Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
:~1.2~
SUSTAINED RELEA5E TABLETS AND METHOD OF MAKING SAME
This invention relates to new dosage units of
medicinal agents for oral administration in the form of
tablets which provide a controlled rate of release of the
medicament in the gastrointestinal tract. More
particularly, the invention relates to dense compressed
tablets comprising mainly coated pellets processed such
that the tablets disintegrate rapidly in aqueous and
gastric fluids at body temperature. Specially coated
pellets are formulated with disintegrants so that they
hydrate quickly disintegrating the tablets in a short
time to release the medicinal agent contained therein
gradually over a relatively long period of time.
This invention also relates to the method of making
such coated pellets which can be compressed into a tablet
or pill of any size or shape.
Background ~f The Invention
The importance of providing dosage unit forms of
medicinal agents for oral administration which release
the drug slowly and at a uniform rate has long been
recognized and ha6 become more apparent as new drugs are
made available. If the medicament is released too
quickly, the stomach may become upset
;
:
,.~
and the acid environment may have an adverse effect
upon the drug. Sometimes the too rapid absorption of
large amounts of potent drugs results in
unnecessarily high blood levels of the drug which
may, in turn, result in unpleasant side-effects or
even toxic manifestations.
To ensure a controlled, long-lasting, continuous
and not too intensive effe!ctive action of a
therapeutic agent in the human body, it is necessary
to be able to delay the absorption of the therapeutic
agent by the body. This is especially important for
water-soluble medicinal agents which are normally
absorbed by the body fluids immediately after
administration and consequently produce normally only
a very short duration of effective action
Many drugs such a~ potassium chloride for
hypokalemia, aspirin~ acetaminophen and ibuprofen for
rheumatism, nicotinic acid for hypercholestqremia,
theophyllin for the relief of bronchial asthma,
griseofulvIn for the treatment of conjunctivitis,
corneal ulcer and antibiotics for fighting bacterial
and viral infections, function best by maintaining
the concentration of the medicament at the optimum
therapeutic blood level. The traditional method of
taking a capsule every few hours is often
inconveni.ent and results in non-compliance by the
patients.
~9-102/nab
: . . .
: ~ . ,: , :
.
~2~L5
As one example for the need for a dosage unit
form which releases the drug at a relatively constant
rate over a longer period of time, reference may be
made to the treatment of hypokalemia with potassium
salts such as potassium chloride, potassium
bicarbonate, potassium citrate and potassium
glycolate and others. In this treatment, relatively
large amounts of potassium salts are given orally,
often resulting in unpleasant side effects such as
gastric ulceration, occasional bleeding and others.
Analgesics and antiarthritic drugs when taken in
large doses often give rise to similar undesirable
side effects. Single smaller doses of antibiotics
and antihacterial agents taken to fight infections
have the desired therapeutic effect for no more than
a few hours whereas lar~e doses tend to result in
severe abdominal cramping/ upset stomach, vomiting,
nausea, diarrhea and occasional hepatic dysfunction.
Up to now one of the methods commonly used in an
!~ attempt to provide prolonged duration of therapeutic
agent i~ to mix the agent with insert waxy materials,
e.~., calcium, mag~nesium and aluminium soap salts
.
and~or othei lnsoluble or partially soluble
materials. This mass is then granulated using
aqueous or non-aqueous solvents to provide granules,
which are then compressed into tablets or pills. The
i ~
.~
~; sustained release effect is achieved when the tablet
:: ~
. ~ .
09-102/nab
: :,
:
~-
,
.
.
. . ; : . ,
erodes or dissolves gradually in the gastrointestinal
tract. This method is exempliEied in U.S. Patent
Nos. 2,793,979, 3,065,143, 3,102,845, 3,184,386,
3,437,726 and 3,558,768.
The dosage form described above has a biy
disadvantage in that the tablet or pill travels
through the intestinal tract as one large mass
constantly in physical contact with gastrointestinal
tissue, thereby causing intestinal and gastric
ulceration, occasional bleeding and other undesirable
side effects such as nausea, vomiting, epigastric
distress and oval thrush.
Anothe. commonly employed method for obtaining a
so-called "prolonged" therapeutic effect from a
pharmaceutical formulation is to coat a drug onto an
innocuous core, e.g., nonpareil seeds (tiny sugar
pellets) or onto drug crystals themselves. The thus
coated druy pellets are then over~coated with several
layers of retarding waxes or insoluble mixes in such
fashion that, when filled into gelatin capsules, the
release of the active drug from the capsule during
; its passage through the stomach and intestinal tract,
is even and gradual. This method is exemplified in
Uo5~ Patent Nos. 3,383,283, 2,921,883, 3,365,365,
3,220,925 and 3,119,742.
Such a sustained release dosage form is limitedt
however, by the amount of coated beads or pellets
09-102/nab
, , :
. . :
.
.
' ' . , '
that can be put into a size capsule that is
convenient Eor swallowing. For example, a #l hard
gelatin capsule holds approximately 400 mg. of
finished sustained release pellets and a #0 size
capsule approximately 600 mg.
