Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 1312800 ~ ~
Description
. ~
The invention relates to a therapeutic system for-applying
active substances to the skin, with a backing layer remote
from the skin, at least one active substance depot, an
active substance distribution device which is linked with
the active substance depot, an active substance delivery
control device controlling the delivery of the active
substance through the system and a contact adhesive fixing
device for the therapeutic system on the skin, its use and
process for the production thereof.
- .
Therapeutic systems for the transdermal administration of
medicaments supply one or more active substances at a
predetermined rate and in continuous manner over a fixed
period to a given application point on the skin. These
systems are therapeutic precision instruments ensuring a
continuous active substance release.
.
Such therapeutic systems can have both a topical and a
systemic action and the large number of active substances
which can be applied in this way and their different che-
mical, physical and pharmalogical characteristics make
ever new demands on the production of such systems.
: . .-. .
: ;~ .::
Conventionally these transdermal systems have at least one
active substance reservoir, where the active substance is
present in solid, li~uid or molecular disperse form and an
adhesion layer through which the system is closely connec-
ted with the skin and through which active substance
transfer takes place, a control membrane and
protective/covering layers which are substantially imper-
meable for the active substance.
~':
l3l2sao
The known systems are difficult to manufacture and have a
complicated structure. One problem of conventional systems
is that of being able to process readily volatile active
substances, because the evaporation of the active sub-
stance is difficult to control during production.
Thermally sensitive active substances can only be used to
a limited extent in the system in the case of matrices or
therapeutic systems which have to be thermally tleated and
which are produced with heat treatment stages.
Attempts have already been made to introduce pure active
substance in fine-grained form into a contact adhesive
polymer, so that the finely divided, fine-grained active
substance dissolves with time as depot crystals in the
adhesive matrix layer (DE-OS 35 00 508). This process is
not suitable for volatile and thermally sensitive active
substances, because it includes thermal treatment stages.
Another attempt to increase the capacity of such therapeu-
tic systems comprises embedding in a contact adhesive
layer of such a system active substance depots in the form
of microcapsules, which are surrounded by a control mem-
brane (cf US patents 3 598 123 and 3 731 683). The produc-
tion of such control membrane-surrounded microcapsules is
extremely complicated and expensive and cannot be perfor-
med for many active substances. The mixing of the active
substance-containing microcapsules under a reservoir mate-
rial constitutes a further difficult process stage, during
with the micro capsules can easily be damaged or
destroyed, which can lead to an unsatisfactory constancy
of the active substance content in the finished therapeu-
tic system. The process of US patent 3 598 123 is diffi-
cult to perform for liquid active substances,
-3- 13128~0
: -,
particularly if the liquid substance is present in readily -
volatile form.
.
German patent 3 424 837 discloses a depot plaster, which
can be used for liquid materials and has a covering film,
a liquid active substance in an outwardly bulging region
of the covering film and a control membrane covering the
active substance and permeable for the latter. Between the
covering film and the control membrane is provided an
active substance distribution device, namely a non-woven
fabric, which unlformly distributes the active substance
liquid on the control membrane and which is effective over
a large surface area. In the case of the depot plaster of
German patent 3 424 837 the covering film and the control
membrane are welded together in their outer regions in
order to prevent an out flow of the liquid active substan~
ce.
..-"~'':
However, the known depot plaster is disadvantageous in
that the liquid therein flows freely and can easily run
out if the adhesive or welded edges are damaged and also
requires an expensive control membrane, which must be
provided in addition.to the active substance distribution
device in order to kinetically control the delivery of the
active substance.
The problem of the present invention is consequently to
provide a novel therapeutic system with active substance
depot for the administration of the active substance,
which can be manufactured less expensively and more relia-
bly than the prior art systems and which is also suitabl.e
for processing volatile and/or thermally unstable compo-
nents.
- --, . . .. " ., , , , . , . .. ,, . ` . , ~ ", ~ . . .. . . . . . . . .
` 13128~
According to the invention this problem is solved by a
therapeutic system, which is characterized in that the
active substance distribution device and the active
substance delivery control device is a reservoir matrix
having one or more discrete active substance depots
arranged in spatially defined manner with respect to one
another and having a higher active substance concentration
than in the reservoir matrix. During the production of the
therapeutic system, the reservoir matrix can be free from
active substances and is only enriched therewith over a
period of time, i.e. during the storage of the system or,
in the case of highly volatile substances, during the
production of the system. Thus, it is an advantage of the
invention that now active substances, which are thermally
unstable and/or volatile can be introduced during
manufacture into transdermal systems in the form of a depot
and without any thermal stressing. There is no need for
stages, such as the mixing of the reservoir matrix material
with the active substance and instead said material becomes
saturated with the active substance at room temperature
during the storage of the therapeutic system. Production
is simplified due to the omission of the production stages
for the active substance-saturated matrix.
