Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1310ql8
GAMMA IRRADIATION OF COLLAGEN/MI~ERAL MIXTURES
Technical Field
The invention relates to preparation of
implants and prostheses for hard tissue repair composed
of collagen and a mineral. In particular, mixtures of
ateloeeptide fibrillar reconstituted collagen are mixed
with a calcium phosphate mineral and the mixtures are
treated with ~ irradiation to improve both biological
and handling properties.
Backaround Art
A wide range of material6 has been proposed for
use in cepairing hard tissues. For weight-bearing
areas, prostheses which are capable of withstanding
stress have ranged from metal rods to reconGtituted
animal bone. Various packing materials have also been
used for augmentation of bony structures, such as the
use of cross-linked collagen for alveolar ridge
augmentation. It is desirable to have available a
variety of materials suitable for the various types of
skeletal repair, as each application has its unique set
of parameters to determine the optimum implant. In
addition, the physical handling properties of the,
material as it is manipulated by the medical
practitioner is significant in permitting the
practitioner to achieve a successful result, in part
because the ease of manipulation determines the ability
to succeed.
Attempts have been made to compose suitable
materials of the chief organic and inorganic components
of bone, namely, collagen and calcium phosphate
mineral. Reports of attempts to use the
collagen/mineral combination are numerous. For example,
l~Uql~
Lemons, J., et al., reported at the Second World
Congres~ of Biomaterials in Washington, D.C., 27 April-l
May 1984, on attempts to utilize collagen along with
commercial hydroxyapatite and calcium phosphate to
repair artificially created lesions in rabbits. The use
of these mixtures did not result in reunion of the
lesions. A control experiment using fresh autogenous
bone, however, was successful in producing a union. -~
Similarly, Levy, P., et al, J Periodontal (1981),
50:303-306, were unsuccessful in their attempts to
utilize collagen/mineral gel implants to repair
intra-bony defects in root canals of canine or monkey
teeth. Gross, B.C., et al., Oral Surq (1980), 49:21-26,
reported limited success in using mixtuces of
reconstituted lyophilized calfskin collagen in admixture
with a hydroxyapatite preparation to induce bone growth
through subperiosteal implants in monkeys. Various
others have reported use of forms of collagen which
clearly contain telopeptides, a major source of
immunogenicity of collagen, in combinaticn with minerals
in bone repair. See, for example, Hayashi, K. et al.,
Arch OrthoP Traumat Surq (1982) 99:265-269: Battista,
U.S. Patent 4,349,490 (using a hydrated gelatin); Cruz,
Jr., U.S. Patent 3,767,437 (using a calcium-precipitated
form of collagen); and Battista, et al., U.S. Patent
3,443,261 (utilizing, in addition to calcium phosphate,
a "new form" of collagen which contains microcrystals of
aggregated tropocollagen units.
Miyata, et al., U.S. Patent 4,314,380, utilized
a mineral backbone prepared directly by treatment of
animal bone to remove all organic materials, which was
then coated with an atelopeptide collagen. Jaeanese
Application J5B~058041, published 6 April 1983,
discloses a spongy porous calcium phosphate material
13~0918
having pores treated with atelopeptide collagen. The
collagen derives from collagen-in-solution having a
concentration of not more that 2~ by weight. The Japanese
application reports the advance of osteoblasts into the pores
of the material and new bone growth. European patent
application, Publication No. 030583, published 24 June 1981,
discloses use of Collagenfleece* in admixture with
hydroxyapatite in bone repair. This collagen material is a
commercial product, is obtained from animal hide by
proteolytic digestion, and is lyophilized and sterilized by
gamma irradiation. This collagen preparation forms a soft
membrane-like material but does contain telopeptides and is
partially degraded by the processing.
