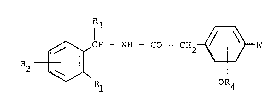Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
13~7~ 27169-119
Phenylacetic acid derivatives
The present invention relates to new certain new phenyl-
acetic acid derivatives, their preparation and pharmaceutical
compositions containi.ng them.
Certain new phenylacetic acid derivatives have been
found to possess valuable pharmacological propert.ies, particularly
an effect on intermediate metabolism, more particularly a hypo-
glycaemic effect.
According to one aspec-t of the invention therefore we
provide compounds of formula I
2 ~ CH -- NH - CO ~ CH2~
OR4
(wherein Rl represents 5- to 7-membered saturated nitrogen-
containing heterocyclic ring, which ring is attached via the
nitrogen atom group with 4 to 6 carbon atoms, optionally substitu-
ted by one or two alkyl groups each having 1 to 3 carbon atoms;
R2 represents a hydrogen or halogen atom or a methyl or
methoxy group;
R4 represents a hydrogen atom, an alkyl group with 1 to 3
carbon atoms or an allyl group; and
R3 represents a hydrogen atom, an alkyl group with 1 to 7
carbon atoms, a phenyl group optionally substituted by a halogen
atom or a methyl or methoxy
.. ~..
~32~723
2 27169 119
group, an alkyl ~roup wlth 1 or 2 carbon atoms substltuted by a
hydroxy, a Cl_3 alkoxy, a C2_3 alkanoyloxy, a tetrahydrofuranyl, a
tetrahydropyranyl, a C3_7 cycloalkyl or a phenyl group, an alkenyl
group with 3 to 6 carbon atoms, an alkynyl yroup with 3 to 5 car~
bon atoms, a carboxy group or an alkoxycarbonyl group with a total
of 2 to 5 carbon atoms, and
W represents a ~yanomethyl, 2-cyano-ethyl, 2-cyano-
ethenyl, carboxymethyl, 2-carboxyethyl, 2-carboxyethenyl, alkoxy-
carbonylmethyl, 2-alkoxycarbonylethyl or 2-alkoxycarbonyl~-ethen~l
group, in which any alko~y molety contains from 1 to 4 carbon
atoms and may optionally be substituted by a phenyl group; or
R3 represents an alkyl group with 1 or 2 carbon atoms
substituted by a hydroxy, a Cl_3 alkoxy, a C2_3 alkanoyloxy, a
tetrahydrofuranyl, a tetrahydropyranyl, a C3_7 cycloalkyl or a
phenyl group, an alkenyl group with 3 to 6 carbon atoms, an
alkynyl group with 3 to 5 carbon atoms, a carboxy group or an
alkoxycarbonyl group with a total of 2 to 5 carbon atoms, and
W represents a methyl, hydroxymethyl, Eormyl, carboxy or
alkoxycarbonyl group, ln which the alkoxy molety contalns 1 to 4
carbon atoms and may optionally be substituted by a phenyl group),
and
the enantiomers and salts thereof.