An examination of other prior art reveals
numerous attempts to solve the problems defined
above, with only limited success. None of the noted
prior art employs my new inventive process and
so~ution of the problem. For example, Press U.SO
Patent 2,953,497 describes a basic timed release
composition and a method of preparing such
composition. The patent describes therapeutically
active ingredients which are coated on or mixed with
a sugar/corn starch mixture to form granules. The
therapeutically active ingredient-containing or
coated granules are thereaeter coated with shellac
and/or a cellulose acetate phthalate solution to
reduce the rate of release of the therapeutlcally
active ingredient. The patent does not disclose my
method of forming a tablet containing disintegrant-
coated medicament-containing controlled release
particles or such a tablet itself.
Barry U.S. Patent 2,996,431 is directed to a
procedure for making a friable tablet and the tablet
produced thereby. According to the patent, the
tablet can be broken into small sized particIes by
09-102/ndb
. ~.,.~, ~ .. .. .. . ... . ..
,
- :
'
application of the pressure of the thumb or other
finger on the tablet against a surface, such as that
of a table, in a single operation. The tablet is
composed of granules or spheroidal pellets composed
of sugar/corn starch with a coating of medicinal
agent. The medicinal agent-containing pellets are
then coated with, inter alla, shellac or cellulose
esters, such as the ace~ate and acetate phthalate.
The granules or pellets are then incorporated in a
matrix or binder and pressed into the desired
shape. The patent does not describe my use of a
thin, uniform coating of a disintegrant on a
medicament containing pellet, nor my process of
forming a compressed tablet containing such
disintegrant-coated pellets.
Halley U.S. Patent 3,044,938 describes a
sustained action pharmaceutical tablet and a process
of making the same. The disclosure indicates that at
least some portions o a medicament incorporate an
ingredient which simultaneously functions as an
"action-retardant" and as a binder. By including a
plural1ty of such special action-retarding and
binding ingredients into the same tablet or by
incorporating action-retarding and binding
ingredients and medicament in unaltered form,
sustained-release action may be obtained. The patent
does notr however, describe my process, or a tablet
09-1~2/nab
.
,
': '
.
' `
formed thereby, in which retarded or coated pellets
of medicament are further coated with a layer of
disintegrants prior to compressing the tablet.
Hermelin U.S. Patent 3,115,441 discloses timed
release tablets in which a plurality of small
particles of an analgesic drug coated with a
solution-resistant coating is dispersed throughout a
compressed matrix including particles Oe the same
analgesic drug in uncoated form. There is no
discussion in the patent of my use of disintegrants,
nor of my process for forming disintegrant-coated
medicament-containing particles in a compressed
tablet.
Berger U.S. Patent 3,344,029 describes a
sustained release preparation which includes a
plurality of resilient cores, each of which is formed
from a cohesive intimate mixture of finely divided,
therapeutically active material in powder form and an
ingestible material which is resistant to
disintegration in the gastrointestinal tract. A
portion of the core is coated with alternating
coatings of therapeutically active materials and
ingestible material. The patent does not disclose
the use of a disintegrant layer on a sustained
release medicament-containing particle, nor a
compressed tablet including a plurality of such
disintegrant-coated particles, as my invention
09-102/nab
' ' '
.
~2~
describes.
Noyes U.S. Patent 536,155, Mori et al. Japanese
Patent Publication 53-127,821, and Wilen U.S. Patent
2,297,599 disclose various forms o* coated particles of
medicament but they do not describe my uniform coating of
a retarding material on a medicament- containing particle
overlaid with a uniform coating of disintegrant material
and then compressed into a dense tablet that breaks up
quickly in the body.
Summary Of The Invention
In accordance with an aspect of the invention, in a
dense, compressed pharmaceutical tablet for oral
administration which is characterized by the ability to
disintegrate quickly and spontaneously into coated
pellets which release medicament at a controlled rate
over a period o~ several hours in the yastrointestinal
tract, the improvement consists essentially of a first
coating of a retarding material adapted to slow the
release rate of said medicament, a second thin, uniform
disintegrant coating applied in a dry state on the said
retardant coated pellets capable of rapidly absorbing
water from the aqueous fluids of the gastrointestinal
tract and disintegrating, said coated pellets adapted for
compression into a dense tablet.
In accordance with another aspect of the invention,
a dense, compressad pharmaceutical tablet for oral
administration comprises a plurality of medicament-
containing pellets, each pellet having coated thereon a
first coating of a retarding material adapted to slow the
release rate of said medicament, a second thin, uniform
disintegrant coating applied in a dry state on said first
coating, said disintegrant coating capabls of rapidly
absorbing water from aqueous fluids in the
gastrointestinal tract and disintegrating, thereby
dispersing said medicament-containing pellets, said
coated pellets adapted for compression into a dense
tablet.
.. .
,
~ his unique composition, according to the present
invention, provides a dosage unit form for the controlled
administration of medicaments which releases the
madicament at a relatively uniform rate over a sustained
period of time. This dosage unit form not only avoids
the above-mPntioned difficulties, but provides various
advantages in addition thereto. For instance, the
equivalent of two or three normal doses of a drug in the
form of coated pellets may be incorporated into one
tablet which may be taken only once every eight to twelve
hours. Thus, it is possible to maintain the action of
the drug during the night while the patient is asleep as
well as during the day when he may find it inconvenient
to take frequent doses of
-
g a
rli
~-g~
medication. F'urthermore, since the tablets rapidly
disintegrate, releasing the individual coated
pellets, the gastric residence time of the dosage
form is significantly reduced, thereby minimizing the
side effects.