EPO application Publication No. 164,483, published 18
December 1985, discloses a process which is asserted to
provide biocompatibility of a mineral/collagen mixture. In
this mixture, solubilized collagen is cross linked either in
the presence of, or before the addition of, a calcium
phosphate mineral component just to the point wherein it
retains its resorbability and absorptive capacity with
respect to body fluids, rather than permitting the
cross-linking to proceed to completion. U.S. 4,516,276 to
Mittelmeier discloses the combination of a nonfibrillar,
nonreconstituted collagen along with hydroxyapatite.
U.S. patent No. 4,795,467 issued 11 January 1989, and
Canadian patent No. 1,260,391 issued 26 September 1989, both
assigned to the same assignee as the application herein,
disclose novel compositions containing reconstituted
fibrillar atelopeptide collagen in admixture with a calcium
phosphate mineral. Various methods are also disclosed for
strengthening the
(*) Trademark
1310918
--4--
composition, which methods include incubation of the
mixture at specified temperatures and times, and the
treatment of the dried mixture with heat. The
preparation of the referenced application6, in order to
S be non-infective to treated subjects, must be prepared
under aseptic conditions, as there i8 no provision in
the disclosed procedure6 for dicect sterilization.
Typically, asceptic processing resul~s in products with
sterility assurance levels (i.e., probability of a
non-sterile product unit) between 10 and lO
The material which results after the various
curing treatments disclosed in the above-referenced
applications has a compressibility above 6 Newtons per
square centimeter (N/cm ). Both this strength and
further improvement in the compressibility indices are
achievable by the curing processes disclosed therein.
The art offers no suitable composition for bone
defect repair which is readily and efficiently
sterilizable while retaining the efficient handling
properties desired to eermit effective insertion of the
implant. The material should be resistant to
compression, and yet sufficiently resilient to permit
shaping into place, or, alternatively, if to be used in
a weight-bearing area, should be suitably rigid. The
procesg and resulting product of the present invention
remedies this omission in the art.
The invention takes advantage of an irradiation
erocess which has previously been disclosed with regard
to its impact on physical properties only in regard to
preparations containing collagen alone. A summary of
the effect of y-ray irradiation on collagen sutures,
for example, is found in Artandi, Technical Report #149,
Intl Atomic Energy Agency, Vienna, Manual on Radiation
Sterilization of Medical & Bioloaical Material~ (1973)
1310918
chap. 15, and a review of the effect of radiation on
collagen as a tissue component is published by Bailey,
A.J., in Internat Rev Connect Tis (1968), pp.233-281.
In addition, PCT application W081/00963 discloses that
collagen materials can be increased in physical strength
by heat treatment and by subjecting them to treatment
with gaseous hydrogen halide. However, Applicant i~
aware of no disclosure in the art which shows the effect
of y-ray ieradiation on the physical properties and
handling properties of collagen/mineral mixtures,
although y-ray irradiation has been used to Eterilize
the lyophilized preparations disclosed in EP0
publication No. 164,483 (Eupra) without further comment
concerning either properties or further use.
Disclosure of the Invention
The invention provides a process whereby
collagen/mineral preparations can be efficiently
sterilized and simultaneously have conferred upon them
eroperties which are especially favorable for handling
of the material in defect repair, and for their behavior
as implants. The heart of the process is irradiation of
the preparation with sufficient total energy to effect
sterilization to the required level, wherein the ,
collagen/mineral preparation is furnished in such form
that the irradiation also provides a satisfactory
compressibility modulus as well as the resilience and
rigidity combination desired. A range of desired
properties iB available, depending on the adjustment of
the condition oc status with regard to celevant
parameters of the collagen/mineral sample during the
irradiation period.
