Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 307784
1 --
13~-alkylgonanes~ their prepa~ation and
pharmaceutical preparations containing them
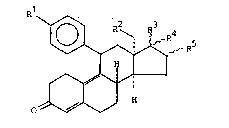
The present invention provides 13~-alkylgonanes of the
general formula
R1 R5 (I)
. :
O~ ~ ~_~ H
in which
Rl represents RI
a group of the general formula -N in which
\ II
RI and RII which may be the same or different,
each represents a hydrogen atom or
an alkyl group having from 1 to 4
carbon atoms, or
RI and RII together with the nitrogen atom to
which they are attached, represent a
saturated 5- or 6-membered ring,
which may, if desired, contain a
further hetero atom, or
X 3~
1 307784
ORIII in which
RIII represent~ a methyl, ethyl, propyl,
isopropyl, methoxyphenyl, allyl or ~-
dimethylaminoethyl group,
R2 represents a hydrogen atom, a methyl group or
an ethyl group,
R3 represents a group of the general formula
-(CH2)n-CH3 in which
o n represents O or an integer from 1 to 4, a
group of the general formula -(CH2)n-CH2-
ORIV or -(CH2)n-CH2-SRIV in which
n represents O or an integer from 1 to 5, and
RIV represents a hydrogen atom or
an alkyl or alkanoyl radical each
having from 1 to 4 carbon atoms,
a group of the general formula -CH=CH-(CH2~n-ORV in
which
n represents an integer from 1 to 4, and
RV represents a hydrogen atom or
an alkyl or alkanoyl radical each
having from 1 to 4 carbon atoms,
a group of the general formula -C~C-X in which
X represents a hydrogen atom or an
alkyl radical having from 1 to 4-carbon
atoms or a halogen atom,
1 3~7784
a group of the general formula -(CH2)n-CH2CH in
which
n represents 0 or an integer from 1 to 3, or
a group of the formula 1¦ in which
-C-CH2Y
Y represents a hydrogen atom of a
group of the general formula ORV in which
RV has the meaning given above,
R4 represents a hydroxy group or an alkoxy or
alkanoyloxy radical each having from 1 to 4 carbon
atoms or
R3 and R4, together with the carbon atom to which they are
attached, represents a group of the formula
l~ ~ -
and R3 is in the ~-configuration and
R4 is in the ~-configuration or
R3 is in the ~-configuration and
R4 is in the ~-configuration
with respect to the steroid structure,
and
R5 represents a hydrogen atom or an alkyl radical having
from 1 to 4 carbon atoms and being in the ~-or ~-
configuration,
X
..
1 307784
and the corresponding tertiary N-oxides and physiologically
tolerable acid addition salts, of such compounds in which
represents a group of the general
/
formula -N
\ RII These compounds have a strong
affinity for the gestagen receptor without themselves having
gestagenic activity. They are competitive antagonists of
progesterone (antigestagens) and are suitable for inducing
abortions since they expel from the receptor the progesterone
necessary to maintain pregnancy. The compounds are therefore
valuable and interesting with regard to their use for
postcoital (p.c.) fertility control.
Corresponding compounds in the oestrane series have
already been described as antigestagenically active compounds
in Fertility and Sterility 40 (1982), page 253.
The structure-action relationships known hitherto for
competitive progesterone antagonists indicate that a-l,3-
diaxial arrangement of an ll~-aryl radical and a 13~-alkyl
group are absolutely necessary for the development of
antigestagenic activity. All the more surprising, therefore,
is the strong and selective antagonistic action of the 13~-
alkylgonanes of the general formula I which has a completely
different molecular topography from the oestrane series.
An alkyl radical represented by RI and/or RII has from 1
to 4 carbon atoms, the methyl group and the ethyl group being
preferred. Preferably RI and RII are the same.
,
1 307784
The group N \ may also represent a
R
saturated 5- or 6-membered ring which, apart from carbon
atoms and the nitrogen atom shown may also contain a further
hetero atom, such, for example, as O, N or S; examples of
such groups are the pyrrolidino, piperidino, piperazino,
morpholino, oxazolidino and thiazolidino and thiadiazolidino
rings. RI
/
The -N \ radical may also be in the form
RII
of a tertiary N-oxide, such as, for example, dimethylamino-N-
oxide, and pyrrolidino-, piperidino-, piperazino-,
morpholino-, oxazolidino-, thiadiazolidino and thiazolidino-
N-oxide.
An alkyl radical represented by X and/or R5, an alkyl or
alkanoyl radical represented by RIV or RV and an alkoxy or
alkanoyloxy radical represented by R4 each has from 1 to 4
carbon atoms; methyl, ethyl, acetyl and propionyl are
preferred.
The following groups should especially be mentioned:
for R1: N(CH3)2, N(C2H5)2~ OCH3
for R2: H, CH3,
for R3: C_CH, C-CCH3, CH2CH2OH, CH=CHCH2OH, CH2CN and COCH3,
for R4: OH and, especially when R3 represents COCH3, OCOCH3,
for R5: H, C2H5.
.
.., . ~._
1 307784
A 13~-alkylgonane of the general formula I or N-oxide or
acid addition salt thereof may be prepared by irradiating a
compound of the general formula
R ~
(II),
H
~O /~~.'~
OH
in which R1, R2 and R5 have the meanings given above and Z
represents an ethylene or 2,2-dimethylpropylene group, or
other ketone-protecting group, or an N-oxide or acid addition
salt thereof, with ultraviolet light, and converting the
resulting 13-epi-steroid of the general formula
~ l ~ ~ ~ r i f
(III),
O ~ H
OH
in which R1, R2, R5 and Z have the meanings given above, or
an N-oxide or acid addition salt thereof into a compound of
the general formula I, for example by a method known er se
by nucleophilic addition to the 17-ketone group, cleavage of
1 30778~
the 3-ketal protection and the splitting off of water from
the 4,5-position.