Accordingly, one embodiment of the instant
invention provides a therapeutic system for applying active
substances to the skin, comprising a backing layer remote
from the skin, an active substance depot containing a
liquid active substance or a liquid formulation of active
substance, a matrix controlling the active substance
release, and a pressure sensitive adhesive arrangement for
fixing the therapeutic system to the skin, wherein the
active substance depot has at least one auxiliary substance
with a supporting and distributing function in the form of
a planar textile material and is surrounded on all sides by
the matrix.
..... .
.. ': ' '':
. : .
; 13~2800
4a -
Due to the fact that here a reservoir matrix with .
its own control function is used, which is inter alia
determined by the migration speed of the material through ~;
5 the matrix, there is not need to provide a control :~
membrane, which requires additional process stages and
membrane material during protection. The depot can consist .
of pure active substance, which can be solid or fluid, but -
also inert adjuvants. The term "inert" is here understood : :
10 to mean that active substance and adjuvant do not react - : ~-
with one another. An "inert" adjuvant can also be a : :
substance ha- .
C ' ' ' .
,~ .. .
_5~ 2 8 a~
ving physiological effects, such as e.g. DMSO or the like,
which e.g. increases the permeability of the skin. The
adjuvants can also be constituted by support materials,
which make the active substance depot insensitive with
respect to pressure and tension application, as well as
carriers.
It is possible to use active substances which can be app-
lied in transdermal manner and typical examples of these
are given below.
-
Nicotine
Corticosteroids: hydrocortisone, prednisolone, beclometha-
sone-proprionate, flumethasone, triamcinolone, triamcino-
lone-acetonide, fluocinolon, fluocinolin-acetonide, fluo-
cinolon-acetonide acetate, clobetasol-proprionate, etc.
Analgesics, anti-inflammatory agents: acetaminophen, me-
fenamic acid, flufenamic acid, dicyclofenac, dicyclofenac-
sodium-alclofenac,o~yphenbutazone, phenylbutazone, ibupro-
fen, flurbiprofen, salicylic acid, 1-menthol, camphor,
sulindac-tolmetin-sodium, naproxen, fenbufen, etc.
.
Hypnotically active sedatives: Phenobarbital, amobarbital, --
cyclobarbital, triazolam, nitrazepam, lorazepam, halope-
ridol, etc.
Tranquilizers: fluphenazine, thioridazine, lorazepam,
flunitrazepam, chloropromazine, etc.
Antihypertensives: pindolol, bufralol, indenolol, nipadi-
pin, lofexidin, nipradinol, bucumolol, etc.
-6- 131280~ ~
Antihypertensively acting diuretics: hydrothiazide, ben-
droflumenthiazide, cyclobenthiazide, etc.
Antibiotics: penicillin, tetracycline, oxytetracycline,
fradiomycin sulphate, erythromycin, chloramphenicol, etc.
Anesthetics: lidocaine, benzocaine, ethylaminobenzoate,
etc.
Antimicrobiological agents: benzalkinium chloride, nitro- -
furazone, nystatin, acetosulfamine, clotrimazole, etc.
Antifungal agents: pentamycin, amphotericin B, pyrrolni-
trin, clotrimazole, etc.
Vitamins: vitamin A, ergocalciferol, chlolecalciferol,
octotiamine, riboflavin butyrate, etc.
Antiepileptics: nitrazepam, meprobamate, clonazepam, etc.
Coronary vasodilators: dipyridamole, erythriol tetranitra-
te, pentaerythritol tetranitrate, propatylnitrate, etc.
Antihistaminics: diphenyl hydromine hydrochloride, chlor-
pheniramine, diphenylimidazole, etc.
Antitussives: dertromethorphan (hydrobromide), terbutaline
(sulphate), ephedrine (hydrochloride), salbutanol (sulpha-
te), isoproterenol (sulphate, hydrochloride), etc. ;
Sexual hormones: progesterone, etc.
Thymoleptics: doxepin, etc.
, . . . . .. . . .. .. . .. . . . . . . . . . .. . . . ~ . .... . . .