Accordingly, in one aspect, the invention
relates to a method for conferring desired physical
1310918
--6--
properties and stsrility level6 on a collagen/min2ral
mixture, which proce~6 compri~es irradia~ing the mixture
with a sterilizing amount of y radiation, typically
between 0.5 and 4 Mrad, wherein the mixture compri6e6
about 60-90% of a calcium phospbate mineral and 2-40% of
an atelopeptide fibrillar reconstituted collagen
exclusive of moisture. During the irradiation, it is
important that the collagen portion of the preparation
undergo or have undergone sufficient cros6-linking to
stabilize the physical properties. Thi6 can be achieved
in a variety of way6, for example, by preheating the
sample to effect partial cros6-linking or by adjusting
the humidity under which irradiation occur6 so that the
radiation itself effect6 the desired level of
cro66-linking. Thus, under these conditions, not only
does 6terilization to a 6terility assurance level of at
lea~t as low as 10 take place, but al60 adjustment
of the phy6ical properties occurs by achieving a balance
between cross-linking and degradation due to the
radiation
Another aspect of the invention is to provide
a biocompatible collagen/mineral composition which
comprises a mixture containing 2-40% reconstituted
fibrillar atelopeptide collagen and 60-90% calcium
phosphate mineral by weight exclusive of mois-ture,
which composition has a compressive modulus of at least
10 N/cm and a sterility assurance factor of at least
as low as 10 6,
.~
1310918
-6A-
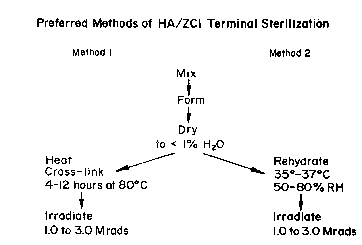
Brief DescriPtion of the Drawina
Pigure 1 ~how~ a diagram of alternative method~
for carrying out the invention.
Figure 2a shows the effect of moisture content
of collagen/mineral mixtures on compres6ible modulus at
various levels of irradiation Figure 2b shows this
effect on trypsin sensitivity.
Figures 3a and 3b show the results of
independent determinations similar to those of Figures
2a and 2b.
V
t 3 1 09 1 8
Modes of carlYinq Out the Invention
The method of the invention is applicable to
collagen/mineral mixtures of defined composition. There
follows first a discussion of the nature of the
individual components and the manner in which they are
formed into mixtures.
The Mineral Component ~~
The composition6 of the invention can use a
variety of calcium phosphate mineral component
materials. As used herein, ~calcium phosphate mineral~
materials refers to those materials compo~ed of Ca
and phosphate ions, regardles6 of the microstructure,
protonation status of the phosphate. or extent of
hydration. Calcium phosphate mineral materials include
a variety of forms, such as the commercially available
forms of tricalcium phosphate, for example,
Synthograft~ tricalcium phosphate, or of
hydroxyapatite such as Periograf~, Alveograf~,
Interpore~, OrthoMatrix~ HA-1000~, or
OrthoMatrix~ HA-500~ hydroxyapatite particulate
preparations. The hydroxyapatite or tricalcium
phosphate may also be prepared by known methods, such as
those disclosed by Termine, et al., Arch Biochem BiophYs
(1970) 140:307-325, or by Hayashi, K. et al., Arch
Orthop Trauma Surq (1982, supra). In any event, the
mineral i6 generally and preferably of nonbiological
origin and is supplied as a powder of appropriate mesh.
Preferred particle sizes are in the range of 100-2000
~. While the mineral content of bone could be
harvested and purified for this purpose, more
economically prepared and controlled compositions are
preferable, both as a matter of cost and of quality. If
1310918
-B-
solid blocks are desired, these are prepared from the
particulate form as described below.
The Collaqen
The collagen component of the composition is
critical to it~ efficiency. The collagen suitable for
use in the invention i8 a purified atelopeptide
fibrillar reconstituted collagen: it is typically
prepared from skin.
Numerous forms of collagen have been prepared
and they differ in their physical properties as well as
in their biocompatibility. Where it is not intended to
seecify the particle size within the range of diameters
over which a mixture will be a solution, colloid, or
suspension, a single generic term, "collagen dispersion"
is used. This term refers to any collagen preparation
in aqueous medium where the collagen particle size is
not specified -- i.e., the preparation may be a
solution, suspension, or gel.