The present invention provides a process for the
preparation of a compound of the general formula I or an N-
oxide or salt thereof from a compound of the general formula
II or N-oxide or salt thereof by the process described above.
The present invention also provides a process for the
preparation of a compound of the general formula I or an N-
oxide or salt thereof from a compound of the general formulaIII or N-oxide or salt thereof by the steps of nucleophilic
addition to the 17-ketone group, cleavage of the 3-ketal
protecting group and the splitting off of water from the 4,5-
position.
If desired, a compound of the general formula I or salt
thereof may be converted to a different compound of the
general formula I or salt thereof or N-oxide thereof.
Nucleophilic addition to the 17-ketone group ma~be
performed before or after the splitting off of water;
advantageously the nucleophilic addition is performed and
then de-protection of the 3-ketal group and the splitting off
of water are performed simultaneously.
Accordingly, the present invention further provides a
process for the preparation of a compound of the general
formula I, in which Rl, R2, R3, R4, R5 and Z have the
meanings given above, or an N-oxide or salt thereof from a
compound of the general formula
1 307784
~ ~ ~ ~ R5
~1
,,- ~ H
0~ .
or N-oxide or salt thereof, which comprises cleavage of the
3-ketone protecting group and the splitting off of water from
the 4,5-position,
or from a compound of the general formula
R ~ R5
~ (vr
Z~ ~ H
or N-oxide or salt thereof, which comprises nucleophilic
addition to the 17-ketone group and cleavage of the 3-ketal
protecting group.
If desired, a compound of the general formula IV or salt
thereof may be converted to a different compound of the
general formula IV or salt thereof or N-oxide thereof.
X
1 3077~
The present invention further provides a compound of the
general formula IV or an N-oxide or acid addition salt of
such compound in which Rl represents a group of the general
formula
--N / RI I
The present invention also provides a compound of the
general formula V or an N-oxide or acid addition salt of such
a compound in which R1 represents a group of the general
formula
RI
-N
\RII
The present invention also provides a compound of the
general formula III or an N-oxide or acid addition salt of
such compound in which Rl represents a group of the general
formula
- N \ RII
1 307784
-- 10 --
and a process for its preparation by irradiation of a
compound of the general formula II or N-oxide or acid
addition salt of such compound in which Rl represents a group
of the general formula
with W light.
By irradiation with ultraviolet light, a 13~-alkyl
steroid of the general formula II is converted, with a good
yield, into the 13-epi-steroid (13~-alkyl steroid) of the
general formula III.
The good yield of the conversion product is surprising.
Although it has long been known that 17-oxo steroids of the
normal series can be converted by W irradiation into 13-epi-
steroids (A. Butenandt et al., Ber. Deutsch. Chem. Ges. 74,
1308 (1941)), mixtures of the starting material and the
epimerised compound were always obtained, the irradiation
times were several hours and the yields were extraordinarily
low. The search for an alternative chemical means of
obtaining the 13-epi series is therefore still going on, as
more recent work by Barton et al., J.C.S. Perkin I, 2163
(1977) shows. This alternative is, however, thought to be
unsuitable for the manufacture of compounds of the general
formula I. We have found that, under certain conditions, the
irradiation of compounds of the general formula II (or their
N-oxides or salts) is considerably more successful than in
the series of ll-unsubstituted 17-oxo steroids: the average
X
._ ....
1 307784
-- 11 --
irradiation times are only from 10 to 30 minutes and the
yields of 13-epi-steroid may be from 60 to 80 %. If desired,
the irradiation products may be reacted further without
chromatographic purification. optimum results are believed
to depend on a suitable choice of solvent, the concentration
of the substrate to be irradiated, and exact adherence to the
period of irradiation. These parameters must be ascertained
individually for each substrate.
The irradiation may be carried out with the full light
of a Hg-high pressure lamp in a quartz glass apparatus. The
temperature of the reaction solution may be, for example,
approximately 25C and the concentration of the solution
preferably from 0.1 to 1.0 % by weight. Tetrahydrofuran and
dioxan are preferably used as solvents, but it is also
possible to use non-polar aprotic solvents, such, for
example, as hexane, cyclohexane, benzene, toluene, and
mixtures thereof. The period of irradiation is
advantageously from 10 to 50 minutes.
A 13-epi-steroid of the general formula III or N-oxide
or salt thereof may then be converted into a compoun~ of the
general formula I or N-oxide or salt thereof for example
acoording to customary processes as described above, for
example by nucleophilic addition to the 17-ketone, de-
protection of the 3-ketone group and splitting off of water
at the 4,5-position, and including, if desired, conversion of
a radical represented by R3 into another such radical and/or
of a radical represented by R4 into another such radical, at
any suitable stage in the process.
