Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
English translation of Swedish priority application No.
8703718-0, filed September 28, 1987
~3~ 1Z
A method in the activation of llgnocelluloslc material wlth
a qas containlnq nitroqen dloxlde
Technlcal fleld
The present lnvention relates to a method in whlch llg-
nocelluloslc material ls sub~ected to a dellgnlfylng treat-
ment. By lignocelluloslc materlal ls meant prlmarily dlffe-
rent llgnocelluloslc pulps, preferably such pulps as thoseln which, e.g., wood has been converted to cellulose pulp
either completely or partially wlth the ald of chemlcals.
The lnventlon ls particularly sulted for application with
chemical cellulose pulps produced in accordance with both
alkaline methods and the sulphite method. Among alkallne
dlgestlon methods can be mentloned the sulphate method, the
polysulphlde method and the soda (= sodlum hydroxlde)
method, wlth or wlthout chemlcal additives of, e.g, the
qulnone compound type. Although the lnventlon ls preferably
applled with unbleached cellulose pulp, the lnventlon may
also be applled successfully to cellulose pulp whlch has
previously been bleached and/or treated ln some other way,
e.g. alkall treated.
Backqround Prlor art
It has been found that by treatlng aqueous llgnocellu-
losic materlal wlth a gas contalnlng nltrogen dloxlde
(NO2) ln one or more so-called actlvatlon stages prlor to
one or more dellgnlfylng stages lt ls posslble to carry out
dellgnlflcatlon of the celluloslc materlal ln a hlghly se-
lectlve manner to a much greater extent than was prevlous-
ly consldered posslble wlthout the use of chlorlne or chlor--
lne compounds as a dellgnlfying agent.
~;
, .
2 ~ .tl~l~
The actlvatlon process ls lnfluenced by a number of
factors. These factors lnclude pulp consistency, the amount
of nitrogen dioxlde charged, time and temperature. Dlfferent
temperature proflles, i.e. different temperatures at diffe-
rent stages of the actlvation process also lnfluence the endresult. In addltlon, the nltrate content and the hydrogen-
-lon content durlng activatlon also have a declslve signi-
ficance on the activation process. The need to supply expen-
slve nltrogen dloxide can be drastlcally reduced by supp-
lying nitrate lons or hydrogen lons to the activatlon stage.The selectivlty ln the deligniflcatlon of the cellulose pulp
can also be optimiæed, by optimizing, lnter alla, the afore-
mentioned parameters. Thls can be utlllzed to carry out an
extremely extensive dellgnlflcation.
The activation process can also be influenced by intro-
ducing an oxygen-containing gas, for instance gaseous oxy-
gen. From an environmental aspect lt ls always advantageous
to add gaseous oxygen, since it has been found that when
activation is completed the gas will always contain a low
proportion of both nitrogen dloxide (NO2) and nitric oxide
(NO). Furthermore, the case is that the addition of gaseous
oxygen during activation of the cellulose pulp will under
certain conditions contribute to improved selectivity in the
delignlflcatlon of cellulose pulp.
SummarY of the inventlon
Technlcal Problem
It has been found, however, that under other conditions
the addition of excesslve quantities of oxygen can result in
impaired selectivity in the delignification of the cellulose
pulp. A low oxygen addition results in turn in environmental
problems, since the gas in such cases contains a high pro-
portion of nitric oxide (NO) in particular upon completion
of the activation process.
-`- 13f~1~1.Z
Solutlon
The present inventlon solves these problems and relates
to a method in the manufacture of cellulose pulp, ln which
aqueous lignocellulosic material ls actlvated with a gas
containing nitrogen dioxide (N02) ln at least one stage,
subsequent to and/or while adding an oxygen-containing gas
to the material, followed by delignification of the llgno-
celluloslc materlal in at least one step, and ln which gas
is separated from the lignocellulosic material during and/or
subsequent to the activatlon process. The method ls charac-
terized by controlling the supply of oxygen-containing gas
such that the separated gas will contaln at least 2 kg nit-
ric oxide (NO) calculated on lOOO kg absolutely dry ligno-
cellulosic material, and by reacting the separated gas in
one or more steps with an absorption solution whose original
pH-value lies within the range 3-13.5, and by releasing the
gas purified with the ald of said absorptlon solution to
atmosphere, optionally after further purification of the
gas, or passlng the gas to a destructlon plant.