Native collagen consists mainly of a triple
helical structure containing repeating triplet sequences
composed of glycine linked to two additional amino
acids, commonly proline and hydroxyproline. Native
collagen contains regions at each end which do not have
the triplet glycine sequence, and thus do not form
helices. These regions are thought to be responsible
for the immunogenicity associated with most collagen
preparations, and the immunogenicity can be mitigated by
the removal of these regions to produce "atelopeptide~'
collagen. This can be accomplished by digestion with
proteolytic enzymes, such as trypsin and pepsin. The
nonhelical telopeptide regions are also responsible for
natively occurring cross-linking, and atelopeptide
1310918
- 9 -
collagen must be cro6s-linked artificially if
cross-linking is desired.
Naturally occurring collagens have been
subcla66ified into about ten types, depending on the
amino acid sequence in the individual chains, the
carbohydrate content, and the presence or absence of
disulfide cro6s-links. The most common subtypes are
Type I, which is present in skin, tendon, and bone, and -~~
which is made by fibroblasts; and Type III, which is
found primarily in skin. Other types reside in
specialized membranes or cartilage, or at cell
surfaces. Types I and III contain similar numbers of
amino acids in their helices and have a high degree of
homology; however, Type III, but not Type I, contains
two adjacent cysteine6 at the C-terminal ends of the
triple helix, which are capable of forming inter-chain
cross-link6.
Therefore, collagen preparations may differ
from each other by virtue of their initial compositions,
which is a function of their origin, or by virtue of
their modes of preparation. Collagen derived from bone,
for example, contains exclusively Type I collagen; while
collagen derived from skin also contains Type III.
Also, the process of preparation may or may not remove
the telopeptides. Thus both unaltered and
"atelopeptidell collagen are po6sible. Cros6-linking may
be effected deliberately or accidentally. Sterilization
by ~-ray irradiation or by high heat may result in
cross-linking without control of extent or nature and
result6 in partial degradation of the triple helix;
deliberate cros6-linking may be carried out by a variety
of means, including treatment with glutaraldehyde.
Differences arising from perhap6 more 6ubtle cause6 are
perhap6 the result of variations in the details of the
1310918
--10--
preparation procedure. For example, the collagen may be
solubilized and reprecipitated, or may simply be finely
divided and kept in 6uspension. When the solubilized
material is reaggregated, the aggregation may be done in
S ways BO as to form nonspecifically bonded solid6, or the
collagen may be reconstituted into fibers which simulate
the native form. Also, of course, the degree of purity
may vary.
As used herein, "free from impurities" or
"purified~ as regards collagen preparations refers to
those impurities which are normally associated with
collagen in its native state. Thus, collagen prepared
from calfskin is free from impurities when other
components of calfskin have been removed; that from bone
when other components of bone are eliminated.
"Reconstituted" collagen refers to collagen
which has been disassembled into individual triple
helical molecules, with or without their telopeptide
extensions, brought into solution and then regrouped
into "fibrillar" forms. In this form, the fibrils
consist of long, thin collagen molecules staggered
relative to one another by multiples of about one-fourth
their length. Thus results in a banded structure which
can be further aggregated into fibers.
Collagen which is "substantially free from
cross-linking" refers to collagen which has had the
atelopeptides removed, and thus lacks the native
capàcity for cross-link formation. These preparations
remain substantially cross-link free if not deliberately
cross-linked by, for example, being treated with
glutaraldehyde or subjected to treatment which itself
results in cross-linking--for example, treatments often
used for sterilizing purposes, such as high temperature
-11- 131091~
and the y-radiation described herein when conducted
under appropriate conditions.