Thus, for example, the process may include one or more
of the following steps as appropriate:
(i) introduction of a group represented by R3 of the general
formula -(CH2)nCH3 in which n represents 0 or an integer
X
1 307784
from 1 to 4 or -(CH2)nCH2CN where n represents O or an
integer from 1 to 3 by an alkali metal-alkyl or alkali
metal-alkylnitrile compound,
(ii) introduction of a group represented by R3 of the
general formula -C--CX in which X represents a hydrogen
or halogen atom or an alkyl radical having from 1 to 4
carbon atoms, by means of a compound of the general
formula MC-CX in which X has the meaning given above
and M represents an alkali metal or by means of an
alkali metal and a compound of the general formula
HC_CX in which X has the meaning given above,
(iii) hydration of a triple bond in a group of the general
formula -C_CH represented by R3 to form a group of the
group of the general formula COCH3,
(iv) introduction of a group represented by R3 of the
general formula -C=C(CH2)nOR in which R represents a
hydroxy-protecting group and n represents an integer
from 1 to 4, by means of a compound of the general
formula NC-C(CH2)nOR in which R and n have the meanings
given above and M represents an alkali metal, and
hydrogenating the resulting compound to form the
corresponding hydroxyalkenyl or hydroxyalkyl radical of
the general formula -CH=CH-(CH2)nOH or -CH2CH2(CH2)nOH,
(v) oxidising a compound in which R3 represents a 3-
hydroxypropyl group and R4 represents a hydroxy group
..,
to form, with the carbon atom to w~ich they are
attached, a group of the formula
1 307784
- 13 -
(vi) introducing a CH2CN group represented by R3 via
formation of a spiro compound and splitting the
spiro compound with HCN,
(vii) introducing a CH20H or COCH20H group represented by
R3 by converting the 17-ketone to the corresponding
17- halo-17-alkoxycarbonyl compound, converting the
halo group to an alkoxy group and reducing ~he 17-
alkoxycarbonyl group to a CH20H group, and, if
desired, converting the 17-alkoxy group to a 17-
hydroxy group and also, if desired, converting the
CH20H group to a COCH20H group,
(viii) converting a hydroxy group represented by R4 into
an alkoxy or alkanoyloxy radical and/or converting
a hydroxy group in a radical represented by R3 into
an alkoxy or alkanoyloxy radical, I
~ R
(ix) converting a compound having a -N ~
\RII
group represented by R1 into an acid addition salt
thereof.
Nucleophilic addition at the C-17 position generally
results in formation of both possible isomers; these are,
however, readily separable by chromatography or fractional
crystallisation. In many cases, both isomers are
pharmacologically active, even though there may be
differences in their strengths of action.
The nucleophilic addition of, for example, acetylene
(ethyne) or propyne may be carried out using an agent that
X
1 307784
- 14 -
yields the radical -C-CH or -C~-CH3. Such agents are, for
example, alkali metal acetylides, such as, for example,
potassium and lithium acetylide or methylacetylide.
The organometallic compound may also be formed in situ
and reacted with the 17-ketone of the general formula III.
For example, acetylene and an alkali metal, especially
potassium, sodium or lithium, may be made to act on the 17-
ketone in a suitable solvent in the presence of an alcohol or
in the presence of ammonia.
To introduce a 17-alkyl or 17-cyanoalkyl group, an
alkali metal alkyl compound, for example methyl- or butyl-
lithium, or alkali metal alkylnitrile, e.g. Li(CH2)nCH2CN,
may be used for reaction with the 17-ketone. Suitable
solvents are, especially, dialkyl ethers, tetrahydrofuran,
dioxan, benzene and toluene.
A 17-ethynyl-17-hydroxy compound may be hydrated in
alcoholic solution with mercury salt catalysis to form a 17-
acetyl-17-hydroxy compound (Chem. Ber. 111 ~1978) 3086 -
3093).
A 3-hydroxypropyl or 3-hydroxypropenyl radical may be
introduced into the 17-position by reacting the corresponding
17-ketone with a metallated derivative of propargyl alcohol,
for example with l-lithium 3-tetrahydropyran-2'-yloxyprop-1-
yne, to form the 17-(3-hydroxyrop-1-ynyl)-17-hydroxy compound
which is then hydrogenated to form the 17-(3-hydroxyropyl or
3-hydroxypropenyl)-17-hydroxy compound. The hydrogenation
must be carried out under conditions that ensure that only
the C-C triple bond is affected and that the tetrasubstituted
9(10)-double bond is not saturated. This is possible, for
example, if the hydrogenation is carried out at room
X
1 307784
- 15 -
temperature and normal pressure in a solvent such, for
example, as methanol, ethanol, propanol, tetrahydrofuran
(THF) or ethyl acetate with the addition of a noble metal
catalyst, such, for example, as platinum of palladium.
A compound with Z-configured double bond in the
hydroxypropenyl group may be formed, for example, by
hydrogenating the acetylenic triple bond with a deactrvated
noble metal catalyst (J. Fried, J.A. Edwards: organic
Reactions in Steroid Chemistry, Van Nostrand Reinhold Company
1972, page 134, and HØ House: Modern Synthetic Reactions
1972, page 19). Suitable deactivated noble metal catalysts
are, for example, 10 % palladium on barium sulphate in the
presence of an amine or 5 % palladium on calcium carbonate
with the addition of lead(II) acetate. The hydrogenation may
be discontinued after the absorption of one equivalent of
hydrogen.
A compound with E-configured double bond in the
hydroxypropenyl group may be formed, for example, by reducing
the acetylenic triple bond in a manner known ~ se. A whole
series of methods for converting alkynes into trans-olefins
are described in the literature, for example reduction with
sodium in liquid ammonia (J. Am. Chem. Soc. 63 (1941) 216),
with sodium amide in liquid ammonia (J. Chem. Soc. 1955,
3558), with lithium in low-molecular weight amines (J. Am.
Chem. Soc. 77 (1955) 3378), with boranes (J. Am. Chem. Soc.
93 (1971) 3395 and 94 (1971) 6560), with diisobutylaluminium
hydride and methyllithium (J. Am. Chem. Soc. 89 (1967) 5085)
and especially with lithium aluminium hydride/alcoholate (J.
Am. Chem. Soc. 89 (1967) 4245). A further possibility is the
reduction of the triple bond with chromium(II) sulphate in
the presence of water or dimethylformamide in weakly acidic
medium (J. Am. Chem. Soc. 86 (1964) 4358) and generally
1 307784
- 16 -
reduction by the action of transition metal compounds with a
change in the oxidation stage.