13128~0
Further medicaments/pharmaceuticals: 5-fluorouracil, fen-
tanyl, desmopressin, domperdon, scopolamine (hydrobromide),
peptide, etc. -
. :
Obviously this list is not exhaustive.
Advantageously the active substance reservoir matrix can be
built up in layer form, the layers being the same or
different. The reservoir matrix can be pressure sensitive
adhesive and can e.g. be a rubber material, such as
styrene/isoprene/styrene block copolymers, silicone rubber
or synthetic resins, such as poly(meth)acrylate,
polyurethane, polyvinylether, polyester, etc. - a list of
suitable matrix materials appearing e.g. in DE-OS 35 00 508,
to which reference is made. It can be advantageous if the
reservoir matrix is pressure sensitive adhesive, because
this can obviate the need for providing a separate pressure
sensitive adhesive fixing device in the system. The use of
such a pressure sensitive adhesive matrix is inter alia
dependent on the compatibility of the matrix material with
the active substance. Pressure sensitive adhesive matrix
materials are known.
~ ' '''. '
Preferred non-pressure sensitive adhesive matrix materials
are polymers comprising poly(meth)acrylate, polyvinyl-
pyrrolidone, ethylcellulose, hydroxypropylcellulose,
hydroxypropylmethylcellulosephthalate, polyvinylalcohol or -
copolymers thereof with vinyllaurate or maleic acid, vinyl-
acetate or copolymers thereof with vinyllaurate or maleic
acid, polyvinylether, butylrubber and polycaprolactam.
For example the active substance depot or depots can be
introduced between a backing side reservoir matrix layer and
a skin side reservoir matrix layer, the thickness ratio of
the reservoir matrix layers preferable being between
approximately X:Y=1:1 to 1:20 and in particularly preferred - ~
manner 1:1 to 1:5. ~-; -
: ': ', ',-
~': :,''''
13128~
It can be appropriate in other cases if the reservoir matrix
or reservoir matrix layers from which said matrix is formed,
to be provided at least on one side with pressure sensitive
adhesive coatings. -
According to a further advantageous development of the
inventive system the active substance depot can be arranged
between the reservoir matrix and the backing layer, which is
e.g. suitable for solid active substances.
In a preferred embodiment of the invention the fixing device
can be formed by adhesive portions embedded in the reservoir
matrix. such as e.g. an all-round adhesive edge or adhesion
points.
In conventional manner, it is possible to provide a detach-
able protective layer for the surfaces of the therapeutic
system facing the skin.
The sum of the active substance in the depot and reservoir -
matrix is advantageously up to 20 times the therapeutically
necessary active substance quantity. ~
A particularly preferred process for producing such systems - -
comprises the reservoir matrix being formed from two
reservoir matrix layers, which can be the same or different,
between which is introduced the active substance depot. The
reservoir matrix layers can be joined together by the ~-
application of pressure and/or heat. The depot can also be
introduced into the reservoir matrix under pressure
application, e.g. by injecting a predetermined quantity or
pressing in a active substance body into a soft matrix
layer.
A further preferred process embodiment comprises forming at
least part of the therapeutical system by strewing on
particles. - ~;
~ . . .
.
9 13128~
It is also possible to produce a multilayer active substance
matrix. The covering and reservoir matrix layer can also be
joined by heat or pressure. The reservoir matrix layer or
layers can at least partly be produced from liquid
materials, e.g. from a dispersion, a melt or solutions.
The inventive therapeutic system is in particular suitable
for local or systematic transdermal active substance
application in human or veterinary medicine or can also be
used in cosmetics.
The invention is described in greater detail hereinafter
relative to non-limitative embodiments of the inventive
therapeutic system and the attached diagrammatic drawings,
in which:
Fig. 1 shows a section through a preferred embodiment of an
inventive therapeutic system;
Fig. 2 shows a section through a further preferred
embodiment of the inventive system, in which the active
substance reservoir is embedded between matrix layers; and
Fig. 3 shows a section through a web-like semifinished
product according to the invention.
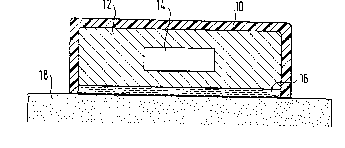
Fig. 1 depicts a section through an inventive therapeutic
system, which is fixed to the skin 18 by a fixing device 16,
e.g. a porous pressure sensitive adhesive layer or the like.
on the fixing device 16 is located reservoir matrix 12
which, at the time of production, is preferably free from
active substance (active substance saturation taking place
during storage). In the reservoir matrix is embedded a
depot 14, which is represented here as a solid active
substance, which dissolves in the reservoir matrix material
and is supplied to the skin 18 by fixing device 16. The
therapeutic system is sealed off from the outside by means
..~.. ,: :
' - ::, ' '
13~28~
of a backing layer 10, which is impermeable for the active
substance and preferably also to moisture and simultaneously
provides a support function for the system.