One collagen preparation which i6 suitable for
the mixtures of the invention is an atelopeptide
collagen which is reconstituted into fibrillar form and
supplied as a dispersion of 5-100 mg/ml, preferably
around 50-70 mg/ml. Such dispersions as Zyderm~
Collagen Implant (ZCI), which is commercially available -~~
in preparations containing 35 mg/ml collagen or 65 mg/ml
collagen in saline, manufactured by Collagen
Corporation, Palo Alto, California, are appropriate.
For use in the compositions of the inventions, the ZCI
or other collagen dispersions are used without lidocaine
or other sedative drugs. As used herein, "ZCI" refers
to the aqueous collagen dispersion, rather than to the
; collagen component per se.
The Collaqen/Mineral Mixtures
The compositions of the invention which are
eventually subjected to irradiation are generally
initially prepared by mixing 50-85% by weight of calcium
phosphate mineral component, preferably 65-75% mineral
component, with the balance as a collagen dispersion in
aqueous medium, such as ZCI. In terms of the
mineral/collagen ratio (excluding the water content of
the collagen dispersion), the mixtures are 60-98%
mineral, preferably 75-98% mineral and the rest
collagen. The composition may be prepared simply by
thoroughly mixing the two components into a cohesive
mass. The mixture can also be cast into a desired shape
(e.g., blocks, square6, sheets). Cross-linking can be
superimposed using, for example, glutaraldehyde to a
level of 0.001-0.1% for either a dry or wet product, as
further described below.
1 3 1 09 1 8
-12-
The mixtures are then dried to less ~han 1%
moisture content and either rehydrated or heat treated
before subjecting them to the sterilizing radiation
procedures of the invention described below. The
percentage compositions of the collagen/minera-l and
moisture content are calculated as follows: percentages
of collagen and mineral are given as dry weights
relative to the total weight of these two components
alone, not including water. Percent moisture i5 the
weight water divided by the total weight (water +
collagen + mineral) times 100.
The sterilized material resulting from the
radiation process may be used as mineral/collagen per se
or may be mixed with additional components, which are
also stecilized, as aepropriate, for administration to
the subject. The preparations, while being described in
terms of collagen and mineral, are always supplied to
the subject in a wetted condition and contain either the
inherent moisture of the original mixture or are
rewetted with sterile water or saline before
administration. In addition, components designed to
increase the efficacy of the compound may be added, such
as blood or bone marrow. As stated above, the
percentages of collagen and mineral reflect their,
relative amounts, and the collagen/mineral mixture can
focm as little as 10% of the total preparation applied
in some instances. Any additives must them6elves also
be sterilized, or be derived from such source that
sterilization is irrelevant, as is the case for blood,
for example.
Desired ProPerties of the Mixture
The collagen/mineral mixture itself, depending
on its application, needs to exhibit certain physical
1310918
-13-
properties. Specifically, it needs to be resilient
enough to permit some shaping, but at the same time must
be sufficiently rigid to resist total disorganization
when stres6ed. Resistance to compression can be
measured as the compressive modulus, using commercially
available equipment, such as Instron Universal Testing
Instrument Model 4202, and according to guidelines for
measurement of compres6ive modulus as published by the
American Society for Testing Materials (ASTM).
To conduct this measurement, the mixtures are
first soaked for 5-24 hours in physiological saline.
This gives more relevant data, as the material will be
wetted when implanted. The soaking is done for a
sufficient time to insure complete wetting; the mixture
is then placed in the test apparatus. If the material
is resilient, it will compres6 easily until a point is
reached wherein, in order further to compres6 the
material, it is necessary to disrupt the inherent
structure at the microscopic level. If the material is
rigid, this point will be reached with les6 deformation
than for resilient material. For collagen/mineral
mixtures, the microscopic organization is maintained
first by the triple helix per se, but also by
interaction between the collagen triple helical po,rtions
of the individual components of the fibrils as well as
binding of the fibrils to each other. Compression
disrupting any of these levels of organization will be
more difficult than general compression which decreases
the volume of empty space. Of course, the more highly
organized and cross-linked the collagen chains in the
composition, the more difficult this microscopic
compression is.