The mixture of lignocelluloslc material (herelnafter
called cellulose pulp) with water shall be such that durlng
the activation process the pulp conslstency will lle wlthln
the range of 2-80%, suitably 3-40%, preferably 5-30%.
Nltrogen dioxide (N02) is introduced to the activating
stage elther as substantlally pure nltrogen dioxide, or ls
allowed to form immedlately before or in the activating
reactor, by supplying nitric oxide (NO) and oxygen. Both
nitrogen dioxide and nitric oxide can be introduced into one
and the same cellulose pulp. The term nltrogen dloxide ls
also meant to include dinitrogen tetroxide (N204) and
other polymeric forms of nitrogen oxides. One mole of dinit-
rogen tetroxide is calculated as two moles of nitrogen
dioxide. Additlon products whlch lnclude nitric oxlde are
calculated ln the same way as nitric oxide. Thus, di-
nitrogen trioxide (N203) is calculated as one mole ofnltric oxide and one mole of nitrogen dloxide. Addltlon
4 l~
products whlch include oxygen are probably present as lnter-
medlates. Slmilarly, nltrous acld (HN02) ls calculated as
actlve nitric oxide. Similar to dlnltrogen trloxlde, nltrous
acld ls volatlle and dlfflcult to separate analytically from
nltrogen dloxide and nitric oxide. Dinltrogen oxlde (N20)
on the other hand ls not calculated as an actlve nltrogen
oxlde.
The amount of nltrogen oxldes charged to the system ls
adapted, inter alia, according to the lignln content of the
cellulose pulp, tolerable attack on the carbohydrates of the
pulp, and the desired degreee of delignification. Calculated
as monomers, the amount of nitrogen oxides charged is nor-
mally from 0.1-2 kilomoles for each 100 kg of lignin in the
cellulose pulp.
The temperature during the activating process can be
chosen relatively freely, e.g. within the range of 20-110C.
If the actlvatlng process is carried out in a slngle stage,
the optlmum temperature wlll lie within the range of
50-95C. When the activating process ls divided into two
stages, the preferred temperature will lle wlthin the range
of 25-40C in the first stage, whereas the temperature ln
the second stage will lie within the range of 80-100C.
The time is partly contingent on the temperature. If the
pH 1~ very low and the temperature high, lt is necessary to
choose a short activating period. In other cases, the actl-
vatlng result ls normally improved when the actlvatlng pro-
cess is carried out over a long time period.
The amount of oxygen charged prior to and/or during the
activation of the cellulose pulp shall be kept low. Accord-
ing to one preferred embodiment of the inventlon no oxygen-
-contalnlng gas is lntentionally charged durlng the actlva-
tlon of the cellulose pulp. Unless special preventative
measures are taken, a certaln amount of air wlll always
accompany the cellulose pulp lnto the actlvatlng reactor,
and the oxygen contalned ln the alr ls often sufflclent. In
so-e cases it ay even be necessacy to reduce the a-ount oi
~- .
.
:` ~
141Z
alr accompanying the cellulose pulp. Alr can be removed by
compressing the cellulose pulp prlor to introduclng the pulp
lnto the actlvatlng stage, or by heatlng and/or evacuatlng
the cellulose pulp. When nitrlc oxlde (NO) ls charged as an
actlve nltrogen oxlde, oxygen ls preferably charged solely
in a quantity below the stolchlometrlc quantlty required to
oxidize nltric oxlde (NO) to nltrogen dloxide (N02).