If an end product in which CR3R4 represents
f ~ \
is desired, then a 17-(3-hydroxypropyl)-17-hydroxy compound
may, for example, be oxidised in a manner known E~ se. The
oxidation conditions generally depend on the nature of the
substituent Rl in formula I. If Rl represents, for example,
a dialkylamino group, then chromic acid reagents are
generally unsuitable for oxidation since they attack
primarily the dialkylamino group. In such cases an oxidation
agent such, for example, as silver carbonate/Celite (a trade
mark) (Fetizon reagent; M. Fetizon and M. Golfier, Compt.
rend. 267 (1968) 900) or platinum/oxygen (H. Muxfeldt et al.,
Angewandte Chemie, Int. Ed. 1 (1962) 157) may be used. If,
on the other hand, Rl represents an alkoxy radical, ~t is
also possible to use an oxidising agent such, for example, as
Jones reagent, chromic acid-pyridine, pyridinium dichromate
or pyridinium chlorochromate.
A 17-cyanomethyl side chain may be introduced, for
example, in a manner known per se from the 17-ketone of the
general formula III, for example via the 17-spiroepoxide and
by cleaving the spiroepoxide with HCN according to Z. Chem.
8 (1978) 259 - 260.
The introduction of a 17-hydroxyacetyl side chain may
also be carried out according to methods known ~er se, for
_.
1 3077~4
example according to the method described in J. Org. Chem. 47
(1982), 2993 - 2995.
If desired, a free hydroxy group represented by R4 in
the 17-position may be esterified or etherified, for example,
in a manner known per se. Similarly, a free hydroxy or
mercapto group in the radical represented by R3 may, if
desired, be etherified or esterified.
Splitting off of water, with the formation of the 4(5)-
double bond, and simultaneous cleavage of the ketal group inthe 3-position (and removal of any other protecting groups
present that can be split off with acid) may be effected with
acid or an acidic ion exchanger.
The acid treatment may be carried out in a manner known
~er se. For example, the compound of the general formula IV
which contains a 3-ketal group and a 5~-hydroxy group (and,
in some cases, an optionally O-protected 17-hydroxy group
and/or hydroxy-substituted 17-aliphatic hydrocarbon radical)
is dissolved in a water-miscible solvent, e.g. aqueous
methanol, ethanol or acetone, in the presence of a catalytic
quantity of a mineral or organic, e.g. sulphonic, acid, e.g.
hydrochloric acid, sulphuric acid, phosphoric acid,
perchloric acid, p-toluenesulphonic acid or acetic acid,
until water has been split off and protecting group(s) have
been removed. The reaction, which generally proceeds at a
temperature of from O to lOO~C, may also be carried out with
an acidic ion exchanger. The course of the reaction may be
followed by analytical methods, for example by thin layer
chromatography of samples taken.
The 13~-alkylgonanes of the general formula I and their
N-oxides and physiologically tolerable salts may be used in
X
,
1 3077~4
- 18 -
the form of pharmaceutical preparations. The preparations
may be prepared, for example, according to methods of
galenical pharmacy known ~er se by mixing with organic or
inorganic inert carrier material suitable for enteral,
percutaneous or parenteral administration.
In the case of human beings, the dosage of the active
ingredients according to the invention may be, for example,
from 10 to 1000 mg per day.
Thus, the present invention provides a pharmaceutical
preparation which comprises a compound of the general formula
I or an N-oxide or physiologically tolerable acid addition
salt of such compound in which Rl represents a group of the
general formula
RI
--N/
~ II
in admixture or conjunction with a pharmaceutically suitable
carrier. Preferably, the pharmaceutical preparation is in
dosage unit form containing, for example, 10 to 100 mg of
active ingredient per dosage unit.
The following Examples illustrate the invention.
X
1 307784
-- 19 --
Examples
A. Preparat_on of compounds of the invention
Example 1
a) A solution of 2.0 g of 11~-(4-dimethylaminophenyl)-3,3-
(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-9(10)-oestr-en-17-
one (m.p. 143-145-C) in 300 ml of absolute tetrahydrofuran
(THF) is irradiated for 16 minutes at 25C with a Hg-high
pressure lamp (Philips HPK 125, immersion lamp, quartz glass
reactor). The solvent is then distilled off in vacuo and the
residue is chromatographed over aluminium oxide (Merck,
neutral, stage III) with hexane/ethyl acetate. 1.46 g of
11~-(4-dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-
dioxy)-5~-hydroxy-13~-methyl-9(10)-gonen-17-one are obtained
in the form of a colourless oil.
b) At 5CC, absolute THF (248 ml) is saturated with
acetylene by introducing the latter over a period of 30
minutes. 51 ml of a 15 % solution of n-butyllithium in
hexane are then slowly added dropwise and the whole is
stirred for a further 15 minutes while cooling with ice-
water. A solution of 2.7 g of 11~-(4-dimethylaminophenyl)-
3,3-(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-13~-methyl-9-
gonen-17-one in 40 ml of absolute THF is then added dropwise
over a period of 15 minutes to the suspension of the lithium
acetylide and stirring is carried out for a further 2 hours
at room temperature. For working up, the whole is poured
into ice-water and extracted with ethyl acetate. The
resulting crude product (2.85 g) is used in the following
stage without being further purified.
- 1 307784
- 20 -
c) 2.8 g of the crude product obtained under b) are
suspended in 29 ml of 70 % aqueous acetic acid and stirred
for 3 hours at 50C. After cooling, the suspension is
diluted with approximately 100 ml of water and adjusted to a
pH of 10.5 by adding concentrated aqueous NH3 solution.
After extracting with ethyl acetate, an oily mixture of
isomers is obtained that is separated by column
chromatography over silica gel with hexane/ethyl acet~te.