The followlng are examples of atoms or groups whlch
comply with the definltions of the groups Rl to R4 and W glven
hereinbefore:
Rl may represent a pyrrolldino, plperidlno, hexamethy-
leneimlno, methyl-pyrrolidino, dimethyl-pyrrolidino, ethyl-
pyrrolidino, 2-methyl-plperldlno, 3-methyl-plperldlno, 4-methyl-
13~723
3 27169-J.19
plperidlno, 3,3-dlmethyl-plperldlno, cls-3,5-dimethylplperldino,
trans-3,5-dlmethyl-piperldlno, ethyl-plperldlno, dlethyl-plper-
idlno, methyl-ethyl-plperidino, propyl--piperldlno, methyl-propyl-
plperldino, or isopropylpiperidino group,
R2 may represent a hydros~en, fluorlne, chlorine or
bromlne atom or a methyl or methoxy group,
R4 may represent a hydroS~en atom, a methyl, ethyl, n-
propyl, lsopropyl or allyl group,
R3 may represent a hydro~en atom, a methyl, ethyl, n-
propyl, lsopropyl, n-butyl, lsobutyl, sec.butyl, tert.butyl, n-
pentyl, 2-methyl-n-butyl, 3-methyl-n-butyl, 2,2-dlmethylpropyl, n-
hexyl, 4-methyl-n-pentyl, n-heptyl, l-propen-l-yl, 2-methyl-1-
propen-l-yl, 3-methyl-2-buten-2-yl, 2-propen-1-yl, 2-methyl-2-
propen-l-yl, 2-buten-1-yl, 2-methyl-2-buten-1-yl, 3-methyl-2-
buten-l-yl, 3-buten-1-yl, 2-methyl-3-buten-1-yl, 3-methyl-3-buten-
l-yl, 2-hexen-1-yl, l-propyn l-yl, 2-propyn-1-yl, 2-butyn-1-yl, 2-
pentyn-l-yl, phenyl, ~luorophenyl, chlorophenyl, bromophenyl,
methylphenyl, methoxyphenyl, hydroxymethyl, l-hydroxy-ethyl, 2-
hydroxy-ethyl, methoxymethyl, ethoxymethyl, n-propoxymethyl,
isopropoxymethyl, l-methoxy-ethyl, 2-methoxy-ethyl, l-ethoxy-
ethyl, 2-ethoxy-ethyl, 2-n-propoxy-ethyl, 2-lsopropoxy-ethyl,
acetoxymethyl, proplonyloxymethyl, l-acetoxy-ethyl, 2-acetoxy-
ethyl, l-propionyloxy-ethyl, 2-proplonyloxy-ethyl, tetrahydro-
furan-2-yl-methyl, 2-(tetrahydrofuran-2-yl)-ethyl, tetrahydro-
~uran-3-yl-methyl, tetrahydropyran-2-yl-methyl, 2-~tetrahydro-
pyran-2-yl)-ethyl, tetrahydropyran-3-yl-methyl,
~ "7~
~3~7?J3 27l6g-ll9
cyclopropylmethyl, cyc]obutylmethyl, cyclopentylmethyl, c~clo-
hexylmethyl, cycloheptylmethyl, 2-cyclopropyl-ethyl, ~-cyclobutyl-
ethyl, 2-cyclopentyl-ethyl, 2-cyclohexyl-ethyl, 2-cyclohep-tyl-
ethyl, benzyl, l-phenyl-ethyl, 2-phenyl-ethyl, carboxy, methoxy-
carbonyl, ethoxycarhonyl, n-propoxycarbonyl, isopropoxycarbonyl,
n-butoxycarbonyl, sec.butoxycarbonyl, isobutoxycarbonyl or
tert.butoxycarbonyl group,
and
W may represent a methyl, hydroxymethyl, formyl, carboxy,
methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl, isopropoxy-
carbonyl, n-butoxycarbonyl, sec.butoxycarbonyl, isobutoxycarbonyl,
tert.butoxycarbonyl, benzyloxycarbonyl, 1-pheny]ethoxycarbonyl,
2-phenylethoxy-carbonyl, 3-phenylpropoxycarboxyl, cyanomethyl,
2-cyano-ethyl, 2-cyano-ethenyl, carboxymethyl, methoxycarbonyl-
methyl, ethoxycarbonylmethyl, n-propoxycarbonylmethyl, n-butoxy-
carbonylmethyl, tert.butoxycarbonylmethyl, 2-methoxycarbonyl-
ethyl, 2-ethoxycarbonyl-ethyl, 2-n-propoxycarbonyl ethyl, 2-
isopropoxycarbonyl-ethyl, 2-n-butoxycarbonyl-ethyl, 2-tert.butoxy-
carbonyl-ethyl, 2-methoxycarbonyl-ethenyl, 2-ethoxycarbonyl-
ethenyl, 2-n-propoxycarbonyl-ethenyl or 2-tert.butoxycarboxyle-
thenyl group.