The gas separated from the actlvatlng stage - thls gas
havlng a certain lowest content of nltrlc oxide (NO) - is
recovered for treatment. This treatment process comprises at
least two phases, namely the lntroductlon of an oxygen-con-
talning gas and the reactlon of the gas wlth an absorptlon
solutlon. The oxygen addltlon ls normally made flrst, al-
though it is fully conceivable to carry out both phases ln
one and the same treatment stage.
In order to achleve an optlmum result, lt has been found
that the amount of oxygen charged shall be from 0.10 to
0.35, preferably 0.20-0.28 mole 2 calculated per mole
nitrlc oxide (NO) in the separated gas.
The ab60rptlon solutlon may be any suitable solutlon
havlng a pH wlthln the range of 3-13.5 and the ability to
remove the nitrogen oxldes to a very high degree from the
separated gas.
According to preferred embodiments of the invention each
of two solutlons ls used as the absorption solution. It is
partlcularly expedlent to use one of the solutlons ln one
absorption stage followed by a second absorption stage in
whlch the other of said solutions is used.
One of the solutions may be weakly acid, up to neutral,
and comprlses waste llquor derived from the actlvation of
the cellulose pulp. The solution may either comprise solely
waste liquor of this kind or also a mixture of sald waste
liquor with some other liquid, e.g. a llquld whlch lncreases
the pH of the resultant solutlon. The other solutlon will
have a pH wlthin the range of 7-13.5, and suitably comprises
6 13Q141Z
waste llquor derived from the dellgnlflcatlon of the cellu-
lose pulp with alkall. A partlcularly preferred waste llquor
ls one obtalned from an alkallne oxygen-gas bleachlng stage,
and particularly a waste llquor obtained from an alkallne
oxygen bleachlng process ln whlch the cellulose pulp has
been activated with nitrogen dloxide (N02) in accordance
with the invention. This results in a low consumptlon of
active nitrogen oxides and avoids the precipltation of llg-
nin. These absorption solutions are recovered and charged to
the cellulose pulp prior to and/or during the activating
process. The solutions may be used advantageously for im-
pregnating and/or diluting the cellulose pulp immediately
prior to and particularly during the activation process,
l.e. the treatment with gas containlng nitrogen dloxlde
(N02)-
The pH-values recited in this document refer to measure-
ments made with glass electrodes on samples which were
cooled to room temperature (approx. 20C) in the absence of
vaporlzatlon. In the case of samples taken durlng the actl-
vatlon process, the cellulose pulp was separated out prlorto determining the pH. When samples were taken at a pulp
conslstency above 8%, the consistency was brought down to 8S
by dllutlng wlth pure water, whereafter the cellulose pulp
was separated. The pH-values recited with regard to absorp-
tlon solutlons, e.g. various waste liquors, relate to cooledundlluted samples.
Wlth regard to the purlflcation of the separated gas,
good results were obtalned when the relative quantltles of
the separated gas and the absorption solution, and optlon-
ally the amount of oxygen (alternatlvely oxygen-contalnlng
gas) charged to the system were adapted so that the pH of
the absorpt~on solution used was wlthln the rage 5-12. The
recovery of active nitrogen oxide was particularly favoured
when using an absorption solutlon within this pH-range. When
recovering this absorption solution lt is preferred to use
the solutlon solely for impregnation and/or dllutlon of the
cellulose pulp prlor to commenclng the activating process.
1412
Advantaqes
When practising the method according to the lnvention it
has been found posslble, as dlstlnct from prlor art tech-
nlque, to maintaln the consumptlon of both oxygen and newly
charged nltrogen dloxlde (alternatlvely nltric oxlde) at a
low level while malntalnlng hlgh selectivlty durlng dellgnl-
flcatlon of the cellulose pulp.
When using certaln condltlons it has even been found
possible to achieve a slightly improved quality, e.g. im-
proved strength properties, despite a low consumption of
nitrogen oxides, as distinct from the prior art techniques.
These advantages are obtained with no effect or only a
slight effect on the environment.