There are obtained in the order of elution: -
1. 530 mg of 11~-(4-dimethylaminophenyl)-17~-ethynyl-17~-
hydroxy-13~-methyl-4,9-gonadien-3-one having a melting
point of 120 - 123C (ethyl acetate/diisopropyl ether)
and
2. 1.33 g of 11~-(4-dimethylaminophenyl)-17~-ethynyl-17~-
hydroxy-13~-methyl-4,9-gonadien-3-one having a melting
point of 201 - 204C (ethyl acetate).
Analogously to b) and c), there are obtained using
methylacetylene instead of acetylene: -
1. 11~-(4-dimethylaminophenyl)-17~-hydroxy-13~-methyl-17~-
propynyl-4,9-gonadien-3-one in the form of an oil.
2. 11~-(4-dimethylaminophenyl)-17~-hydroxy-13~-methyl-17~-
propynyl-4,9-gonadien-3-one in the form of an oil.
d) After the addition of 0.87 ml of concentrated sulphuric
acid, a suspension of 1.02 of mercury oxide (HgO, red) in 20
ml of water is stirred for 30 minutes at 60C. 9 ml of this
mercury salt solution are added to a solution of 3.25 g of
11~-(4-dimethylaminophenyl)-17~-ethynyl-17~-hydroxy-13~-
methyl-4,9-gonadien-3-one in 32 ml of glacial acetic acid.
_~ , . .
1 3a7784
- 21 -
The whole is then stirred for 2 hours at 60 C. For working
up, the cooled reaction solution is poured into ice-water,
adjusted to a pH of 10.5 by adding concentrated aqueous NH3
solutiGn and extracted with ethyl acetate. The resulting
oily crude product is crystallised from methylene
chloride/diisopropyl ether. 2.37 g of 11~-(4-
dimethylaminophenyl)-17~-hydroxy-13~-methyl-18,19-dinor-4,9-
pregnadiene-3,20-dione having a melting point of 224 --225 C
are obtained.
e) A suspension of 2.3 g of 11~-(4-dimethylaminophenyl)-
17~-hydroxy-13~-methyl-18,19-dinor-4,9-pregnadiene-3,20-dione
in 58 ml of toluene is stirred for 20 hours at 25-C after the
addition of 11.6 ml of acetic anhydride and 5.8 g of 4-
dimethylaminopyridine. The suspension is then poured into
saturated NaHC03 solution and extracted with ethyl acetate.
The crude product is chromatographed over 200 g of silica gel
with hexane/ethyl acetate. After crystallising the main
fraction from hexane/ethyl acetate, 1.71 g of 17~-acetoxy-
11~-(4-dimethylaminophenyl)-13~-methyl-18,19-dinor-4,9-
pregnadiene-3,20-dione having a melting point of 194-- 195-C
are obtained.
Example ?
a) A solution of 1.8 g of 11~-(4-diethylaminophenyl)-3,3-
(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-9-oestren-17-one
(m.p. 223 - 226C) in 300 ml of THF are irradiated for 26
minutes under the conditions of Example 1 a). After
chromatography of the crude product, 1.58 g of 11~-(4-
diethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-5~-
hydroxy-13~-methyl-9-gonen-17-one are obtained in the form of
a colourless oil.
____ ~
1 3077~
b) At 0C, the organo-lithium compound is prepared from
3.94 g of 3-tetrahydropyran-2'-yloxy-1-propyne in 85 ml of
absolute THF and 23.1 of a 15 % solution of n-butyl-lithium
in hexane. A solution of 3.53 g of the product described
under 2 a) in 71 ml of absolute THF is then added dropwise
and the whole is stirred for 4 hours at room temperature.
The reaction solution is afterwards poured into ice-water and
extracted with ethyl acetate. The crude product (3.85 g) of
11~-(4-diethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-
dioxy)-13Q-methyl-17~-[3-(tetrahydropyran-2-yloxy)-1-
propynyl]-9-gonene-5~,17~-diol and 11~-(4-
diethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-13~-
methyl-17~-[3-(tetrahydropyran-2-yloxy)-1-propynyl]-9-gonene-
5~,17~-diol is used for hydrogenation without being further
purified.
c) After the addition of 400 mg of 10 ~ palladium/carbon,
3.85 g of the crude product obtained under 2 b) are
hydrogenated in 95 ml of ethanol at room temperature and
normal pressure. After 191 ml of hydrogen have been taken
up, the catalyst is filtered off and concentration is carried
out.
d) The crude hydrogenation product (3.85 g) obtained under
2 c) is stirred in 30 ml of 70 % acetic acid for 2 hours at
60-C. After cooling, working-up is carried out as under 1 c)
and the resulting mixture of isomers is chromatographed.
There are obtained in the order of elution:
1. 410 mg of 11~-(4-diethylaminophenyl)-17~-(3-hydroxy-
propyl)-17~-hydroxy-13~-methyl-4,9-gonadien-3-one in the
form of a yellowish oil.
W (methanol): ~266 = 19080,~309 = 19110.
~.
_. _ .......
1 307784
- 23 -
2. 1.39 g of 11~-(4-diethylaminophenyl)-17~-(3-
hydroxypropyl)-17~-hydroxy-13~-methyl-4,9-gonadien-3-one
in the form of a solid foam.
Analogously to b) to d), there are obtained when using
11~-(4-dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-
dioxy)-5~-hydroxy-13~-methyl-9-gonen-17-one as starting
material: -
1.) 11~-(4-dimethylaminophenyl)-17~-hydroxy-17~-(3-
hydroxypropyl)-13~-methyl-4,9-gonadien-3-one in the form
of an oil.
2.) 11~-(4-dimethylaminophenyl)-17~-hydroxy-17~-(3-
hydroxypropyl)-13~-methyl-4,9-gonadien-3-one in the form
of an oil.