Preferred compounds include those of formula I wherein
R1 represents 5- to 7-membered saturated nitrogen-containing
heterocyclic ring, which ring is attached via the nitrogen atom to
the benzene ring, the heterocyclic ring being optionally substi-
tuted by one or two alkyl groups each having 1 to 3 carbon atoms,
R2 represents a hydrogen or halogen atom or a methyl or
'~l
, ,,,~
132B7 23
- 4a - 27169-ll9
methoxy group;
R4 represents a hydrogen atom, an alkyl group with 1 to 3
carbon atoms or an allyl group; and
'~' ~3 1
,~. '.! j
1~2~723
5 27169 llg
R3 represents a hydrogen atom, an alkyl group wlth 1 to
7 carbon atoms, a phenyl group optlonally substituted by a halogen
atom or a methyl or methoxy group, an alkyl group wl~h 1 or 2 car-
bon atoms sub6tltu$ed by a hydroxy, a Cl_3 alkoxy, a C2_3 alkanoy-
loxy, a C3_7 cycloalkyl or a pheny]. group, a carboxy group or an
alkoxycarbonyl group wlth a total of 2 to 5 carbon atoms, and
W represent~ a cyanomethyl, 2~cyano-ethyl, 2~cyano-
ethenyl, carboxymethyl, 2-carboxyethyl, 2-carboxyethenyl, alkoxy-
carbonylmethyl, 2-alkoxycarbonyl-ethyl or 2-alkoxycarbonyl-ethenyl
group, ln which any alkoxy molety contalns from 1 to 4 carbon
atoms and may optlonally be substltuted by a phenyl group~ or
R3 represent~ an alkyl group with 1 or 2 carbon atoms
substituted by a hydroxy, a C]_3 alkoxy, a C2_3 alkanoyloxy, a C3_
7 cycloalkyl or a phenyl group, a carboxy group or an alkoxy-
carbonyl group with a total of 2 to 5 carbon atoms, and
W represents a methyl, hydroxymethyl, ~ormyl, carboxy or
alkoxycarbonyl group, in which the alkoxy molety contalns 1 to 4
carbon atoms and may optlonally be substituted by a phenyl group;
and the enantiomers and salts thereof.
Particularly pre~erred compounds include those of formu-
la I whereln
Rl represents a pyrrolldlno, piperldino, 4-methyl-
plperldlno, 3-methyl-plperldino, 3,3-dlmethyl-plperidlno, 3,5-
dlmethyl-piperidino or hexamethylenelmino group;
: ,,,
132~7~3
6 27169-119
R2 represents a hydrogen, fluorlne or chlorlne atom or a
methyl or methoxy group,
R4 represents a hydrogen atom, an alkyl group with 1 to
3 carbon atoms or an allyl group, and
R3 represents a hydrogen atom, an alkyl group with 1 to
6 carbon atoms or a phenyl group olptionally substltuted by a
chlorine atom or a methyl or methoxy group, an alkyl yroup wlth 1
or 2 carbon atoms substltuted by a hydroxy, a Cl_3 alkoxy, an
acetoxy, a proplonyloxy, a tetrahydrofuranyl, a tetrahydropyranyl,
a C3_7 cycloalkyl or a phenyl group, an alkenyl group wlth 3 to 5
carbon atoms, an alkynyl group wlth 3 or 4 carbon atoms, a carboxy
group or an alkoxycarbonyl group wlth a total of Z to 5 carbon
atoms, and
W represents a cyanomethyl, carboxymethyl, 2-carboxy-
ethyl, 2-carboxy-eth~nyl, alko~ycarbonylmethyl, 2-alkoxycarbonyl-
ethyl or 2-alko~ycarbonyl-e~henyl group, ln whlch any alkoxy
molety contalns from 1 to 4 carbon atoms and may be substltuted by
a phenyl group, or
R3 represents an alkyl group with 1 or 2 carbon atoms
substltuted by a hydroxy, a Cl_3 alkoxy, an aceto~y, a propiony-
loxy, a tetrahydrofuranyl, a tetrahydropyranyl, a C3_7 cycloalkyl
or a phenyl group, an alkenyl group with 3 to 5 carbon atoms, an
alkynyl group wlth 3 or 4 carbon atoms, a carboxy group or an
alkoxycarbonyl group wlth a total of 2 to 5 carbon atoms, anc~
~ represents a methyl, hydro~ymethyl, formyl, carboxy or
alkoxycarbonyl group, ln whlch the alkoxy molety contalns 1 to 4
carbon atoms anc~ may be substituted hy a phenyl group,
132~3r~
7 27169-119
and the enantlomers and salts thereof.