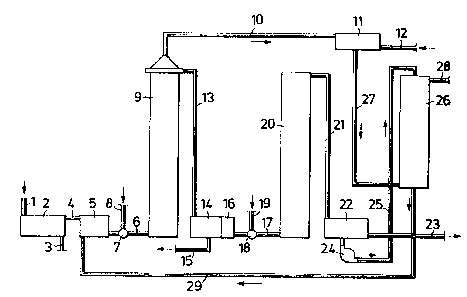
Brief descriPtion of the drawin~s
Fig. l is a flow sheet describing a first embodiment of
the method according to the invention, and Fig. 2 is a flow
sheet describing a preferred second embodiment of the method
according to the lnvention.
Preferred embodlment
A plurality of other parameters slgnificant to the in-
ventive method will be recited in the following description
of the aforesaid flow sheets.
In the case of the inventlve method lllustrated in Fig.
l chemical cellulose pulp, e.g. unbleached chemical cellu-
lose pulp, is passed to a liquor removing apparatus 2, e.g.
a press, through a condult l. The cellulose pulp may be
screened or unscreened, and is normally freed from the ma~or
part of the spent digestion liquor accompanylng the pulp
from the digester. The spent digestion liquor is displaced
normally with waste bleaching liquors, e.g. waste liquor
deriving from oxygen-gas bleaching stages. It is also poss-
ible to use for this purpose a given quantity of waste li-
quor deriving from the activating process. The pulp consist-
ency is normally low (one or a few %) when the pulp is
8 ~3~ 1Z
introduced lnto the press 2. The pulp consistency ls in-
creased in the press 2, e.g. to 30% and above. Llquor
pressed from the cellulose pulp ls carried away through a
conduit 3 and used, for lnstance, to wash and/or dllute
unbleached pulp. The cellulose pulp is passed to a dllutlng
plant 5, through a condult 4. At this location of the pro-
cess, the conduit 4 may be replaced wlth a chute, or a feed
screw. The consistency of the pulp is lowered in the plant 5
to, e.g. 5%. The cellulose pulp is then passed through a
conduit 6 (e.g. with the aid of a pump) to a mixer 7. A
nltrogen oxide, e.g. nitrogen dloxide (N02) is supplied
through a conduit 8. Desired temperature during the activa-
ting process is normally obtained by in~ecting steam into
the cellulose pulp flow. The cellulose pulp is then caused
to pass upstream through an activating reactor 9. The tem-
perature at which activation is permitted to take place is
dependent on a number of other parameters, although in the
case of thls embodiment of the inventlon the temperature
used will normally lle within the range of 50-95C. Tempera-
tures of approx. 90C are partlcularly appllcable ln thecase of the lllustrated slngle-stage method, with regard to
actlvatlon. The helght of the reactor 9 and the rate at
whlch pulp passes through the reactor are determined by the
resldence tlme (the treatment tlme). The resldence tlme ls
normally from 60-360 mlnutes. Resldence tlmes of 180 minutes
have been found to glve good results at temperatures of
approx. 90C.
A gas contalnlng nltrlc oxlde (N0) ls separated from the
cellulose pulp suspenslon at the top of the reactor 9. The
gas ls passed to an oxldatlon reactor ll, through a condult
lO. The gas, whlch contalns lnter alla nltrlc oxide (N0) is
reacted ln the reactor with an oxygen-containing gas, pre-
ferably oxygen gas, which is supplled through a condult 12.
The amount of oxygen charged ls sultably from 0.10-0.35 mole
2 calculated per mole nitrlc oxlde (NO) ln the gas trans-
ferred to the reactor ll.
9 13~1~12
No oxygen-contalning gas ls supplled lntentlonally to
the cellulose pulp when practislng thls embodlment of the
lnventlve method. On the other hand, larger or smaller quan-
tltles of air wlll always accompany the cellulose pulp lnto
the activatlng reactor. Wlth the ald of the apparatus arran-
gement lllustrated ln Flg. 1, lt ls posslble to malntaln the
amount of alr accompanylng the pulp on a sultable level (and
therewlth also the amount of oxygen charged).