Another advantage with the present invention is
that the compressed tablet can be bisected on upper,
or lower, or both sides of the tablets which make it
easier to administer one-half tablet or one-hal~
dose, something which i~ impossible when coated
pellets are dispensed in gelatin capsules.
According to the invention, sustained release
tablets or pills may now be prepared which do not
exhibit the shortcomings enumerated above and which
may contain relatively large amounts of active
ingredients. Thus, according to the present
invention, the active ingredient is first coated onto
non-pareil beads or onto drug crystals or granules.
These pellets are then divided into several groups
and varying amounts of retarding materials are
applied to dlfferent groups. Upon subsequent mixing
of the groups, the combined effect of the total
pellets will provide gradual release of the
medicine. These pellets are then cured in the oven
to stabilize the release rates.
The stabilized pellets are then coated with
severaI layers of disintegrating agent or agents and
09-102/nab
: . ~ : - :
- ~ . ,. . , .~ :: :
. .. . .
, ,
~ : : . ,
~2~
compressed into tablets or pills, after adding a
small amount of lubricants or other inert
ingredients, if necessary. Such a compressed tab].et,
when tested in water or gastric fluid, breaks up
quickly thereby releasing the individual pellets in a
matter of minutes, whereupon they act as independent
pellets releasing their medicine at a predetermined
rate.
By following this method of preparing a drug, a
relatively large amount of the drug can be compressed
into a tablet size which is sti]1 easy to swallow.
As much as 1500 mg. of the drug can now be
administered in a tablet size which is no bigger than
#0 size gelatin capsule. In brief, this process
results in a tablet containing approximately 200% to
250% the amount of medicament that could be placed
into a capsule of equal size. My tablets will
significantly reduce the gastric residence time, thus
eliminating most, if not all, of the gastric related
side effect~ because the smaller size pellets pass
through the pyloric valve swiftly. The non-
disintegrating type tablets, especially sizes bigger
than 7/16" to l/2" diameter pass through the pyloric
valve with difficulty.
The claimed invention, distinguishable not only
on the basis of the method of forming the tablet but
the tablet itself, may be further distinguished from
,
09-102/nab
11
: ' ~ '
;
.,
. :
.
~2~
the prior art based on the effects achieved by the
claimed tablet. Whereas the prior art incorporates
disintegrants into a tablet or a capsule simply by
mixing the disintegrants with coated beads and
granules and thereafter compressing the mixture when
a tablet is formed, the present invention coats the
dlsintegrant onto a pellet and thereafter compresses
a plurality of the dislntegrant-coated pellets into
tablet form. The claimed process and product formed
thereby, which include medicament-containing pellets
uniformly coated with disintegrant, provide very
reliable and predictable disintegration times for the
tablet. This permits more accurate determination of
dosages and intervals between release of medicament.
Another effect which is obtainable by the
tablets of the present invention is to reduce the
gastric residence time. Thus, a tablet according to
the present invention can be made which will
disintegrate rapidly in the stomach into coated
beads. These beads, being small in size, move
rapidly into the inte~tine, thereby reducing the
gastric residence time of the medicament
significantly and the concomitant gastric irritation
associated with many drugs, such as aspirin,
ibuprofen, potassium chloride, antibiotics, etc.
The tablets of the present invention permit the
administration of larger, single doses. As
09-102/nab
12
,,
`~ ` -' ~ - , .
.
distinguished from prior art tablets in which the
disintegrant is mixed with the medicament-containing
beads, the present inventlon is more efficient in
that it allows s~aller amounts of disintegrant to be
used by providing a thin, uniform coating of the
disintegrant on the medicament-containing bead.
Since a smaller portion of the volume of the
compressed tablet of the present invention is
occupied by disintegrant, as compared to compressed
mixtures of the prior art, a greater amount of
medicament may be incorporated in the same volume
tablet. Thus, the present invention permits the
administration of about 1,200 to about 1,600 mg. of
medicated beads in a single dose without effecting
the rate of release of medication as compared to not
more than about 600 to 700 m90 of medicated beads in
a single dose prepared according to the prior art.
The present invention permits a greater
uniformity in effect as a result of a greater
uniformity of coating of the disintegrant, as
~ompared to mixing disintegrant with medicament-
containing beads~ Generally, the disintegrants are
of a much smaller size (lO0 to 300 mesh~ compared to
the coated medicaments which are generally about 10
to 15 times larger (10 to 20 mesh~. As a result,
when simply mlxed together, the large and small
particles tend to separate with the small particles
09-102/nab
13
- -
.,
.
.. . .
~ . ' ' . : .
~2~
settling on the bottom of a container and being non-
uniformly dispersed in the mixture. Thus, the rates
of disintegration may differ for the "mixed" tablet,
not only from one tablet to the next, but also as
compared to what may be obtained according to the
tablets of the present invention.