However, more especially preferred compounds lnclude
those of formula I whereln
Rl represents a pyrrolldlno, plperldlno, 4--methyl-
plperldlno, 3-methyl-plperldlno, 3,3-dlmethyl--plperidino, 3,5-
dlmethyl-piperidino or hexamethyleneimino ~roup.
R2 represents a hydrogen, ~luorine or chlorine atom or a
methyl or methoxy ~roup,
R4 represents a hydrogen atom or an alkyl group with 1
10 to 3 carbon atoms, and
R3 represents a hydrogen atom, an alkyl group wlth 1 to
5 carbon atoms, a phenyl, hydroxymethyl, ethoxy-methyl, acetoxy-
methyl, benzyl or tetrahydrofuranylmethyl group, a cycloalkyl-
methyl group in whlch the cycloalkyl moiety contaln from 3 to 7
carbon atoms, an alkenyl group with 3 to 5 carbon atoms, a pro~
pargyl group or an alkoxycarbonyl group wlth a total of 2 to 5
carbon atoms, and
W represents a cyanomethyl, carboxymethyl, 2-carboxy-
ethyl or 2-carboxy-ethenyl group, or
R3 represents a cycloalkylmethyl group in whlch the
cycloalkyl moiety contalns 3 to 7 carbon atoms, an alkenyl group
wlth 3 to 5 carbon atoms, a propargyl group, an alkoxycarbonyl
group with a total of 2 to 5 carbon atoms, a hydroxymethyl,
ethoxymethyl, acetoxymethyl, benzyl or tetrahydrofuranylmethyl
group, and
~ represents a carboxy, methoxycarbonyl, ethoxycarbonyl
or benzyloxycarbonyl group,
132~72~
8 ~7169-119
but partlcularly those compounds of formula I wherein
Rl represents a piperidlno group,
R2 represents a hydrogen, fluorlne or chlorlne atom,
R4 represents a methyl or ethyl group, and
R3 represents a methyl, ethyl, n-propyl, n-butyl,
lsobutyl or phen~l group and
W represents a cyanomethyl, 2--carboxyethenyl, carbo~y
methyl or 2-carboxy-ethyl group, or
R3 represents a cyclopropylmethyl, cyclobutylrnethyl,
cyclopentylmethyl, cyclohexylmethyl, tetrahydrofuran-2-yl-meth~l,
allyl, methallyl, 2-methyl-vlnyl, 2,2-dlmethyl-vinyl, propargyl,
methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl, lsopropoxy-
carbonyl, hydroxymethyl or ethoxymethyl group and
W represents a carboxy, methoxycarbonyl or ethoxy-
carbonyl group,
and the enantiomers and the salts thereof, partlcularly
the physlologically acceptable salts thereof wlth lnorganic or
organlc aclds or bases.
Especlally preferred compounds include those of formula
I whereln W represents the carbo~y ~roup, and the enantlomers and
salts thereof.