In order to achleve an optlmum actlvatlng effect, lt is
important to monltor and control the pH of the cellulose
pulp prlor to and durlng the actlvatlng process. At posltion
7, i.e. at the locatlon lmmedlately prlor to lntroduclng
nltrogen oxide in some form or another through the condult
8, the cellulose pulp wlll normally have a pH wlthln the
range of 5-12. A pH wlthln the range of 5.5-8 ls preferred.
Subsequent to lntroduclng nltrogen oxlde lnto the pulp the
pH falls, and it has been found that the pH of the cellulose
pulp durlng the flnal stage of the actlvatlng process and
thereafter should lle wlthln the range of 1.5-4.5. Partl-
cularly good re6ult6 have been achieved with a final pH of1.8-2.8. Sultable low pH-values can be obtained ln several
ways. For example, a sultably low pH can be obtalned by
addlng nltrlc acld or 60me other acld, preferably a strong
mineral acld. The addition of a small quantity of oxygen
gas, e.g. 0.5-2 kg for each 1000 kg of llgnln accompanying
the cellulose pulp can also be used to lower the pH.
The cellulose pulp leaves the reactor 9 through the top
of the reactor and i6 passed to a liquor-separating appara-
tus 14, through a conduit 13. This apparatus may have the
form of a pres6 by means of which a conslderable part of the
actlvating liquor can be removed from the cellulose pulp.
Instead of pressing the waste llquor from the cellulose
pulp, the llquor can be dlsplaced from the pulp with the aid
of a liquld, preferably re-cycled actlvatlng waste llquor
12
and/or fresh water. The dlsplaclng llquld may also contain
waste bleachlng llquor, e.g. waste llquor derlved from an
oxygen-gas bleaching stage. The waste llquor ls carrled away
through a condult 15 and passed to the dllutlng apparatus 5
and/or to the cellulose pulp at a posltlon upstream of sald
plant. The cellulose pulp is then passed to the lmpregnatlng
plant 16, in which alkali in some form or another, e.g. 50-
dlum hydroxide, is charged to the pulp. If oxygen-gas
bleaching waste liquor has not already been charged to the
cellulose pulp, it is suitable to introduce such liquor into
the pulp, preferably in con~unction with adding a magnesium
salt. The cellulose pulp is passed through a conduit 17 to
an intensive mixer 18, to which oxygen gas is supplied
through a conduit 19. The oxygen gas disperses in finely
divided form throughout the cellulose pulp suspension, the
consistency of which suitably lies within the range of
5-10%. The suspension passes upstream through an oxygen-gas
bleaching reactor 20. The oxygen-gas pressure at the bottom
of the reactor 20 is, to some extent, determined by the
height of the reactor. The pressure of the oxygen gas supp-
lled can be selected freely, meaning that the oxygen-gas
pressure at the top of the reactor 20 is equal to atmo-
spherlc pressure or hlgher than atmospherlc pressure. It is
also concelvable to use a pulp conslstency greater than 10%
durlng the oxygen-gas bleachlng stage, lmplying so-called
high-consistency oxygen-gas bleaching.
The cellulose pulp is then passed through a condult 21
to a llquor separatlng plant 22, ln which the cellulose pulp
ls freed from oxygen-gas bleachlng waste-llquor in a known
manner, e.g. by pressing and/or washing. The cellulose pulp
leaves the plant through a conduit 23, for further treatment.
13(~41Z
11
A certaln amount of the waste oxygen-bleachlng llquor ls
transported through a conduit 25, wlth the ald of a pump 24,
to the top of a gas absorption plant (scrubber) 26. The
waste oxygen-gas bleaching llquor normally has a pH withln
the range of 9-12. Gas taken from the oxldatlon reactor 11
is passed to the bottom of the scrubber 26 through a condult
27. Contact between the gas and the absorptlon llquld frees
the gas from the ma~or part of lts nltrogen-oxlde content.
