Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to an improved method and apparatus
for detecting the singlet oxygen emission produced during
photoradiation when a chopped source of optical radiation is used
to excite a photodynamic sensitizer.
2. Description of Prior or ContemPOrar~ Art
When certain non-toxic photodynamic sensitizers, such as
hematoporphyrin derivative (HPD) and components thereof, are
injected intravenously into the human body, they are selectively
retained by cancerous tissue. Thus, two or three days after
injection, significantly higher levels of the photodynamic
sensitizer are retained in malignant tissue. The tumor is then
1 ~(3~
exposed to a therapeutic light and this light energy causes the
photodynamic sensltizer to be excited to an energetic metastable
triplet state. Through a direct intermolecular process, the
sensitizer transfers this energy to oxygen molecules present in
the tissue and raises them from the ground triplet to the first
excited electronic singlet state, 102 [symbolic deslgnation of
molecular oxygen in the l~g electronic state]. The singlet
oxyqen, 102, attacks and functionally destroys vital cellular
components ultimately inducing necrosis and destroying the
cancerous iissue. The advances and problems associated with this
cancer treatment are addressed in an article by Thomas J.
Dougherty et al entitled l'Photoradiation Therapy for the Treat-
ment of Malignant Tumorsl' published in Cancer Research, Vol. 38,
pages 2628-2635 (1978).
SU~qMARY OF THE INVENTION
_
The present invention represents an improvement to the
singlet oxygen monitoring apparatus and method described by John
G. Parker and William D. Stanbro in U.S. patent 4,576,173,
entitled "Electro-Optical Device and Method for Monitoring
Instantaneous Singlet Oxygen Contraction Produced During Photo-
radiation Using a CW Excitation Source". The present Applicant
discovered that a phase shift introduced by the photodetector and
associated electronics reduced the sensitivity of the aforemen-
tioned singlet oxygen monitoring apparatus.
The present invention utilizes a two band optical
comparator apparatus and method to compensate for this
undesirable phase shift. The invention utilizes an out-of-band
filter that passes a first band of light adjacent to the singlet
oxygen 1270 nm emission band and, an in-band filter that passes a
second band essentially comprisiny the singlet oxygen 1270 nm
emission band. Optical emission in said f irst band is
essentially composed of a f luorescence emission signal; and
optical emissions in said second band comprises a fluorescence
emission component and a singlet oxygen emission component that
is delayed in time with respect to such fluorescence emission
component. With the first band located spectrally near the
second band, any phase difference between the fluorescence
emission signal detected in said first band and the fluorescence
emission component detected in the second band is minimal.
To compensate for the undesirable phase error an electrical
reference signal is synchronized with the detected signal from
the first band, which is essentially composed of the fluorescence
emission signal. This electrical reference signal is then used
by the signal processing apparatus to separate the composite
electrical signal detected in the second band into the fluores-
cence emission component and the singlet oxygen emission com-
ponent. Separation of these two components is possible because a
component of the composite electrical signal 90 out of phase
from said electrical reference signal will indicate the singlet
oxygen emission component.
BRIEF DESCRIPTION OF T~E DRAWINGS
Figure 1 i~ a graph showing the composite
fluorescence/singlet oxygen emission signals appearing in the
1270 nm band.
Flgure 2 is a phase diagram of the composite fluorescence/
singlet oxygen emission signal appearing in the 1270 nm band.
Figure 3 illustrates, in block diagrammatic form, an
apparatus used to process the composite fluorescence/singlet
oxygen emission signal so as to extract the singlet oxygen
emission signal.
Figure 4 is a phase diagram of the composite fluorescence/-
singlet oxygen emission signal appearing in the 1270 nm band that
illustrates the undesired electrical phase shift.
Figure 5 illustrates, in block diagrammatic form, the
improved singlet oxygen monitoring apparatus that utilizes a two
wavelength optical comparator.
Figure 6 is a graph showing the optical filter characteris-
tics of the in-band filter and out-of-band filter as taught by
the present invention.
1~}~
Fiqure 7 illustrates, in block diagrammatic form, the two
band optical comparator.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Photodynamic therapy involves injecting a patient with a
photodynamic sensitizer, such as hematoporphyrin derivative, and
after the sensitizer has localized at the appropriate biological
site, illuminating that site with optical energy. The resultlng
photodynamic action causes singlet oxygen to be generated. When
the singlet oxygen interacts with molecules at the biological
site, a relatlvely weak collisionally induced emission occurs at
the singlet oxygen 1270 nm emission band. (The singlet oxygen
emission band is centered at 1270 nm and has a full-width-at-
half-maximum (FWHM) of 0.02 micron extending from 1260 nm to 1280
nm. This band shall hereafter be referred to as the singlet
oxygen 1270 nm emission band or 1270 nm band).