Thus, a high compressive modulus (measured in
Ntcm ) indicates a high level of organization at the
13~0918
-14-
microscopic level, specifically, a high level of
cross-linking. A low compressive modulus indicates that
cross-linking i5 low. For appropriate physical handling
properties and for maintenance of integrity a~ an
implant, it is important that the compressive modulu~ be
reasonably high, at least about lO N/cm or more, and
may be as high as 35-45 N/cm . The upper levels of
compressive modulus are imposed by the nature of the
materials, and it is believed that mixtures of this type
cannot, in fact, attain modulus values of much greater
than 100 N/cm under any degree of cross-linking. In
any event, it is significant in maintaining suitable
physical properties for the compositions of the
invention that the compressive modulus be above 10
N/cm , and a preferred range is 10-60 N/cm , most
preferably 25-45 N/cm . The resultant composition
after the treatment according to the process of the
present invention is assessed by this measure in order
to verify that the appropriate compressive resistance
strength is attained.
While the mixture needs to maintain integrity
at a microscopic level, it must also be sufficiently
porous and vulnerable to have biological properties
which permit ingrowth of surrounding hard tis6ue,,and in
some cases should exhibit resorbability when placed in a
subject. However, this is a property that needs ~o be
optimized rather than maximized. It is reflected as a
modest degree of degradation of the collagen fibrils,
which makes them susceptible to biological processes
when placed in the 6ubject.
One in vitro mea6urement of this chaIacteristic
is su6ceptibility to hydrolysi6 by tryp6in or "trypsin
6ensitivity". To effect this measurement, the samples
are treated with the protease trypsin, which is capable
-15- 1310~18
of attacking only fragmented portions of the collagen
protein. The extent of hydrolysis is measured by
fluorescamine assay for solubilized peptides, and the
results are expres6ed as percentage nonhelical
collagen. For example, and for comparison, gelatin
preparations of collagen are 100% non-helical, collagen
in solution is about 10% non-helical, and ZCl is 10%
non-helical. Desirable ranges depend on the use
intended.
An alternative measure of fragmentation at a
microscopic level i8 the transition temperature as
measured by differential scanning calorimetry (DSC).
lowering of the transition temperature indicates an
increase in fragmentation at a microscopic level in a
manner similar to that measured by tcypsin sensitivity.
The process of the invention permits adjustment
of the foregoing parameters to achieve optimum physical
and biological compatibility properties. The process
also results in efficient sterilization of the material,
assuring sterilization levels at least as low as 10
Method of the Invention
Sterilization and optimization of physical
properties are achieved by subjecting the compositions
to irradiation using a y radiation source in the range
of 0.5-4 Mrad, preferably 1-3 Mrad, and most preferably
2.5-3 Mrad. These dosages are known to effect
sterilization of pceparations containing only collagen
(see Artandi, (suPra)). The irradiation process itself
is carried out uging standard procedures known per se in
the art for sterilization of foodstuffs, cosmetics, and
the like. The irradiation is conducted using a
y-emitting source, such as 131I 137C
commonly, Co. These materials are supplied in
.,..:.. -
1310ql8
-16-
standard forms and applied to samples using standard
equipment by AEC licensees according to establi6hed
guidelines. Reference is m2de to Proces6 Control
Guidelines for Gamma Radiation Sterilization of Medical
Devices published by Assoc. for Advancement of Medical
Instrumentation (1984) as ~AMI Recommended Practice.
Reference is made also to Technical Reports Series 149;
"Manual on Radiation Sterilization of Medical
Biological Materials", Intl Atomic Energy Commission,
Vienna 1973.
The significant factors in the effect of the
radiation on the sample are the total dosage (Mrad) and
the state of the sample while being irradiated. Other
factors, such as the rate at which the energy is
supplied, total radiation time, distance of the sample
from the source, and so forth, are generally irrelevant
except as to their combined effect on total dosage.