15 Example 3
a) A solution of 1.75 g of 3,3-(2,2-dimethylpropane-1,3-
dioxy)-5~-hydroxy~ -(4-methoxyphenyl)-9-oestren-17-one in
290 ml of dioxan is irradiated for 19 minutes under the
conditions of Example 1 a). After chromatography, 1.45 g of
3,3-(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-11~-(4-
methoxyphenyl)-13~-methyl-9-gonen-17-one are obtained in the
form of a colourless oil.
b) A solution of 8.2 g of 3,3-(2,2-dimethylpropane-1,3-
dioxy)-5~-hydroxy-11~-(4-methoxyphenyl)-13~-methyl-9-gonen-
17-one in 130 ml THF is added dropwise to a suspension of
lithium acetylide prepared from a saturated solution of
acetylene in 450 ml of THF and 130 ml of a 15 ~ solution of
n-butyllithium in hexane. Sitrring is then carried out for 3
hours at room temperature and the reaction solution is
1 307784
- 24 -
afterwards poured into approximately 3 1 of ice-water and
extracted with ethyl acetate. The crude product is
chromatographed over aluminium oxide with hexane/ethyl
acetate. There are obtained in the order of elution:
1. 1.8 g of 3,3-(2,2-dimethylpropane-1,3-dioxy)-17~-
ethynyl-ll~-(4-methoxyphenyl)-13~-methyl-9-gonen-5~,17~-
diol in the form of a colourless oil.
2. 5.1 g of 3,3-(2,2-dimethylpropane-1,3-dioxy)-17~-
ethynyl-11~-(4-methoxyphenyl)-13~-methyl-9-gonene-
5~,17~-diol in the form of a solid foam.
c) A solution of 3.28 g of 3,3-(2,2-dimethylpropane-1,3-
dioxy)-17~-ethynyl~ -(4-methoxyphenyl)-13~-methyl-9-gonene-
5a,17~-diol in 33 ml of 70 % aqueous acetic acid is stirred
for 30 minutes at 60C. After cooling, the solution is
poured into ice-water and extracted with methylene chloride
and the MeC12 extracts are washed with saturated NaHC03
solution and concentrated. Crystallisation of the crude
product from ethyl acetate yields 2.0 g of 17~-ethynyl 17~-
hydroxy-11~-(4-methoxyphenyl)-13~-methyl-4,9-gonadien-3-one
having a melting point of 186 - 187C.
ExamDle 4
a) 20 minutes' irradiation of 1.84 g of 11~-(4-
dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-5~-
hydroxy-18-methyl-9-oestren-17-one in 280 ml of THF under the
conditions of Example 1 a) results in 1.36 g of lI~-(4-
dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-13~-
ethyl-5~-hydroxy-9-gonen-17-one in the form of a foam.
'`C 7
1 307784
- 25 -
b) 6.1 g of 11~-(4-dimethylaminophenyl)-3,3-(2,2-
dimethylpropane-1,3-dioxy)-13a-ethyl-5a-hydroxy-9-gonen-17-
one are reacted under the conditions of Example 1 b) with
lithium acetylide and the resulting crude product is cleaved
with acetic acid under the conditions of Example 1 c). After
chromatography and crystallisation from ethyl
acetate/diisopropyl ether, 3.2 g of 11~-(4-
dimethylaminophenyl)-17~-ethynyl-13a-ethyl-17a-hydroxy--4,9-
gonadien-3-one having a melting point of 197 - 198C are
obtained.
[] D5 + 450 4O (CHC13, c = 0.505).
c) By hydration, catalysed with mercury salt, analogous to
Example 1 d) and subsequent acetylation analogous to Example
1 e), there are obtained from 1.3 g of 11~-(4-
dimethylaminophenyl)-17~-ethynyl-13a-ethyl-17a-hydroxy-4,9-
gonadien-3-one, after chromatographic purification, 720 mg of
17a-acetoxy-11~-(4-dimethylaminophenyl)-13a-ethyl-18,19-
dinor-4,9-pregnadiene-3,20-dione in the form of a solid foam.
ta] 25 ~ 290.8 (CHC13, c = 0.515).
1 307784
- 26 -
Example 5
a) By reacting 7.3 g of 11~-(4-dimethylaminophenyl)-3,3-
(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-13~-methyl-9-
gonen-17-one with 10.7 g of 3-tetrahydropyran-2'-yloxy-1-
propyne under the conditions of Example 2 b) there are
obtained, after chromatography of the crude product over
aluminium oxide with hexane/ethyl acetate, 4.83 g of 11~-(4-
dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-13~-
methyl-17~-[3-(tetrahydropyran-2-yloxy)-1-propynyl]-9-gonene-
5~,17~-diol in the form of a yellowish foam.
b) After the addition of 210 mg of palladium on barium
sulphate (10 %), a solution of 2.2 g of the adduct obtained
under a) in 67 ml of ethanol and 0.56 ml of triethylamine is
hydrogenated at room temperature and normal pressure. After
83.5 ml of hydrogen have been taken up, the catalyst is
filtered off and concentrated. The resulting crude
hydrogenation product is cleaved with 14 ml of 70 % acetic
acid under the conditions of Example 1 c). After
crystallisation from ethyl acetate/diisopropyl ether, 1.1 g
of 11~-(4-dimethylaminophenyl)-17~-hydroxy-17~-(3-hydroxy-
l(Z)-propenyl)-13~-methyl-4,9-gonadien-3-one having a melting
point of 133 - 135C are obtained.
X~
~~. .