By optically detecting and monitoring this emission it is
possible to determine the instantaneous rate at which singlet
oxygen is being generated and is attacking biological matter.
However, as illustrated in Figure 1, the optical energy emitted
in the 1270 nm band during photodynamic therapy is a composite
signal comprising a first component 10 due to the spectrally
diffuse fluorescence of the sensitizer and auto fluorescence of
the biological matter and, a second component 12 produced by the
emission from singlet oxygen molecules. Both the first component
fluorescence signal 10 and the singlet oxygen emission 12 arise
as a consequence of optical excitation of the sensitizer and both
are inextricably intertwined in the 1270 nm band. There is no
way of spectrally separating the singlet oxygen emissions 12 from
the fluorescence component 10 both appearing in the 1270 nm
singlet oxygen emission band. The fluorescence component 10 in
the 1270 nm band also is generally much larger in magnitude than
the relatively weak singlet oxygen emissions 12~ The central
difficulty to be overcome, therefore, in monitoring the singlet
13()~ '7
oxygen emission is to separate the singlet oxygen emission
component from the fluorescence component.
In U.S. patent 4,576,173~
the present Applicant along with William D. Stanbro
disclosed an invented method and apparatus for separating the
singlet oxygen emission signal from the composite signal. That
invention was based upon the fact that the fluorescence component
occurs simultaneously with the excited light but the singlet
oxygen emission component is delayed in time with respect to the
fluorescence component. This time delay is due to the fact that
formation of the singlet oxygen is not directly coupled to the
optical excitation. Formation of singlet oxygen involves a
collisional transfer of energy from the sensitizer metastable
triplet state, thus delayed with respect to the initiation of the
optical excitation by the time required to bring about collision
of the ground electronic state dissolved oxygen 302 with the
excited sensitizer. Figure 2 contains a phase diagram showing
the phase relationship between the fluorescence component and the
singlet oxygen emission component of the composite optical
signal. The horizontal axis of the phase diagram shows the in-
phase or real component (phase ~ = 0) and the vertical axis
shows the quadrature or imaginary component (phase angle
~ = -90). Since the fluorescence component ~14) is essentially
concurrent with the-excitation light, its phase vector will
appear at phase angle ~ = 0 (e.g., the fluorescence component is
in-phase with the chopped CW excitation signal). However, the
singlet oxygen emission signal, as shown in the above equations,
appears as a vector (16) having a phase delay (e.g., the singlet
oxygen emission signal lags the fluorescence component (14),). The
sum of the fluorescence component vector 14 and the singlet
oxygen emission vector 16 produces a resultant signal vector 18.
The resultant signal vector can be divided into a real component
(phase angle ~ = 0) 20 and a quadrature component (phase angle
O = -90) 22. The real or in-phase component is the sum of the
real sensitizer fluorescence component and the real singlet
13(1 ~
oxygen emission component. However, the imaginary or quadrature
component 22 is only dependent on singlet oxygen emission.
Figure 3 is a block diagrammatic view of the apparatus used
to separate and detect the singlet oxygen component as taught by
the above-referenced patent ~U.S. 4,576,173). A CW light source
24, which may be a laser is chopped by chopper 26 and directed by
a light delivery means 28 onto the biological mass 40. The
chopper may be a mechanieal chopper, an acousto-optic modulator,
or similar devices capable of chopping the excitation beam at a
rate from lkHz to 100 kHz or higher. The chopper 26 has adjust-
ment 30 so that the chopping frequency (fc) can be set at
particular values or scanned across a range of values. The light
delivery means 28 used to direct the chopped CW excitation beam
can be a lens arrangement or a fiberoptic link. A beam splitter
32 and a photodiode 34 work in conjunction to produce an electri-
cal reference signal 36, which is used to synchronize the lock-in
amplifier 38. Alternatively, an electrical signal produced
directly by the chopping circuit or by a timing circuit can act
as a reference signal 36, to synchronize the lock-in amplifier.