Detailed Desri~tlon Of The Invention
In one embodiment of this invention, the tablet
composed of 85% to 98% of the drug-containing,
coated, spherical pellets, the remainder being
composed of binder and lubricants, preferably between
90% to 100% of the tablet. The pellets are
predominantly of a size ranging from 12 to 30 mesh,
with sizes 16 thru 24 mesh being preferred and they
all may be the same size or of different sizes within
that range.
The spherical drug pellets may be coated using
conventional coatings to simply retard the release
rate of the medicament, or they may be enteric coated
so that they will not release the drug in the
intestines. The coating materials used may also be
such that the coated pellets will release the drug at
selected locations within the gastrointestinal
tract. This result can be achieved by using specific
en2ymes or specific chemlcals which interact with the
~i
09-102/nab
14
,
.
~ .,. . :
~%~
enzymes present or dissolve at the prevailing pH
within the gastrointestinal tract.
A compressed tablet prepared using ~he above-
mentioned technique may contain only a single drug
component or it may contain different pellets
containing different drugs, which are mixed together
before compressing into tablets. A mixture of
different pellets of different drugs coated
differently, i.e., wax retarded, enteric coated and
site specific coated will provide a tablet which is
capable of providing more than one medicament at
several locations within the gastrointestinal tract
at a controlled specific release rate for each
medicament.
The film coating material may be applied by any
procedure which achieves a continuous film o~
essentially uniform thickness. One method of film
coating involves rotating a bed of uneoated beads
(e.g., nonpareil) in a conventional tablet coating
pan and applying a solution or dispersion of the
coating agent in a suitable solvent by pouring or
spraying onto the moving beads, care being taken to
uniformly coat each bead and to avoid incomplete film
coating such as is caused by bead agglomeration,
etc. Drying of the coated beads is accomplished by
exposure to warm, dry air. The coating procedure
conveniently i8 continued until the desired film
09-102/nab
. .
,
,, . :
: ;, .. :
~ `` ' `
:`
9~
thickness is obtained. The resulting film coated
beads are then cured if necessary with heat (air
drying, baking or force drying), polished and
finished as desired. Other coating procedures such
as fluid bed coating, vertical spray coating, etc.,
can also be employed.
Although my novel formulation is peculiarly
adapted to the use of "water soluble" medicaments, it
may be readily modified to include "water insoluble"
therapeutics, singly~ in combination with each other,
or in combination with water soluble medicaments, and
it is intended that this modification be included
within the scope of the present invention. Inasmuch
~ as a water insoluble drug can be made available for
; absorption from the intestinal tract only by being
directly exposed to the intestinal mucosa, such
exposure can be accomplished by incorporating in the
formulation an appropriate amount of hydrophilic
gum. The quantity of the hydrophilic gum should be
such that the pellets erode slowly while traveling
through the intestinal tract, thereby rendering the
water insoluble drug available for absorption.
~ or a more detailed explanation of the
invention, re~erence is made to FIGS. 1-6. FIG. 1
represents, in enlarged form, a pellet 10 graphically
as a sphere although in reality not all are perfect
spheres but rather a variety oE spheroidal forms.
09-102/nab
16
~: :: - ' : .: : : :
:
: . : ; . :
-
. . . : ..
'' :: : . ` '
~2~
FIG. 2 represents the pellet 10 to which a
medica~ent 11 has been applied by coating. The
medicament is exemplified as continuous and uniform
in thickness, but in reality the medicament may be
deposited in somewhat less than complete uniformity.
FIG. 3 represents the pellet after one or more
coating applications 12 of retarding wax such as
stearic acid, carnauba wax or a mixture thereof are
applied. ~he coating 12, if desired at all, is
deposited as described above over the surface of the
medicated pellet.
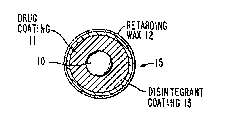
FIG. 4 represents the pellet 15 after
applications of a coating o~ disintegrant materials
13 over the retarding wax 12 shown.
FIG. 5 represents an enlarged cross-section of a
compressed tablet 14 consisting of the coated pellets
lS exemplified in FIG. 4. The spheroidal pellets 15
are actually somewhat flattened by the eompression
force of the tablet press d~ring tabletting, but due
to the nature of the coating materials and the layer
of disinte~rants, the intesrity of the retarding
coating i9 essentially maintained. The exterior
surface of the tablet 16 is not an applied coating as
such, but is exemplified herein to show the hard
compressed surface area which results from the force
o~ the tablet pre~s on the pellets 15.
FIG. 6 shows an enlarged cross-section of the
09-102/nab
17
.. ~ .. . , . ~ ..,
,
.
tablet 14 after application of finish coatings to
make a coated tablet 17. Depictecl here are two
layers of coating materials, 18 and 19. This number
could be zero to four depending upon the need to coat
the tablet or the type of coating applied.
The nature of the coating of my invention will
vary fram pellet to pellet within the same batch, as
well as in uniformity and thickness, as is known to
; persons skilled in this art. Some pellets will have
more direct and continuous layers Eormed by the
orientation of the powder particles than other
pellets and the same disintegrants may create weak
spots in the coating of some pellets but not in
others.