The condition of the sample subjected to the
radiation is of the utmost importance, and forms the
basis for the invention herein. The sample must either
be provided with a desired level of cross-linking before
being subjected to the radiation, or must be placed in a
condition ducing the radiation so as to permit the
radiation itself to effect this cross-linking, or,a
combination of these factor6 must be used.
In one preferred method of carrying out the
invention, the mixture is assured to contain a moisture
content of 1-6%, preferably 1-2%, during the application
of the y-radiation. This is most conveniently
achieved by first drying the mixture to a moisture
content of less than 1% by dry heat at 35-45C,
preferably 35-37C, and then rehydrating the mixture by
treating it for 6-24 hours at 35-45C at 50-95% relative
humidity (RH), preferably 35-37C at 50-80% RH, to
1 3 1 09 1 8
achieve the desired equilibrium moisture content. The
moisture content can be measured by standard techniques
such as that described by Fischer, K., Anqew. Chem.
(1935) 48:394 to assure that the desired range i6
achieved. Other protocols to achieve the desired level
of moisture can also be used, and the water content
verified as described. When the mixture has the desired
level of moisture, it is subjected to the radiation
dosage described. Cross-linking to the desired level
then occurs during the irradiation.
In an alternative embodiment, cross-linking is
induced by heating prior to irradiation. In one
preferred protocol, the sample is first dried, to a
moisture content of less than 1%, or preferably 0.5-1%
as above, and then heated for 4-24 hours at about
60-90C, preferably 70-80C at 20-80% relative humidity,
preferably 50-60% relative humidity to effect a desired
level of cross-linking, as measured by the compressive
modulus. Suitable values for the compressive modulus
are 10-45 N/cm . Alternative means to achieve this
level of cross-linking are also available, including
treatment with cross-linking agents, such as
glutaraldehyde or formaldehyde. In any case, the sample
is subjected to these cross-linking treatments un,til a
suitable measure of cross-linking by compressive modulus
is attained. The sample is then subjected to the
radiation.
Thus, in the first embodiment above,
cross-linking is believed to occur during the radiation
process due to the presence of moisture in the sample;
in the second approach, the cross-linking is effected
prior to the radiation treatment and is not greatly
increased during sterilization. However, it is clear
that a combination of the two foregoing treatments can
-18- 1310918
be employed by reducing the degree of cross-linking in
the preradiation treatment and adjusting the moisture
content of the sample during radiation so as to complete
the desired process. The general aspects of the
foregoing preferred procedures are set forth in Figure 1.
For the irradiation step, the compositions,
suitably prepared for radiation treatment as above, are
packaged in materials compatible with y radiation to
preserve the sterilization of the samples contained, and
are then subjected to 0.5-4 Mrad of radiation, according
to standard procedures. The samples as then packaged
are in a form suitable for reconstitution under sterile
conditions and application to ths subject. For such
use, the sample is removed from the package under
sterile conditions and soaked in sterile saline or mixed
with blood or bone marrow, as desired, and used for its
desired purpose.
Use of the Composition
The resulting composition is used to augment
bone and fill bony defects, for example, periodontal
bony pockets, tooth extraction sockets, and jaw cysts.
An important example of onlay procedures includes
alveolar ridge augmentation. The procedures for tpe
surgical implantation are known in the art. For
alveolar ridge augmentation, the composition is inserted
under the periosteum in places where augmentation is
desired. In orthopedic and reconstructive applications,
mineral in the form of porous blocks may also be
indicated, particularly where the graft must bear
stress. Implantation of the collagen-impregnated blocks
is also effected by standard surgical techniques.
1310918
-19- -
Examples
The following example~ are meant to illustrate
the invention, but not intended to limit its scope.