The biologieal mass or tumor 40 which has absorbed a photodynamic
sensitizer, such as hematoporphyrin derivative or components
thereof, and which is optlcally irradiated emits a composite
signal composed of a fluorescence component and a sin~let oxygen
emission eomponent. A light collecting means 42, which may be a
lens arrangement or fiberoptic link, collects the light. The
collected light is then filtered by filter means 44 which passes
light in the singlet oxygen 1270 nm emission band. The filtered
light is then directed to a photo detector 46 which converts the
composite fluorescence/singlet oxygen emission signal, both
appearing in 1270 nm emission band, into an electrical signal 48.
The eleetrical signal 48 is amplified by preamplifier 50;
photodeteetor 46 and preamplifier 50 together forming an eleetro-
optieal means 45 for detecting optieal emissions. The output of
the preamplifier 50 is fed to lock-in amplifier 38. The inven-
tors have used an EG&G PAR Model 124A lock-in amplifier, but
* Trade Mark
other known lock-in amplifiers or synchronous detectors can be
used as well. The lock-in arnplifier 38 has as input the refer-
ence signal 36 and the output from preamplifier 50. The imagi-
nary or quadrature component (phase angle ~ = -90) of the
composite signal is processed by the lock-in amplifier 52.
Indicator 54 connects to output terminal 52 and displays the
magnitude of the processed signal which is directly proportional
to the magnitude of the instantaneous singlet oxygen concentra-
tion generated in the biological mass 40 by photoradiation.
The present invention represents an improvement to the
apparatus shown in Figure 3 and claimed in U.S. patent 4,576,173.
Applicant discovered that a small phase shift was occurring
between the electrical composite signal 56 from the detec-
tor/preamplifier and the reference electrical signal 36. The
phase relationship between the fluorescence component vector 14
and the singlet oxygen emission vector 16, as shown in Figure 2,
was maintained. However, if the horizontal axis is synchronized
with the reference electrical signal 36, the additional phase
delay would cause vectors 14, 16, and 18 to appear rotated from
the horizontal axis. A graphic representation of this rotation
caused by the additional phase shift in the electrical signal
appears in Figure 4. As a result, the quadrature electrical
component (~ = 90) would include a small component of the
fluorescence signal component, which would introduce an error in
the singlet oxygen measurement. (Note: In a biological medium
the fluorescence component vector 14 is many times larger than
the singlet oxygen emission vector 16. Therefore, any slight
rotation of the fluorescence component vector from the horizontal
axis will produce a component along the quadrature axis that may
mask the singlet oxygen quadrature signal.)
Applicant believes this phase shift is an electronic phase
shift that occurs in the detector 46 and preamplifier 50. It is
believed that the electronic circuit in the preamplifier adds a
phase delay. It is also believed that a phase shift occurs in
the detector. The phase shift occurring in the photodetector
1306~7~7
appears to vary with wavelength. Therefore, the photodetector
will, for instance, introduce a different phase shift for the
excitation wavelength as it would for optical radiation in the
singlet oxygen 1270 nm emission band. Further, Applicant
believes that the electrical phase shift changes as a function of
a change in intensity of the received optical radiation.
Applicant has discovered a method and apparatus for compen-
sating for the electrical phase shift generated in the detector
and preamplifier circuits. The invented method and apparatus
adjusts the electrical reference signal 36 so that it is coinci-
dent with the electrical signal generated by the fluorescence
signal component. The improved photodynamic therapy monitoring
apparatus is shown in Figure 5. The apparatus contains the same
basic components as described earlier: filter means 44, photo-
detector 46, preamplifier S0 and lock-in amplifier 38 (alterna-
tively, a synchronous detector or similar clrcuit can be used).
However, the filter means 44 has been modified to include two
alternate filters (58, 60). Filter 58 is a narrow band filter
that passes light only in the singlet oxygen 1270 nm emission
band. Again, emissions_in this band is a composite signal
containing the fluorescence component as well as the singlet
oxygen component. Filter 60 is a narrow band filter that passes
light just outside the 1270 nm singlet oxygen emission band.
Therefore, the emissions passing through this filter would be
almost exclusively the fluorescence component. The general
characteristics of filter 58 and 60 are shown in Figure 6. Band
width deviations from that shown in Figure 6 are acceptable as
long as one filter contains the singlet oxygen emission and the
other filter is outside the oxygen emission band. The filter
ou~siae the singlet oxygen band should be spectrally near the
singlet oxygen band so that there will be little or no change in
the phase of the fluorescence signal component in these two bands
caused by the photodetector. Therefore, filter 58 shall here-
inafter be called the "in band filter"; and, filter 60 should be
called the "out-of-band filter".