The overall effect, however, is as stated more
fully herein, and reproducibility can be obtained
from batch to batch and from samples within a batch.
The rate of release of medicament from a
particular dose can be controlled by:
(a) Varying the relative solubility of the
drug;
(b) Varying the drug concentration per pellet;
tc~ Varying the number of pellets (population)
per unît dose;
(d) Varying the thickness of the coating;
(e) Varying the amount and ratio of retarding
wax;
(f) Varying the characteristics of the fatty
;~ acids used in retarding wax;
09-102/nab
18
- . : .
~: ~ ., . ~ `.
: ' ~ : : ` ' '` '
(g) Blending two or more batches of pellets
with different release rates;
(h) The amount and type of disintegrants used.
Because the present method for providing
sustained release dosage units and the compos,itions
produced thereby are not limlted by the physical or
chemical properties of the drugs utilized, a large
number of drugs of various physical and chemical
properties may be embodied therein. Without
intending to exclude any useful pharmaceutical, the
following is a list of representative pharmaceuticals
or drugs by generic or chemical name which may be
used to prepare sustained release dosages according
to the present invention:
Anal~esic or Antipyretic A~ents
, . Aspirin~
: Acetaminophen
5alsalate
Antibiotics/Antibacterial Aqents/Vermicidal
Agents
~ Penicillin Griseofulvin
,: Tetracycline Dicloxacillin Sodium
: Chlortetracycline Erythromycin and its salts
:: Oxytetra:cyclinePiperazine Citrate &
Neomycin Hexahydrate
ChloramphenicolMethanamine Hippurate & ',
CephradlneMandalate Salts
Nalidixic AcidSulfasoxizole and other
CephloseporinsSulfonamide Salts
-~ Antiepileptlc A~ents
Ethotoin (Peganone)~
~, Trimethadione (Tridione)~
: Phenytoin
: Paramethadione (Paradione)~
.
19
,
~` ~ ~ '
'~:` :-' - '
: '
: :
Sodium Valproate
Sodium Hydrogen Valproate
Dietary Sup~ ents
Nicotinic Acid
Ferrous Sulfate and other Fe Salts
Urinary Acidifiers ~ Alkalizers
Potassium Acid Phosphate
Sodium Acid Phosphate
Potassium Acetate
Bronchodilators/Vasodilators
Theophyllin
Oxytriphylline
Potassium Supplements
Potassium Chloride
Potassium Citrate
Potassium Gluconate
Potassium Bicarbonate
Sedative~ and Hy~__tics
Pentabarbital Carbromal
Phenobarbital Barbital
Secobarbital Amobarbital
Codeine Butabarbital
Bromisovalum Methocarbamol
, ::
: Sulfonamides
; ~Sulfamethoxy~iazine
Sulfaethiodole
: Sulfasoxizole & other
Sulfonamide Salts
~: ::
~ Cardiovascular Drugs
`: :
: : : Papaverine ~ydrochloride
Anti-Inflar~atory~Antiarthritis~Drugs
Diflunisal
Ibuprofen
Indocin
~:~ : Procainamide Hcl
:: :
09-102/nab
~: ` ' : .' - . :
~ ,~' , '- ' ~ : ' ` :
: ' `~ ' ' ' ' :
': . ~; ' '
.
3L2~
Anti-Parkin~on's Disease
~ _ .
Levo-Dopa
The disintegrating or swelling agents which may
be used according to the invention are substances
which by capillary action or by process of hydration
upon contact with water help .in disintegrating the
compressed tablet into individual pellets. Specific
examples of such swelling agents, which may be used
alone or in any sui.table mixture with other members
oE the group, are the following: Various starches,
such as corn starch, potato starch, rice starch,
sodium carboxymethyl starches, pregelatinized
starches, sodium starch ~lycolate, cellulose powder
and cellulose ethers, such as carboxymethyl cellulose
tCMC), methylcellulose, hydroxymethyl cellulose,
polyacrylic acid (Carbopol~934), sodium alginate and
alginic acid, plantago ovata seed husk, modified
cellulose gums (AcDiso~ and pecti.n. Particularly
preferred are sodlum carboxymethyl starches,
pregelatinized qtarches and corn starch or
combination of above starches with ceLlulose
etheri. The dlsintegrant component is employed in
~ amounts between about 2 percent to about 15 percent
;~ ~ by weight of the tablet, preferrably between about 5
percent to 10 percent by weight.
The well known water-insoluble polymers may be
!: ~ . ~ employed, a preferred polymer being a polyvinyl-
:
21
~y
.... .. ..
.~ .
acetate of medium viscosity.
Another method of preparlng a rapidly
disintegrating tablet made up of coated beads employs
an effervescent mixture, whereby approximately one-
half of the beads are coated with sodium bicarbonate,
potassium bicarbonate or similar salts and the other
half with organic acids, such as citric acid,
tartaric acid or both. A compressed tablet
consisting of such beads in contact with water or
gastric fluids will generate effervescent action and
disintegrate the tablet into beads.