If a very high degree of absorption is achleved ln the
scrubber 26, the treated gas may be dlscharged to atmosphere
through a conduit 28. This is not to be preferred. Instead,
and alternatlvely, the gas is transported to a soda recovery
unlt in which, inter alia, cooklng waste liquor is combust-
ed. In those cases when the requirements placed on the
envlronment are very high, it may be necessary to further
purify the gas in the conduit 28 prlor to dlscharging the
gas to atmosphere or introducing the gas lnto the soda
recovery unit.
The absorptlon liquid, which contalns several nltrogen
compound6, ls removed from the scrubber 26 through the
bottom thereof, and is transported to the dilutlng plant 5,
through a conduit 29. A larger or smaller amount of the ab-
sorption liquid can also be used for other purposes. It is
preferred, however, to lntroduce the absorption liquid into
the cellulose pulp at a position upstream of the activating
reactor 9, 80 as to use the nitrogen compounds present dur-
ing the actlvatlng process.
It may be advantageous to recycle both the gases and
liquids through the various stages, even though thls ls not
shown in the figure. For example, part of the gas flow in
conduit 10 and/or conduit 27 can be returned to the activa-
tlng reactor 9. Furthermore, it is preferred to recycle to
the activatlng reactor 9 part of the gas carried through the
conduit 28. Oxygen can be supplied to said gas flow, during
passage of the flow to the reactor 9. Furthermore, part of
the absorptlon solutlon at the bottom of the scrubber 26 can
be cycled back to the scrubber, and preferably to one or
more locations along the outer shell of the scrubber in the
vertlcal extenslon thereof.
12 13~141Z
The preferred embodlment of the lnventlve method lllu-
strated ln Flg. 2 colncldes lnltlally wlth the embodlment of
the inventlve method descrlbed wlth reference to Fig. 1.
Chemical cellulose pulp ls transported through a condult
30 to a llquor removal apparatus 31. Llquor extracted from
the cellulose pulp is carried away through a conduit 32. The
cellulose pulp is transported through a conduit 33 to a dl-
luting plant 34. The cellulose pulp ls then transported
through a condult 35 to a mlxer 36, ln whlch the cellulose
pulp is brought into contact with nitrlc oxlde (N0) and oxy-
gen (2) vla a condult 37. The molar proportion between
these gases, may. e.g., be 2.5:1.
The introduction of these gases lnitiates the activating
process. In this case the activating process is divided into
two stages wlth intermediate dilution of the pulp suspen-
sion. Cellulose pulp havlng a consistency of e.g. 10-15% is
passed upstream through a first activatlng reactor 38. The
temperature in this stage is advantageously comparatively
low and the time comparatlvely short. For example, a tempe-
rature of 35C and time of 20 minutes can be used. Thecellulose pulp is then transported through a conduit 39 to a
dilutlng arrangement 40, ln which the cellulose pulp is
thlnned, e.g. to a conslstency of 4-9%. The cellulose pulp
is then transported through a conduit 41 to a second activa-
ting reactor 42. This second activating stage shall becarried out at a high temperature (e.g. 90C) and over a
long period of time (e.g. 180 minutes). This second treat-
ment stage can be referred to as the ripening stage. The
cellulose pulp is then conducted through a conduit 43 to a
gas separator 44, e.g. a cyclone separator. The nitrogen-
-oxide containing gas separated from the cellulose pulp
suspension is passed through a conduit 45 to an oxldation
reactor 46, to which there is connected a conduit 47 for
supplying oxygen gas to the reactor.
:
13 ~ 2
Subsequent to thls gas extraction, the cellulose pulp ls
transported to a llquor separatlng plant 49, through a con-
dult 48. The cellulose pulp ls then lmpregnated ln a plant
50 with alkali, e.g. in the form of sodium hydroxide, ne-
cessary for oxygen-gas bleaching, and optionally a protec-
tor, e.g. in the form of a magnesium salt. Waste oxygen-gas
bleaching liquor may also be supplied to the cellulose pulp
at positions 49 and 50. The cellulose pulp ls then transpor-
ted through a condult 52 to an intenslve mlxer 51, to whlch
oxygen gas under overpressure ls supplled through a condult
53.