Fig. 2 shows another preferred embodiment, in which an
inventive therapeutic system is fixed to the skin 18 by
means of adhesive particles or portions embedded on the skin
side in the active substance reservoir matrix material. The
active substance reservoir layer 12 here comprises an upper
layer X and a lower layer Y, between which is introduced the
active substance, which is e.g. here in liquid form. The
provision of two reservoir matrix layers X, Y is
advantageous if a system is being produced in such a way
that firstly the lower active substance reservoir layer is
provided, optionally with an already coated on covering film
or the like and then in accordance with a predetermined
pattern the active substance reservoir layer X is added and
finally in conventional manner the backing layer or
optionally various adhesive layers are applied to complete
the system. It may also be appropriate to firstly place the
two active substance reservoir layers X, Y on top of one
another, then inject a predetermined active substance
quantity between the two reservoir layers and in this way
keep evaporation of the active substance to a minimum.
Fig. 3 shows a precursor of an inventive transdermal system,
such as is obtained during a preferred production process.
A web-like protective coating material, such as e.g. waxed
paper or the like is covered by a reservoir matrix layer Y,
which is here constructed of pressure sensitive adhesive and
has located thereon in accordance with a predetermined
pattern active substance depot bodies. Matrix layer Y is
covered by a second matrix layer X, which can e.g. comprise
a material differing from that of layer Y. The second
matrix layer Y is terminated by a backing film 10. Along
the arrows are located the parting or separating lines,
along which the intermediate product is cut or punched
i.- . .- -
3~ .,
13128~
11
during the production of the inventive transdermal systems
and then prepared in the usual way.
Typical thicknesses for inventive transdermal systems are in
the case of a total thickness of approximately 123 to
5550~m, preferably 285 to 1550 ~m; thickness of the backing
layer 8 to 150~m and preferably 15 to 100 ~m; thickness of
the reservoir 100 to 5000 ~m, preferably 200 to 1330 ~m; ~-
thickness of the protective layer 15 to 400 ~m, preferably - -
70 to 150~m.
For special applications it is also possible to market the
"semifinished product" as such, so as to enable users to
carry out the separation of the systems, so that the
semifinished product acts in the manner of a "storage pack".
.:
Preferred examples of the invention are described below.
Example 1 -
PRODUCTION OF A NICOTINE PLASTER ~-
A nicotine plaster according to the invention may be
produced as follows.
: ' .:::: ':
A pressure sensitive adhesive material comprising 2.0825 kg
of a 40~ solution of a self-crosslinking acrylate copolymer ` -~
(DUROTAC *280 - 2416 of the firm National Starch/Chemical
B.V.) in a mixture of ethyl acetate, ethanol, hexane and - ~
methanol, 147 g of an acrylic resin of dimethylaminoethyl- -~ ~-
methacrylate and neutral methacrylate (EUDRAGIT *E 100 of
the firm ROHM PHARMA), as 20 g of a mixed acid triglyceride -
of fractionated C8-CIo coconut fatty acids (Miglyol 812 of
the firm Dynamit Nobel) are applied to a protective layer ~ -
* - Trade-mark
', '~'~'.
~,, .
13~ 28~
vacuum-deposited with aluminium on one side and adhesively
finished on both sides and the solvent is evaporated at 50
to 80C. An approximately 300 g/m2 layer is obtained. From
the thus produced pressure sensitive adhesive layer are
punched round blanks with a diameter of 65 mm. The
projecting edges are worked and centrally to the same is
applied in each case one circular blank of a non-woven
fabric (fibrous mixture of viscose staple fibre/cotton 50:50
with a substance weight of 80 g/m2 - PARATEX II/80 of
LOHMANN GMBH ~ CO KG) and with a diameter of 40 mm. To this
is applied nicotine as the active substance in solution (140
g nicotine in 100 g of an acrylic resin of dimethylamino-
ethylmethacrylate and neutral methacrylates (EUDRAGIT E 100
of the firm ROHM PHARMA) in 102 mg dose/blank. The thus
produced patches are immediately laminated with a nicotine
impermeable backing layer, a 15 ~ thick polyester film on
one side of which aluminium is vapour deposited and sealed
in a four-edge sealing bag of a suitable packing material.