1 307784
- 27 -
Example 6
Manufacture of 11~-(4-dimethylaminophenyl)-17~-ethynyl-
16~-ethYl-17~-hydroxy-13~-methyl-4.9-gonadien-3-one
a) A suspension of 29.3 g of 3,3-(2,2-dimethylpropane-1,3-
dioxy)-5(10),9(11)-oestradien-17-one and 28.6 g of bis-
dimethylamino-tert.-butoxymethane is stirred under argon for
60 minutes at 160C. After cooling, the crude product is
triturated with approximately 50 ml of ethyl acetate,
filtered, and the filtration residue is recrystallised from
ethyl acetate. In this manner, 27.6 g of 16-
dimethylaminomethylene-3,3-(2,2-dimethylpropane-1,3-dioxy~-
5(10),9(11)-oestradien-17-one having a melting point of 208 -
211C are obtained.
b) 85 ml of a 5 % solution of methyllithium in diethyl
ether are added dropwise while cooling with ice-water to a
solution of 14.4 g of 16-dimethylaminomethylene-3,3-(2,2-
dimethylpropane-1,3-dioxy)-5(10),9(11)-oestradien-17-one in
220 ml of toluene. When the addition is complete, the whole
is stirred for 15 minutes at +5 to +10C, excess reagent is
decomposed by the careful addition of approximately 20 ml of
water and the reaction solution is then poured into
approximately 3 1 of ice-water and extracted with methylene
chloride. The crude product is chromatographed over neutral
X
_.. , ,. _
1 307784
~ 28 -
aluminium oxide with hexane/ethyl acetate. After
crystallisation of the main fraction from ethyl acetate, 13.0
g of 3,3-(2,2-dimethylpropane-1,3-dioxy)-16(E)-ethylidene-
5(10),9(11)-oestradien-17-one having a melting point of 121 -
123C are obtained.
c) 4.3 ml of 30 % hydrogen peroxide are added dropwise
while cooling with ice-water to a solution of 9.4 g of-3,3-
(2,2-dimethylpropane-1,3-dioxy)-16(E)-ethylidene-5(10),9(11)-
oestradien-17-one in 43 ml of methylene chloride, 0.34 ml of
hexachloroacetone and 0.01 ml of pyridine and then the whole
is stirred for 16 hours at 25C. For working up, the
reaction solution is diluted with approximately 100 ml of
methylene chloride and washed in succession with 5 % Na2S203
solution and water; the methylene chloride phase is dried
over Na2S04 and concentrated. The resulting 5,10-epoxide
mixture is chromatographed over A1203, neutral, stage III,
with hexane/ethyl acetate. 4.7 g of 3,3-(2,2-dimethylpropane-
1,3-dioxy)-5~,10~-epoxy-16(E)-ethyliden-9(11)-oestren-17-one
having a melting point of 139 - 141C (ethyl
acetate/diisopropyl ether) are obtained.
1 307784
- 29 -
d) After the addition of 930 mg of palladium/carbon (10 %),
a solution of 8.2 g of 3,3-(2,2-dimethylpropane-1,3-dioxy)-
5~,10~-epoxy-16(E)-ethyliden-9(11)-oestren-17-one in 400 ml
of ethanol is hydrogenated at room temperature and normal
pressure. After 510 ml of hydrogen have been taken up, the
catalyst is filtered off and concentration is carried out in
vacuo. 7.7 g of 3,3-(2,2-dimethylpropane-1,3-dioxy)-5~,1Oa-
epoxy-16~-ethyl-9(11)-oestren-17-one are obtained in the form
of a colourless oil.
e) An organo-magnesium compound is prepared from 1.4 g of
magnesium, 0.05 ml of methyl iodide and 17.9 g of 4-bromo-
N,N-dimethylaniline in 150 ml of absolute THF. After the
addition of 344 g of CuCl, the whole is stirred for 15
minutes at 0C and a solution of 7.7 g of the product
obtained under d) in 70 ml of absolute THF is then added
dropwise. Afterwards, the whole is stirred for 3.5 hours at
room temperature. For working up, the reaction solution is
poured into a mixture of ice-water and NH3 solution and
extracted with ethyl acetate. After chromatography of the
crude product over aluminium oxide with hexane/ethyl~~acetate
and crystallisation of the main fraction from diisopropyl
ether/ethyl acetate, 6.5 g of 11~-(4-dimethylaminophenyl)-
3,3-(2,2-dimethylpropane-1,3-dioxy)-16~-ethyl-5~-hydroxy-
9(10)-oestren-17-one having a melting point of 180 - 181C
are obtained.
X
. ..... ~. .
1 307784
- 30 -
f) A solution of 4.0 g of the product obtained under e) in
600 ml of dioxan is irradiated under the conditions of
Example 1 a). After crystallisation of the crude irradiation
product from diisopropyl ether, 1.74 g of 11~-(4-
dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-16~-
ethyl-5~-hydroxy-13~-methyl-9(10)-gonen-17-one having a
melting point of 192 - 194C are obtained.
g) 1.4 g of the product obtained under f) are reacted with
lithium acetylide under the conditions of Example 1 b).
After crystallisation of the crude product from ethyl
acetate/diisopropyl ether, 960 mg of 11~-~4-
dimethylaminophenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-17~-
ethynyl-16~-ethyl-13~-methyl-9(10)-gonene-5~,17~-diol having
a melting point of 132 - 134~C are obtained.
h) 760 mg of the product obtained under g) are reacted with
8 ml of 70 % acetic acid under the conditions of Example 1
c). Crystallisation of the crude product from hexane/diethyl
ether yields 460 mg of 11~-(4-dimethylaminophenyl)-17~-
ethynyl-16~-ethyl-17-hydroxy-13~-methyl-4,9-gonadie~-3-one
having a melting point of 195 - 197C.
..w~.~.....