There is no restriction on the inclusion of
other commonly employed excipients in the formulation
of the novel combination of this invention. Thus,
one may employ as diluents r in wha~ever quantities
are indicated, such components as dibasic calcium
phosphate, lactose, mannitol and others. One may
also include as binders, to ensure additional
cohesive properties over and above those exerted by
P.V.P., such gums as acacia or tragacanth.
Example No. 1:
Nonpareil seed (sugar pellets~, 20.0 kg., all
passing through a No. 30 U.S. mesh screen, 90~
passing through a No. 35 U.SO mesh screen, and not
over 10% passing through a No. 40 U.S. mesh screen
are placed in a 48~inch coating pan. The pan is set
09-102/nab
22
.. . . .. .
: . :
..
~2~37~
Jl
in rotation and coatlng solution is sprayed slowly
onto the pellets in order to wet them evenly. Then
400 gm. of Potassium Chlorid~e powder, containing 5
talcum powder, are sprinkled on the wetted mass of
nonpareil seeds. The pellets are dried in war~
air. The addition of the coating solution, coating
powder and the drying procedure are repeated to apply
additional coats until all the Potassium Chloride
powder is used up. Two final coatings are added,
each coat consisting of coating solution followed by
talcum powder. ~he pellets are thoroughly dried and
screened through a No. 12 mesh screen and on a No~ 20
mesh screen.
The screened pellets are divided into three
equal parts and returned to three separate coating
pans and coated with coating solution followed by
dusting with a mixture of stearic acid, carnauba wax
and talc which work as retarding wax. The pellets in
the first pan are thus coated with 3~3 kg. of
retarding wax, the second pan with 4.95 kg. of the
retarding wax and the third with 6.6 kg. of retardiny
wax. All three groups are thoroughly dried. The
medicament release rate of each group is checked and
then the three yroups are mixed together and the
whole is divided into two sections and each is
returned to a separate coatin~ pan.
Now the coating solution is sprayed into each
09-102/nab
23
,. ~ ., , . ~ ~
.. : " ~ .
.
.
. . ` ~ -
70~5
pan, followed by dusting of a mixture of corn starch
and sodium starch glycolate (disintegrant). This
process is continued until 6% W/W of the disintegrant
has been added. The pellets are thoroughly dried,
and screened through a 12 mesh screen and onto a 30
mesh screen. These pellets are then compressed on a
tablet press to a weight which provides 750 mg. of
Potassium Chloride per table~.
These tablets are then film-coated using
conventional coating techniques for improving
appearance and acceptability.
The coated tablets thus obtained released the
active ingredient at a sustained rate over a period
of six to seven hours under simulated physiological
conditions.
The disintegration time of these tablets was
determined by U.S.P. method using the Stoll-Gershberg
apparatus. Six tablets were placed in the basket of
the apparatus, and the basket was lowered into an
lOOO ml. beaker containing 900 ml. simulated gastric
fluid maintained at 37 C. The apparatus was then
operated in the prescribed manner.
Example No. 2:
Potassium Chloride Granules, 40.0 kg., all
passing through a No. 20 U.S. mesh screent 90%
passing through a No. 30 mesh screen, and not over
09-102/nab
24
s
~ ~:
:
' ` ` : ` ' ~ ' ` '
: ,
10~ passing through a No. 40 U.S. mesh screen are
placed in a 48-inch coating pan. The pan is set in
rotation and coating solution is ~prayed slowly onto
the pellets in order to wet them evenly. Then 400
gm. of Potassium Chloride powder containing 5~
povidone powder are sprinkled on the wetted mass oE
nonpareil seeds. The pellets are dried in warm
air. The addition of the coating solution, and
coating powder and the dryincl procedure are repeated
to apply additional coats until all the Potassium
Chloride powder is used up. Two final coatings are
added, each coat consisting o~ coating solution
followed by talcum powder. The pellets are
thorou~hly dried and screened through a No. 16 mesh
screen and on a No. 30 mesh screen. The yleld is
approximately 100 kg.
The screened pellets are divided into three
equal parts and returned to three separate coating
pans and coated with coating solution followed by
dusting with a mixture of stearic acid~ carnauba wax
and talc which work as retarding wax. The first pan
is thus coated with 3.3 kg. of retarding wax, the
second pan with 4.95 kg. of the retarding wax and the
third with 6.6 kg. of retarding wax. All three parts
are thoroughly dried. The release rate of each part
is checked and the three parts are mixed together and
again divided into two sections and returned to the
09-102/nab
~5
,,
~ . . -
: ;, ::
coating pans.
Now the coating solution is sprayed onto each
pan, followed by dusting of mixture of alginic acid
and sodiu~ carboxymethyl starch (disintegrant). This
process is continued until 10~ w/w of the
disintegrant has been added. The pellets are
thoroughly dried, and screeni~d through a 12 mesh
screen and onto a 30 mesh screen. The yield is
approximately 125 kg. These pellets are then
co~pressed on a tablet press at a weight which i5
equal to 750 mg. of Potassium Chloride per tablet.
These tablets are then film-coated using
conventional coating techniques for improving
appearance and acceptability. The coated tablets
thus obtained released the active ingredient at a
sustained rate over a period of six to seven hours
under simulated physiological conditions.
