through
one atomizer while a solution of 1 N H2SO4 was pumped through the other swirl
atomizer. It is well known that strong acid-strong base neutralizations are
virtually instantaneous. The phenolpthalein was used as an indicator of
neutralization between the acid and base. In basic solution phenolpthalein is red,
whereas it is clear in acidic solutions. However it was not possible to see the red
color of the phenolpthalein regardless of its concentration because of the extreme
thinness (30 microns and less) of the liquid sheets formed. To test for
~2~63~
neutrali~ation a 0.5 mm probe was immersed directly into the mixed liquid sheet.
This resulted in local premature sheet disruption along the radial line of
immersion, causing liquid to bead up along the probe. These beads are about 100
times or so thicker than the thin sheet and color can be readily seen in them.
For a flowrate of 1450 milliliters/min of 1.0 N NaOH containing phenolpthalein
and at a contact thickness of each sheet of about 2~ microns, the following flowrates
of H2SO4 yielded the following color of the mixed sheet:
1~0N H2S04 flowrate Color of mixed sheet
980 mVmin red
1180 mlhnin red
1410 mVmin light red
1520 mVmin clear
1620ml/min clear
At equal flowrates (14~0 mVm;n) the acid and base should neutralize each other,
and the phenolpthalein indicator should turn clear. Allowing for the uncertaintyin the flowmeter resolution, it is seen that the mixed sheet was clear when an excess
of 5% acid was used. This value of 5% is within the accuracy of the flowmeters
used. No color was noted anywhere within the sheet at the neutralization flowrate.
When the probe was immersed just beyond the well-defined mixing zone where the
liquid sheets contacted, the color at the neutralization flowrate was still clear just
as it was over 5 cm further away in the mixed liquid sheet.
The time of travel 0.5 mm beyond the mixing zone for sheets with a velocity of
about 12 meter/sec is approximately 4 x 10-5 second. Therefore the time required for
mixing yielding neutralization is of order 100 microseconds or less for the low
viscosity tluids tested. The reader will note that this mixing time is less than the
mixing time for Kletenick's device discussed earlier. This is expected since thepresent invention contacts two liquid sheets which are thinner than the sheets
produced by Kletenick's device. The mixing time of the present invention is lessthan the mixing time claimed by Kletenick for his device, and further, much
higher liquid flowrates can be used in the present invention.
Example 2: Mixing of two viscous liquid sheets.
Two swirl atomizers of the type shown in Fig. 2 producing conical sheets at an
angle greater than 150 degrees were positioned so that their thin liquid sheets would
contact and mix. Through both atomizers soybean oil ~viscosity of about 50
centipoise) was pumped at a pressure drop of 50 psi. Each sheet alone was clearly in
laminar flow as evidenced by the glassy appearance of the liquid sheets. When the
sheets impinged, the mixed sheet length actually increased by 25% from about 20
cm to about 30 cm compared to the length of the sing]e sheets.
Although the degree of turbulence in the mixed sheet was low due to the low
atomizer pressure drop and high liquid viscosity, the mixed sheet was waYv and
contained ripples of fluid. This waviness and rippling is often used as a criterion
for the onset of turbulent flow. The waviness and rippling clearly indicate mixing
in the direction of thickness of the mixed sheet. Higher pressure drops than that
used ;n this e~ample, would only serve to increase the turbulence of the mixed sheet
nnd would not effect the contacting or impingement process. Viscous fluids tend to
produce longer and more stable sheets that are more resistant to disruption than low
viscosity fluids.
Example 3: Absorption of a gas in a mixed liquid sheet.
Two swirl atomizers of the type shown in Fig. 2 producing conical sheets at an
angle of about 150 degrees were positioned so that their liquid sheets would contact
and mix as shown in Fig. 3. Through both atomizers water initially free of carbon
dioxide was pumped at a pressure drop of 20 psi into a pressure vessel substantially
containing carbon dioxide. The flowrate of water in the mixed liquid sheet was
about 11.0 Iiter/min. Water exiting the pressure vessel was found to contain
carbon dioxide in an amount equal to 58% of the equilibrium or in this case
maximum amount possible. Carbon dioxide concentration in the water was
determined by titration with standard solutions.
When the two atomizers were positioned far apart from one another so that a
mixed liquid sheet could not form, the approach to equilibrium of a single liquid
sheet was 54%. Even though the total flowrate for the mixed liquid sheet was twice
as high as the single sheet, the increased turbulence within the mixed liquid sheet
allowed for a higher approach to equilibrium. The mixed liquid sheet although
twice as thick as the single sheet, had a degree of turbulence that was much higher
than the turbulence in the single sheet.
Thus the reader will see that the liquid sheet mixer provides extremcly rapid and
uniform mixing of liquids. While the above description contains many
specificities, these should not be construed as limitations on the scope of the
invention, but rather as an exemplification of one preferred embodiment thereof.Many variations on the preferred embodiment are possible. For example, as
mentioned earlier, the angle of contact can vary from just greater than 0 degrees to
almost 90 degrees. Accordingly, the scope of the invention should be determined
not by the embodiment illustrated, but by the appended claims and their legal
equivalents.