Example 1
Preparation of a Basic ComPosition
A mineral/collagen preparation was obtained by
mixing 65 parts by weight of OLthoMa~rix~ HA-1000
hydroxyapatite with 35 parts by weight of Zyderm~
Collagen Implant (65 mg/ml) without lidocaine. tSince
ZCI is a 6.5% collagen-in-saline preparation, the final
composition is 65 parts HA, Z.3 parts collagen (0.065 x
35) and 32.7 parts (35-2.3) saline, all by weight).
Th~ mixture was thoroughly mixed, and portions
measuring 0;.55 ml were extruded into blocks and dried
under a laminar flow hood for about 48 hr at 36-37C.
The resulting preparation had a moisture content of
0.87%, as measured by the method of Fischer, K., Anqew.
Chem. (1935) 48:394. The composition is thus 0.87%
20 water, 3.37% collagen, and 95.76% mineral, all by weight
as defined above.
Example 2
Effect of Moisture Content
The blocks prepared to according to Example 1
were set into vials for rehumidification. Twenty vials
were incubated at 75% relative humidity, 35C for about
24 hr to obtain blocks with a moisture content measuring
1.86%. Ten of these were further subjected to 95%
30 relative humidity at 36-43C for 15-1/2 hr to obtain a
moisture content of 5.9%.
The dry and rehumidified samples were subjected
to varying levels of total radiation ranging from 0.5 to
3 Mrad. The results of the radiation on the compression
13~0918
-20-
modulus are shown in Figure 2a, and the effect on
trypsin sensitivity is shown in Figure Zb. These
results show that samples containing 1.86% moisture
content were strengthened by the radiation procedure in
terms of compression modulus, while their trypsin
sensitivity was not markedly increased. In contrast,
samples not rehumidified showed considerable
fragmentation during irradiation, and the compressive
strength was not measurably improved. (All samples
showed a modest decrease in the transition temperature
when measured by DSC.)
The foregoing procedure was repeated, this time
rehumidifying the samples to 1.28% and 1.62% moisture
content, and gave comparable results, as shown in
Figures 3a and 3b, respectively. Again, the samples
containing a higher moisture content exhibited less
fragmentation during irradiation, according to the
trypsin sensitivity assay (Figure 3b), but markedly
increased in compression modulus during radiation, as
shown in Figure 3a.
ExamPle 3
Effect of Pretreatment with Heat
The samples prepared as in Example 1 werç
placed in vials and 16 vials stoppered and treated at
80OC at 50-70~ RH for 48 hrs. The effects of radiation
on these heat-treated samples was compared to samples
not heat treated, but containing the original 0.87%
moisture content. The trypsin sensitivity of the
heat-treated samples increased from a value showing 10%
non-helical collagen for unirradiated samples to 60%
nonhelical content for samples irradiated with 3 Mrad,
in contrast to a relatively low fragmentation increase
of 3% nonhelical character to about 25% at 3 Mrad for
1310918
-21-
the samples not heat-treated. The compres6ive 6trength
of the 6ample was measurably increased by the heat
treatment, measuring about 35 N/cm before radiation
and maintaining this level throughout the dosage range.
In a separate experiment, samples containing
0.87~ moisture heated for only 6-1/Z hrs at 80C and
50-70% RH also showed a compressive modulus of 35
N/ 2
Thus, it appears that heat-treated materials
maintain their capacity to resist compression after
radiation, while having increased trypsin sensitivity.
ExamPle 4
Effect of Heat Curina Alone
Samples were prepared as in Example 1, except
that the extruded mixture was incubated for 72 hr at
26-34C at 90-95% relative humidity before drying, as
described above, to obtain a moisture content of
0.48-0.49~. When this preincubated mixture was treated
for varying lengths of time at 80C at 50-70% RH it
showed a consistent increase in compressive modulus,
from 15 N/cm with no heat treatment, to 25 N/cm
after 4 hours at 80C, 30 N/cm after 8 hours, and 40
N/cm after 12 hours. Therefore, heat treatment is
- 25 effective in increasing the compressibility of dried
samples as is the application of radiation; however,
sterilization doefi not necessarily result.