In a further aspect the present lnventlon provides a
process for preparing the compounds of the invention, sald process
comprlsing at least one of the followlng steps
~ 3 ~ 3
~ 27169-:L19
a) reacting an amine o~ formula II
R3
~H-NH
2 (II)
~ Rl
(wherein
Rl to R3 are deflned as hereinbefore) with a carboxylic
acld of formula III
HOOC-CH2 ~ W' (III)
OR4
(wherein
R4 ls de~lne~ as herelnbefore and
W' has the meanlngs glven for W hereinbefore, whilst any
carboxy ~roup contained ln the group W may be protected by a pro-
tecting group)
or with a reactive derlvative thereof, optionally pre-
pared in the reactlon mixture, lf necessary with subsequent spllt-
ting of~ of any protecting group used:
b) (to prepare compounds of formula I wherein R3 repre-
sents a carboxy or alkoxycarbonyl group and W has the meanings
given herelnbefore or W represents a carboxy~ carboxymethyl, 2-
carboxy-ethyl, 2-carboxy-ethenyl, alkoxycarbonyl, alkoxycarbonyl-
methyl, 2-alkoxycarbonyl-ethyl or 2-alkoxycarbonyl-ethenyl group
and R3 has the rneanlngs glven herelnbefore)
~L 3 2 ~ r~ 2 ~
2716g-119
hydrolyslng, thermolysing, hydrocJenoly.sing or alcoholyslng a
compound of formula IV
R2 ~ ~CH - NH - CO - CH2 ~ (IV)
OR4
Rl
(whereln
Rl, R2 and R4 are A5 herelnbe~ore defined,
B represents a bond or a methYlenel ethylene or
ethenylene group,
A and L each represent a nitrlle group or a group
convertable by hydrolysls, thermolysis or hydrogenolysls, lnto a
carboxy group, and
L addltionally may have the meanlngs given for R3
herelnbefore);
c) (to prepare compounds of Eormula I wherein R~
represents a hydrogen atom~
splittlng off a protecting group from a compound of
formula V
R3
CH - NH - CO - CH2 ~W
2 ~Rl OR5 (V)
(whereln
Rl to R3 and W are as herelnbefore deflned and
R5 represents a hydroxy group protecting group);
I
- 13~23
-- 11
d) ~to prepare compounds of -Eormula I wherein R4
represents an alkyl group with 1 to 3 carbon atoms
or an allyl grouP)
reacting a compound of formula VI
S~2
IH NH CO C~ ~ W tVI)
R OH
(wherein
Rl to R3 and W are as hereinbefore defined) with
a compound of formula VII
X - R~ (VII)
(wherein
R6 represents an alkyl group with 1 to 3 carbon
atoms or an allyl group, and
X represents a nucleophilically exchangeable group
such as a halogen atom, a sulphonyloxy group or,
lS together with the~ ad~acent hydrogen atom, a diazo
group, if R6 represents an alkyl group with 1 to
3 car~on atoms), i. necessary with subsequent hydrolysis
of the compound thus Gbtained.
e) (to prepare compounds of formula I wherein W
represents a cyanomethyl. or 2-cyano-ethyl group)
reacting a compound of formula VIII
~xc~-Nl~-co-c~r-2~ E-Y
R2 OR4
:,
132~72~
- ]2 -
(wherein
Rl to R~ are as hereinbefore de~ined,
represents a methylene or ethylene group and
Y represents a nucleophilically exchangeable group
such as a halogen atom or a sulphonyloxy group,
e.g. a chlorine, bromlne or iod;ne atom or a methane-
sulphonyloxy or p-toluenesu:Lphonyloxy group)
with an alkali metal cyanide such as sodium or
potassium cyanide.