Example No. 3:
Nonpareil seed (sugar pellets~, 20.0 kg., all
passing through a No. 30 U.S. mesh screen, 90~
passing through a No. 35 mesh screen, and not over
10% passiny through a No. 40 U.S. mesh screen are
placed in a 48-inch coating pan. The pan is set in
rotati3n and coating solution is sprayed slowly onto
the pellets in order to wet them evenly. Then 400
gm. of aspirin powder containing 5% talcum powder are
09-102/nab
26
': .
, ~ : . :
~2~01l~
sprinkled on the wetted mass of nonpareil seeds. The
pellets are dried on warm air. The addition of the
coating solution, and coating powder and the drying
procedure are repeated to apply additional coats
until all aspirin powder is used up. The final
coatings are added, each coat consisting of coating
solution followed by talcum powder. The pellets are
thoroughly dried and screened through a No. 12 rnesh
screen and on a No. 20 mesh screen. The yield i5
approximately 100 kg.
The screened pellets are divided into three
equal parts and returned to three separate coating
pans and coated with coating solution followed by
dusting with a mixture of stearic acid, carnauba wax
and talc which work as retarding wax. The first pan
is thus coated with 3.3 kg. of retarding wax~ the
second pan with 4.95 kg. of the retarding wax and the
third with 6.6 kg. of retarding wax. All three parts
are thoroughly dried. The release rate of each part
is checked and the three parts are mixed together and
again divided into two sections and returned to the
coating pans.
Now the coating solution is sprayed onto each
pan followed by dusting of mixture of potato starch
and modified cellulose gum (AcDisol)
(disintegrant). This process is continued until 8~
w/w of the dlsintegrant has been added. The pellets
09-102/nab
27
.,
. . .
.
. . :
: .~ :.
.
~2~
are thoroughly dried, and screened through a 12 mesh
screen and onto a 30 mesh screen. The yield is
approximately 125 kg. These pellets are then
compressed on a tablet press at a weight which is
equal to 1000 mg. of Aspirin per tablet.
The tablets thus obtained released the active
ingredients at a sustained rate over a period of six
to eight hours under physiological conditions.
Example No~ 4:
Nonpareil seed (sugar pellets), 30.0 kg., all
passing through a No. 30 U.S. mesh screen, 90~
passing through a No. 35 U.S. mesh screen, and not
over 10% passin~ through a No. 40 U.S. mesh screen
are placed in a 48-inch coating pan. The pan is set
in rotation and coating solution is sprayed slowly
onto the pellets in order to wet them evenly. Then
400 gm. of theophyllin anhydrous containing
approximately 5~ talcum powder are sprinkled on the
wetted mass of nonpareil seeds. The pellets are
dried in warm air. The addition of the coating
solutio~, coating powder and the drying procedure are
repeated to apply additional coats until all the
theophyllin anhydrous powder is used up. Two final
coatings are added, each coat consisting of coating
solution followed by talcum powder. The pellets are
thoroughly dried and screened through a No. 12 mesh
09-102/nab
2B
. . .
. ~
screen and on a No. 20 mesh screen. The yield is
approximately 100 kg.
The screened pellets are divided into three
equal parts and returned to three separate coating
pans and coated with coating solution. The coating
solution may be a solution or dispersion of methyl
cellulose, ethyl cellulose or a mixture thereoE in a
solvent system such as a water-ethanol mixture, an
ethanol-methylene chloride mixture or a methanol-
methylene chloride mixture. The first pan is thus
coated with 8% by weight, the second pan with 10~ by
weight and the third with 12% by wei~ht of the dry
solids from the aforesaid solution. All three parts
are thoroughly dried. The release rate of each part
is checked and the three parts are mixed together and
again divided into two sections and returned to the
coating pans.
Now the coating solution is sprayed onto each
pan, followed by dusting mixtures o corn starch and
cellulose powder (Solka Flog) (disintegrant). This
process is continued until 10% w/w of the
disintegrant has been added. The pellets are
thoroughly dried, and screened through a 12 mesh
screen and onto a 30 mesh screen. The yield is
approximately 125 kg. These pellets are then
compressed on a tablet press at a weight which is
equal to 450 mg. o~ theophyllin anhydrous per tablet.
09-102/nab
29
" '
. . . - ' :~
,
.
:
~9'7~ iL5
These tablets are then coated using conventional
coating techniques for improv;ng appearance and
acceptability. The coated tablets thus obtained
released the active ingredient at a sustained rate
over a period of 10 to 12 hours under physiological
conditions.
Exam~ o. 5:
Ibuprofen granules 40 kg., all passing through a
No. ~0 U.S. mesh screen, 90% passing through a No. 30
mesh screen, and not over 10% passing through a No.
50 U.S. mesh screen are placed in a 48-inch coating
pan. The pan is set in rotation and coating solution
is sprayed slowly onto the pellet in order to wet
them evenly. Then 400 gm. of Ibuprofen powder
containing 5~ talcum powder are sprinkled on the
wetted mass of nonpareil seeds. The pellets are
dried in warm air. The addition or the coating
solution, coating powder and the drying procedure are
repeated to apply additional coats until all the
IbuproEen powder is used up. Two final coatin~s are
.
added, each coat consisting of coating solution
followed by talcum powder. The pellets are
thoroughly dried and screened through a No. 16 mesh
screen and on a No. 30 mesh screen. The yield is
approximately lOO~kg.