1 307784
Example 7
Manufacture of 17~-cyanomethyl-11~-(4-dimethylaminophenyl)-
17~-hydroxy-13~-methyl-4~9-aonadien-3-one
a) 2.63 g of potassium tert.-butoxide are added in
portions, while cooling with ice-water, to a suspension of
5.0 g of 11~-(4-dimethylaminophenyl)-3,3-(2,2-
dimethylpropane-1,3-dioxy)-5~-hydroxy-13~-methyl-9(10)-gonen-
17-one and 4.74 of trimethylsulphonium iodide in 60 ml of
dimethylformamide. The whole is stirred for 4 hours at 25C,
then poured into ice-water and extracted with ethyl acetate.
After removing the solvent, the crude product, which is at
first oily, is crystallised from ethyl acetate/diisopropyl
ether and, in this manner, 4.2 g of 11~-(4-dimethylamino-
phenyl)-3,3-(2,2-dimethylpropane-1,3-dioxy)-5~-hydroxy-13~-
methyl-9(10)-gonene-17~-spiro-1',2'-oxirane having a melting
point of 23~ - 236C are obtained.
X
_ ...~... ...
1 307784
b) 2.0 g of the spiro-oxirane obtained under a) are
dissolved in 84 ml of ethanol and, after the addition of 4.6
g of potassium cyanide, the whole is heated under reflux for
4 hours. The cooled solution is poured into saturated NaHC03
solution and extracted with ethyl acetate. ~he crude product
obtained after concentration is taken up in 26 ml of 70 %
acetic acid without being further purified and stirred for 60
minutes at 60C. After cooling, the whole is poured into
ice-water, adjusted to a pH of 10.5 by adding NH3 solution
and extracted with methylene chloride. After chromatography
of the crude product over silica gel with hexane/acetone and
crystallisation of the main fraction from ethanol, 1.4 g of
17~-cyanomethyl-11~-(4-dimethylaminophenyl)-17~-hydroxy-13~-
lS methyl-4,9-gonadien-3-one having a melting point of 135 -
137C are obtained.
~'
1 307784
B. Tec,ts on antiaestaqenic actior
In order to identi~y the antigestacenic action of
the compounds according to tne lnventlor., the abortive
action was investigated at an early stage post
nidationem (Experiment I) and at an advanced stage post
nidationem (Experiment II).
The experiments were carried out on female rats
weighing approximately 200 g. After mating had taken
place, the commencement of pregnancy was ascertained by
the detection of spermatozoa in vaginal smears. The
day on which sperm was detected is desianated day 1 of
gravidity (= d 1 p.c.).
The following were investigated for antigestagenic
action:
A: 11~-(4-dimethylaminophenyl)-17~-hydroxy-17a-pro-
pynyl-4,9-oestradien-3-one (reference substance).
B: 11~-(4-dimethylaminophenyl)-17~-hydroxy-17~-(3-
hydroxypropyl)-13~-methyl-4,9-gonadien-3-one
(compound of the invention).
C: 11~-(4-dimethylaminophenyl)-17~-hydroxy-17~-(3-
hydroxypropyl)-13~-methyl-4,9-gonadien-3-one
(compound of the invention).
The test substances were dissolved in a mixture of
benzyl benzoate and castor oil (ratio 1:4). The volume
of vehicle per individual dose was 0.2 ml. The
treatment was carried out subcutaneously (s.c.).
1 3077~4
- 34 -
The treatment of the animals with the particular
substance to be tested or with the solvent as control was
carried out after the nidation of the blastocysts from day 5
p.c. to day 7 p.c. (Experiment I) and day 13 p.c. to day 15
p.c. (Experiment II). On day g p.c. and day 17 p.c.,
respectively, the animals were killed and the uteri were
examined for implants and absorption sites. Photographs were
taken of all the uteri. The lack of implants was assessed as
abortion.
The results are given in Tables 1 and 2 below.
Compounds B and C of the general formula I of the
invention had a completely abortive action in rats at an
early stage of pregnancy in doses ~ 1.0 mg/day (abortion
rate: 4/4). In contrast, the reference substance A
exhibited maximum abortion-inducing (antigestagenic) action
only at doses of ~ 3.0 mg/day (Table l).
At an advanced stage of pregnancy (day 13 to 15 p.c.),
the percentage of complete abortions with s.c. admin-istration
for 3 days of 3.0 mg/day was 35.4 % for B, 52.3 % for C and
3.5 % for the comparison substance A (Table 2).
X
- 35 - 13077~4
T2~1e 1 (E~eriment I)
.~ortive action at an earlv staae of ~reqnancy in rats
.
Treatment from d 5 p.c. to d 7 p.c., autopsy d 9 p.c.
compound dose rate of 2bortion ~'
mg/animal/day s.c. n abortion/n total (%)
30.0 4/4 (100)
10. 0 -- _
3.0 4/4 (100)
A 1.0 2/4 ( 50)
0.3 0/4 ( 0)
O. 1 0/~ ( O)
10.0 4/4 (100)
3.0 4/4 (100)
B 1.0 4/4 (100)
0.3 0/4 ~ )
o.~ 0/4 ( 0)
I
. 10.0 4/4 (100)
3.0 4/4 (100)
C 1.0 4/4 (100)
0.3 0/4 ( 0)
0.1 0/4 ~ 0)
solvent as control: - 0/4 ~ 0)
0.2 ml benzyl benzoate
+ castor oil ~1 : 4)
n = 4 rats/group
1 307784
- 3
Table 2 (E~:peri~ent II)
bortive action at an advanced staae of pregnancy in rats
~I`reat~ nt with 3.0 mq/d s.c. antigestagen (AG) from
d 13 p.c. to d 15 p.c., autopsy d 17 p.c.
complete abortions
= empty nidation sites
1G0
~ AG
I ( ) nu~ber of rats
_ . 52.3
~0 ! 35.4 ~ ~6
0 % 3.5 %
control A ~ C ~