f) lto prepare compounds of formula I wherein W
represents a cyanomethyl, 2-cyano-ethyl or 2-cyano-
ethenyl group)
dehydrating a compound of formula IX
R2 ~r~ co-c-~2~G-coNH~
(wherein
Rl to R~ are as hereinbeore defined and
G represents a methylene, ethylene or ethenylene
group);
g) ttb prepare compounds of formula I wherein W
represents a 2-cyano-ethenyl, 2-carboxy-ethenyl
or 2-alkoxycarbonyl-ethenyl group~
reacting a compound of formula X
R
~-~H-~0-C-2 ~ CHO (X)
R1 G~.4
132~ ~23
- 13 -
(wherein
Rl to R4 are as hereinbefore defined) with a corresponcling
acetic acid derivative of formula XI
Z - CIT2 - ~ (Xl~
(wherein ~ represents a carboxy, alkoxycarbonyl
or cyano group and
Z represents a hydrogen atom, an alkoxycarbonyl,
dialkylphosphono or triphenylphosphonium halide
group an-d optionally subsequently hydrolysing an~/or
decarboxylating the compound thus obtained;
h) (to prepare compounds of formula I wherein W
represents a 2-carboxy-ethyl, 2-alkoxycarbonyl-
ethyl or 2-cyano-ethyl group)
reducing a compound of formula XII
R2 ~ ~ _C O _ C. 1~2 ~3 W ~ Y I I !
(wherein
Rl to R4 are as hereinbefore defined and
W7' represents a 2-carboxy-ethenyl, 2-alkoxycarbonyl-
ethenyl or 2-cyano-ethenyl group~;
i) (to prepare compounds of formula I wherein R3
represents an alkyl group with 1 or 2 carbon ato~s
substituted by an alkoxy or alkanoyloxy group~
reacting a compound of Formula XTTI
132~7%3
~ 14 -
2 ~ ~ CH-NH-CO-CH2 ~ (XIII)
OR4
R1
(wherein
Rl, R2, R4 and W are as hereinbefore defined and
R3' represents an alkyl group with 1 or 2 carbon
S atoms substituted by a hydroxy group)
with a compound o~ formula XIV
U - R7 ~XIV)
~wherein
R7 represents an alkyl group with 1 to 3 carbon
atoms or an acetyl or propionyl group and
U represents a nucleophi.lically exchangeable group
such as a halogen atom, a sulphonyloxy group, an
acetoxy or propionyloxy group or, together with
the adjacent hydrogen atom, represents a diazo
group if R7 represen.s an alkyl group with 1 to
3 carbon atoms~ and optionally subsequently hydrolysing
the compound thus obtained;
k) (to prepare compounds of formula I wherein R3
represents an alkoxycarbonyl group)
20 reacting a compound of formula X~
COOR
R2 r cH NH cO c~2 ~ ( xv
Rl P'4
~whe~ei.n
~ 3~723
27169-119
Rl, R2, R4 and W are a~ hereinbefore defined and
R8 represents a hydrogen atom or an alkall metal atom)
or a reactlve derivative thereof, optionally prepare~ ln the
reaction mixture with a compound of formula XVI
T - Rg (XVI)
~whereln
Rg represents an alkyl gr.oup with 1 to 4 carbon atoms
and
T represents a hydroxy group or a nucleophlllcally ex-
changeable group such as a halogen atom or a sulphonyloxy group,or together wlth the ad~acent hydrogen atom of the group Rg repre-
sents a diazo group) and optionally by hydrogenolyslng the com-
pound thus obtalned iE W contains a benzyloxycarbonyl group~
1) (to prepare compounds of formula I wherein
R4 represents a hydrogen atom or an alkyl group wlth 1
to 3 carbon atoms, and
R3 represents a hydrogen atom, ~n alkyl group with 1 to
7 carbon atoms, a phenyl group optlonally substltuted by a halogen
atom or by a methyl or methoxy group, an alkyl group with 1 or 2