The screened pellets are di~ided into three
09-102/nab
; 30
:
~ ~ .
: :
~L2~7~
equal parts and returned to three separate coating
pans and coated with coating solution followed by
dusting with a mixture of stearic acid, carnauba wax
and talc which work as retarding wax. The first pan
is thus coated with 4 kg. of retarding wax, the
second pan with 5 kg. of the retarding wax and the
third with 6 kq. of retarding wax. All three parts
are thoroughly dried. The release rate of each part
is checked and the three parts are mixed together and
again divided into two sections and returned to the
coating pans.
Now the coating solution is sprayed onto each
pan, followed by dusting of mixture of alginic acid
and sodium carboxymethyl starch ~disintegrant). This
process is continued until 10% w/w of the
disintegrant has been added. The pellets are
thoroughly dried, and screened through a 12 mesh
screen and onto a 30 mesh screen. The yield is
approximately 125 kg, These pellets are then
compressed on a tablet press at a weight which is
equal to lO00 mg. of Ibuprofen per tabletO
These tablets are then film-coated using
conventional coating techniques for improving
appearance and acceptability. The coated tablets
thus obtained released the active ingredient at a
sustained rate over a period of six to seven hours
under simulated physiological conditions.
09-102/nab 31
~: ,
:,
:
' ' . :
~2~70~
Example No. 6:
Potassium Chloride Granules, 40.0 kg., all
passing through a No. 30 U.S. mesh screen, 90%
passing through a No~ 30 mesh screen, and not over
10~ passing through a No~ 40 U.S. mesh screen are
placed in a 48-inch coating pan. The pan is set in
rotation and coating solution is sprayed slowly onto
the pellets in order to wet them evenly. Then 400
gm. of Potassium Chloride powder containing 5%
povidone powder are sprinkled on the wetted mass of
nonpareil seeds. The pellets are dried on warm
air. The addition of the coating solution, and
coating powder and the drying procedure are repeated
to apply additlonal coats until all the Potassium
Chloride powder is used up. Two final coatings are
added, each coat consisting of coating solution
followed by talcum powder. The pellets are
thoroughly dried and screened through a No. 16 mesh
screen and on a No. 30 mesh screen. The yield is
approximately 100 kg.
The screened pellets are divided into three
equal parts and returned to three separate coating
pans and coated with coating solution followed by
dusting with a mixture of stearic acid, carnauba wax
and talc which work as retarding wax. The first pan
is thus coated with 3.3 kg. of retarding wax~ the
09-102/nab
32
-
~2~70~5
second pan with 4.95 kg. of the retarding wax and the
thlrd with 6.6 kg. of retarding wax. All three parts
are thoroughly dried. The release rate of each part
is checked and the three part:s are mixed together and
again divided into two sections and returned to the
coating pans.
Now the coating solution is spayed onto each
pan, followed by dusting of Potassium Bicarbonate
powder in one pan and citric acid, anyhdrous powder
in the other. This process is continued until 10%
w/w of the powder on each pan has been added. The
pellets are thoroughly dried, and then screened
through a 12 mesh screen and onto a 30 mesh screen.
The yield is approximately 125 kg. These pellets are
then compressed on a tablet press at a weight which
is equal to 750 mg. of Potassium per tablet.
The tablets thus obtained released the active
ingredient at a sustained rate over a period o~ six
to seven hours under physiological conditions.
Example No. 7:
The process of Example No. 1 is carried out
replacing the mixture of corn starch and sodium
starch glycolate with a mixture of metnylcellulose
and sodium carboxymethyl starch.
03-102/nab
33
' ' "
~,
~ '
s
Exam~e No. 8:
The process of Example No. 2 is carried out
replacing the mixture of alginic acid and sodium
carboxymethyl starch with a mixture of potato starch
and sodium carboxymethyl cel:Lulose.
Example No. ~:
The process of Example No. 3 i~ carried out
replacing the potato starch and modified cellulose
gum (AcDisol) with 4% corn starch and sodium starch
glycolate coated onto pellets and another 4~ of the
mixture added in the lubricating step, prior to
compressing the tablet.
Example No. lO:
The process of Example No. 4 is carried out
replacing corn starch and cellulose powder with 5%
sodium starch glycolate.
.,
Example No. ll:
The process of Example No. 5 is carried out
replacing corn~tarch and sodium starch glycolate with
pregelatinized starch and plantago ovata seed husk
powder.
While I have illustrated my invention with the
ai~ of certain specific embodiments thereof, it will
be readily apparent to others skilled in the art that
.
09-lO~/nab
: ~ 34
:: :
'': : '' , ~ .
-: -
~L~9~7~
my invention is not limited to those particular
embodiments, and that various changes and
modifications may be made to achieve comparable
results without departing from the spirit of the
invention or the scope of the appended claims.
,
09-102/nab
.
.
~, , .: .:
~ ., : : `
,