13~ 77
In operation, light emissions from the biological site is
first passed through the "out-of-band filter". The "out-of-band
filter" transmits only the infrared fluorescence component.
Photodetector 46 and preamplifier 50 generate an electrical
signal which is fed to the amplifier 64 and synchronous detector
66 that are associated with the lock-in amplifier 38. The
electrical reference signal 36 passes through a voltage control
phase-locked oscillator 68, phase selector 70 and phase shifter
72 to the synchronous detector 66. The phase selector is set so
that the quadrature component (phase O = -90) of the reference
signal 36 is input to the phase shifter 72. The phase shifter 72
allows one to add or subtract a small phase shift onto the
quadrature component of the reference signal. The synchronous
detector provides an output 78 based on the reference signal
input and the detector signal input 76. Alternative circuitry
can be substituted for the synchronous detector such as a mixer
or phase sensitive detector; such alternative circuitry shall be
generically called a-synchronous detector. Basically, the
synchronous detector multiplies the reference signal input with
the detector signal input 76. The phase shifter 72 is adjusted
until the output of the synchronous detector 66 is zero. The
output of the synchronous detector being zero means that the
detected signal input 76 is concurrent with the reference signal
74. This means that the electrical signal generated only by the
fluorescence emission component is now concurrent with the
reference electrical signal 36 and the phase shift added by phase
shifter 72 now compensates for the undeslrable phase shift
discussed earlier. The fluorescence signal component vector is
now concurrent with the horizontal axis as shown in Figure 2, and
synchronization adjustment is completed.
To measure the singlet oxygen signal, the "in-band filter"
is used to replace the "out-of-band filter" and phase selector 70
and phase shifter 72 are kept at the positions established above.
Since the detector phase (quadrature condition) is established,
the output from the lock-in amplifier 38 will give a non-zero
~ 3 ~
signal because of the ~xistence of the time-delayed singlet
oxygen emission.
This self-referencing two-band comparative method for
establishing the zero quadrature condition for the ~luorescence
only component uniquely enhances the accuracy of the photodynamic
therapeutic monitoring system. The two filters could be replaced
by a spectrometer which provides the same basic function but
would result in greater signal loss. Further, the method can be
automated with the use of a phase adjustment means 80 shown in
Figure 5. The phase ad~ustment means receives an input from the
synchronous detector and controls the phase shift added by the
phase shifter 72. The phase adjustment means 80 could be a
microprocessor control circuit that adjusts the phase shift 72
until the synchronous detector output is zero.
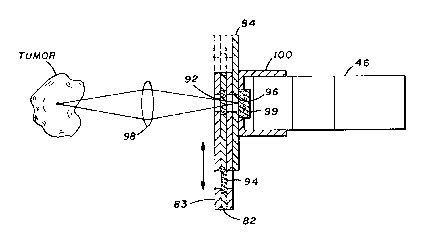
Figure 7 shows a more detailed view of a filter means 44
that provides optimum optical coupling to the detector. A
movable filter carriage plate 82 is held firmly between an
aperture plate 84 and a front plate 83. The filter carriage
plate 82 includes an "out-of-band filter" 92 and an "in band
filter" 94. The filter carriage plate 82 can be moved back and
forth (as indicated by the arrows) thus allowing the "out-of-band
filter" or "in-band filter" to be positioned in front of the
aperture 96. Therefore, light emitted from the biological site
will be collected by either a fiberoptic means or a lensing means
98 and will pass either through the "in-band filter" or the "out-
of-band filter" and then through aperture 96 and band pass filter
99 (1150-1320 nm) to photodetector 46. A light shield 100
prevents stray light from entering the photodetector.
It is to be understood that it is within the scope of the
present invention to use other mechanisms for alternatively
placing the "in-band filter" and the "out-of-band filter" in
front of the detector aperture. A rotating system as well as the
transverse motion system is possible; in addition, an electro-
mechanical mechanism could be used to move the filters in
position as well as a manually controlled mechanism. Further,
~30~ 7
although the above example shows the phase of the reference
electrical signal being adjusted to compensate for the undesired
electrical phase shift, it is also contemplated that the detected
electrical signal could alternatively have a phase shift intro-
duced to it which would compensate for the undesirable electrical
phase shift.
Obviously many modifications and variations of the present
invention are possible in light of the above teachings. It is
therefore to be understood that within the scope of the appended
claims the invention may be practiced otherwise than as specifi-
cally described.