carbon atoms substltuted by a Cl_3 alkoxy, a C2_3 alkanoyloxy, a
tetrahydrofuranyl, a tetrahydropyranyl, a C3_7 cycloalkyl or a
phenyl group, an alkenyl group wlth 3 to 6 carbon atoms, an
alkynyl group with 3 to S carbon atoms, a carboxy group or an
alkoxycarbonyl group wlth a total of 2 to 5 carbon atoms, and
W represents a carboxymethyl, 2-carboxy-ethyl, alkoxy-
~i
132~ ~23
16 27169~carbonylmethyl or 2-alkoxycarbonylethyl group, ln which any alkoxy
moiety contalns from 1 to 4 carbon atoms, or
R3 represents an alkyl group with 1 or 2 carbon atoms
substltuted by a Cl_3 alkoxy, a C2_3 alkanoyloxy, a tetrahydro-
furanyl, a tetrahydropyranyl, a C3.7 cycloalky]. or a phenyl group,
an alkenyl group with 3 to 6 carbon atoms, an alkynyl group with 3
to 5 carbon atoms, a carboxy group or an alkoxycarbonyl group wlth
a total o~ 2 to 5 carbon atoms and
W represents a methyl, formyl, carboxy or alkoxycarbonyl
group, in which the allcoxy moiety contains 1 to 4 carbon atoms)
reacting a compound of formula XVII
~ CH-OH
2 ~ 1~ (XVII)
~/ R
( wherein
R and R2 are as herelnbefore defined and
R3" (lf W ls to represent a carboxymethyl, 2-carboxy
ethyl, alkoxycarbonylmethyl or 2-alkoxycarbonyl-ethyl group, in
whlch any alkoxy moiety contains from 1 to 4 carbon atoms) repre-
sents a hydrogen atom, an alkyl group wlth 1 to 7 carbon atoms, a
phenyl group optionally substltuted by a halogen atom or by a
methyl or methoxy group, an alkyl group with 1 or 2 carbon atoms
substituted by a Cl_3 alkoxy, a C2_3 alkanoyloxy, a tetrahydro-
furanyl, a tetrahydropyranyl, a C3_7 cycloalkyl or a phenyl group,
an alkenyl group wlth 3 to 6 carbon atoms, an alkynyl group wlth 3
to 5 carbon atoms, a carbo~y group or an alkoxycarbonyl group wlth
a total o~ 2 to 5 carbon atoms, or
'~i`i
..1 ~ l,
132~723
17 27169-119
R" (if W ls to represent a methyl, formyl, carbo~y or
alkoxycarbonyl group ln which the alkoxy molety contalns from 1 to
carbon atoms)
represents an alkyl group wlth 1 or 2 carbon atoms
substltuted by C1_3 alkoxy, a C2_3 alkanoyloxy, a
tetrahydrofuranyl, a tetrahy~ropyranyl, a C3_7 cycloalkyl or a
phenyl group, an alkenyl group wlth 3 to 6 carbon atoms, an
alkynyl group wlth 3 to S carbon akoms, a carboxy group or an
alkoxy-carbonyl group wlth a total of 2 to 5 càrbon atoms) wlth a
compound of formula XVIII
l~=C-CH ~ W"' (XVIII)
OR4
~wherein
R4 ls as hereinbefore deflned and
W"' (when R3" in the compound of formula XVII represents
a hydrogen atom, an alkyl group with l to 7 carbon atoms, a phenyl
group optionally substituted by a halogen atom or a methyl or
methoxy group, an alkyl group with one or two carbon atoms
substltuted by a C1_3 alkoxy, a C2_3 alkanoyloxy, a
tetrahy~rofuranyl, a tetrahydropyranyl, a C3_7 cycloalkyl or a
phenyl group, an alkenyl group wlth 3 to 6 carbon atoms, an
alkynyl group wlth 3 to 5 carbon
132~723
- 18 -
atoms, a carboxy group or an alkoxycarbonyl group
with a total of 2 to 5 carbon atoms)
represents a carhoxymethyl, 2-carboxy-ethyl, alkoxy-
carbonylmethyl or 2-alkoxycarbonyl-ethyl group,
in which any alkoxy moiety conta;ns from 1 to 4
carbon atoms, or