The cellulose pulp is then caused to pass upstream
through an oxygen-gas bleachlng reactor 54. The temperature
and tlme are not critical, and these parameters, together
wlth oxygen-gas pressure and alkali charge can be selected
ln accordance with conventional techniques.
The cellulose pulp is transported from the oxygen-gas
bleachlng reactor 54 through a conduit 55 to a liquor sepa-
rating plant 56. Subsequent to extractlng waste oxygen-gas
bleachlng llquor from the cellulose pulp, the pulp ls tran-
sported through a conduit 57 to some further treatment loca-
tlon, e.g. one or more flnal bleachlng stages.
Part of the waste oxygen-gas bleachlng llquor, whlch has
a pH of 9-12, is transported, wlth the ald of a pump 58,
through a conduit 59 to the top of a flrst gas absorptlon
plant 60 (scrubber). The liquld is flnely divlded in a known
manner, e.g. with the ald of spray nozzles, or ls caused to
pass through the scrubber ln the form of a thln llquld fllm
on solld packlng bodles arranged ln the scrubber, e.g. sadd-
le packlng bodles or so-called Raschlg rlngs. The gas ls
transported from the oxldatlon plant 46 through a condult 61
to the bottom of a scrubber 60. Purlfled gas ls removed from
the scrubber 60 and passed through a condult 62 to the
bottom of a second gas absorptlon plant 63 (scrubber).
Further oxygen is supplied through a condult 64, whlch ls
connected to the conduit 62. An advantage is galned when
14 l;~ lZ
part of the gas flowlng through the condult 62 ls removed at
a location immedlately upstream of the connectlng condult 64
or downstream thereof and returned to one (or both) of the
actlvating reactors 38 and 42, through a condult herefor.
Absorption liquid is passed through a condult 65 from the
liquor separating plant 49 to the top of the scrubber 63.
This liquid has a pH which lies within the range 3.5-6.5.
The cellulose pulp suspension introduced into the plant 49
normally has a pH well below 3. When the displacement liquld
used is totally or partially waste liquor from the oxygen-
-gas bleaching stage, the waste liquor will have a pH which
lies within the aforesaid range. When the activating waste
llquor is extracted from the cellulose pulp with the aid of
a press, the extracted waste llquor can normally be mlxed
wlth an alkaline liquid (e.g. waste oxygen-gas bleaching
liquor), so that the mixture will function well as an ab-
sorption liquid in the scrubber 63.
The gas cleansed in two stages can be removed from the
6y6tem, through a conduit 66, and dlscharged, e.g. to atmo-
sphere or to a soda recovery unlt, or to some other form ofcombustlon plant. It 16 also pos61ble to purlfy the gas ln a
thlrd purlflcation 6tage of any form, prior to flnally dls-
charglng the gas from the system. Absorptlon solution from
the flrst scrubber 60 ls passed through the condult 67 to
the dllutlng plant 34, whlle absorptlon solutlon from the
second scrubber 63 18 passed to the dilutlng arrangement 40
through the condult 68.
There 18 obtained wlth the aforedescrlbed method a resl-
dual gas whlch ls extremely pure, l.e. has a practlcally
negllgible content of nltrogen oxldes, whlle at the same
tlme obtalnlng two ab60rptlon solutlon6 whlch can both be
used effectlvely in the actlvatlng stage. By lntroduclng
these solutlons lnto the cellulose pulp prlor to and durlng
i 3n 1 412
activatlon of the pulp together wlth nltrogen dloxlde
(N02) containing gas, there is obtalned an actlvated
cellulose pulp whlch can be delignified in a highly select-
ive manner in a subsequent stage (e.g. an oxygen-gas bleach-
lng stage). It has also been found posslble to lower thellgnln content of the cellulose pulp from a kappa number of
30-35 to 3-4 while maintaining a viscoslty of about
9SO dm3/kg, when applying the aforedescrlbed method.