In this case the non-woven fabric serves as the
supporting layer and to assist the uniform distribution of
the nicotine as an inert adjuvant as defined hereinbefore.
Due to the fact that, according to the invention,
an active substance solution can be rapidly applied to a
matri~ layer and is then covered by an active substance
impermeable covering layer, it is possible for the first
time to obtain in a satisfactory manner well dosed nicotine
plasters.
- . : - .
trademarks
,,~.
~31 2~
13
ICOTINE RELEASE TEST (IN VITRO)
A nicotine plaster produced according to example
1 after removing the protective layer is immersed in 80 ml
of isotonic common salt solution at 37 and the released
nicotine quantity is determined in liquid chromatography
after predetermined intervals. The release medium volume
was chosen in such a way that "sink" conditions are obtained
over the entire test period. The following results were
obtained:
after 2 hours : 23.90 mg/plaster
after 4 hours : 32.34 mg/plaster
after 8 hours : 41.50 mg/plaster -
after 24 hours : 56.54 mg/plaster
.
Example 2
PRODUCTION OF A NICOTINE PLASTER
Another nicotine plaster according to the-~
invention may be produced as follows. ~-
A pressure sensitive adhesive material (adhesive ~
1) comprising 1.9758 kg of a 40~ solution of a self- ~-
crosslinking acrylate copolymer (DUROTAC 280 - 2416 of the - ~
firm Delft National & Chemical B.V.) in a mixture of ethyl -~ - -
acetate, ethanol, heptane and methanol, 189.7 g of an ~ -
acrylic resin of dimethylaminoethylmethacrylate and neutral
methacrylate (EUDRAGIT E 100 of the firm ROHM PHARMA), and
20 g of a mixed acid triglyceride of fractionated C8-CIo -
coconut fatty acids (Miglyol 812 of the firm Dynamit Nobel)
are applied to a protective layer vacuum-deposited with
aluminum on one side and adhesively finished on both sides
and the solvent is evaporated at 50 to 80 C. An ~
approximately 440 g/mZ layer is obtained. From the thus ~ :
produced pressure sensitive adhesive layer are punched round `
blanks with a diameter of 51 mm. The projecting edges are
worked and centrally to the same is applied in each case one ~ -
circular blank of a non-woven fabric (fibrous mixture of
viscose staple fibre/cotton 70:30 with a substance weight of
40g/m2 -PARATEX III/40 of LOHMANN GMBH & CO KG) and with a
"'',
' -
:.
~3~ 28~ :
diameter of 42 mm. To this is applied nicotine as the active
substance in solution (140 g nicotine in 100 g of an acrylic
resin of dimethylaminoethylmethacrylate and neutral
methacrylates (EUDRAGIT E 100 of the firm ROHM PHARMA) in 46
mg dose/blank. The thus produced patches are immediately
laminated with a nicotine impermeable backing layer, a 15
thick polyester film on one side of which aluminum is vapour
deposited having an approximately 110 g/mZ coating of
adhesive 1 and sealed in a four-edge sealing bag of a
suitable packing material.
In this case the non-woven fabric serves as the supporting
layer and to assist in the uniform distribution of the
nicotine as an inert adjuvant as defined hereinbefore.
.
Due to the fact that, according to the invention, an active
substance solution can be rapidly applied to a matrix layer
and is then covered by an active substance impermeable
covering layer, it is possible for the first time to obtain
in a satisfactory manner well dosed nicotine plasters
': '
NICOTINE RELEASE TEST (IN VITRO) - -
A nicotine plaster produced according to example 1 after ;~
removing the protective layer, immersed in 80ml of isotonic
common salt solution at 37 and the released quantity is
determined by liquid chromatography after predetermined
intervals. The release medium volume was chosen in such a -
way that "sink" conditions are obtained over the entire test
period. The following results were obtained:
after 2 hours : 5.1 mg/plaster
after 4 hours : 7.2 mg/plaster
after 8 hours : 10.1 mg/plaster
after 24 hours : 16.5 mg/plaster
':,
::,
; ~3~28~0
It is to be understood that the invention is not limited to :
nicotine plasters and the production thereof with the .
claimed build-up but that other substances as preferred -
substances are mentioned in the specification may be
administered by this new therapeutic system.
'
~', ' .', '
'',,''~''
~ .
` ' ' '
.. . ~