W"' (when R3" in the compound of formula XVII
represents an alkyl group with 1 or 2 carbon atoms
substituted by by a Cl 3 alkoxy, a C2 3 alkanoyloxy,
a tetrahydrofuranyl, a tetrahydropyranyl, a C3 7
cycloalkyl or a phenyl group, an alkenyl group
with 3 to 6 carhon atoms, an alkynyl group with
3 to 5 carbon atoms, a carboxy group or an alkoxycarbonyl
group with a total of 2 to 5 carbon atoms)
represents a methyl, formyl, carboxy or alkoxycarbonyl
group wherein the alkoxy moiety contains from 1
to 4 carbon atoms);
m) (to prepare compounds of formula I wherein
R~ represents a hydrogen atom or an alkyl group
with 1 to 3 carbon atoms,
R3 represents an alkyl group with 1 to 7 carbon
atoms, a phenyl group optlonally substi.tuted by
a methyl or methoxy group, an alkyl group with
1 or 2 carbon atoms substituted by a Cl 3 alkoxy,
a tetrahydrofuranyl, a tetrahydropyranyl, a C5 7
cycloalkyl or a phenyl group, and
W represents a cyanomethyl, 2-cyano-ethyl, carboxy-
methyl, 2-carboxy-ethyl, alkoxycarbonyl-methyl
or 2-alkoxycarbonyl-ethvl group in which any alkoxy
moiety contains from 1 to 4 carbon atoms and may
be substituted by a phenyl group, or
.32~3r~2'~
19 27169-llg
R3 represents an alkyl group wlth 1 or 2 carbon atoms
substituted by a Cl_3 alkoxy, a tetrahydrofuranyl, a tetrahydro-
pyranyl, a C5_7 cycloalkyl or a phenyl group, and
W represents a methyl, hydroxymethyl, carboxyl or
alkoxycarbonyl group, in whlch the alkoxy moiety contalns from 1
to 4 carbon atoms and may be substi.tuted by a phenyl group)
reduclng a compound of formula XIX
2 ~ D - C - CH2 ~ W"" (XIX)
(whereln
Rl, R2 and R4 are as herelnbefore ~eflned and
D represents a group of formula
10 ~ / 11
C R3"'
Il or
/ ~ NH- '~ ~ N-
ln whlch R3"' represents a phenyl ~roup optionally sub-
stltuted by a halo0en atom or by a methyl or methoxy group,
Rlo and Rll together wlth the carbon atom between them
represent an alkylidene group with 1 to 7 carbon atoms, an alkyli-
dene group with 1 or 2 carbon atoms substltuted by a Cl_3 alkoxy,
a tetrahydrofuranyl, a tetrahydropyranyl, a C5_7 cycloalkyl or a
phenyl group, and
~32~723
- 20 -
W"" represents a cyanomethyl, 2-cyano-ethyl, 2-
cyano-ethyl, carboxymethyl, 2-carboxy-ethyl, 2-
carboxy-ethenyl, alkoxycarbonylmethvl, 2-alkoxycarbonyl-
ethyl or 2-alkoxycarbonyl-ethenyl group, in which
any alkoxy moiety contains from 1 to ~ carbon atoms
and may be substituted by a phenyl group, or
Rlo and Rll together with the carbon atom between
them represent an alkylidene group with 1 or 2
carbon atoms substituted by a Cl 3 alkoxy, a tetrahydro-
furanyl, a tetrahydropyranyl, a C5_7 cycloalkylor a phenyl group, and
W"" represents a methyl, hydroxymethyl, formyl,
carboxy or alkoxycarbonyl group in which the alkoxy
moiety contains 1 to 4 carbon atoms and may be
substituted by a phenyl group);
n) converting a compound of formula I into a
salt thereof; and
o) separating out a stereoisomer of a compound
of formula I or salt thereof from a compound of
~ormula I or salt thereof thus obtained.
Examples of reactive derivatives of a compouna
of formula III which rnav be used in step (a~ include
the esters thereof, such as the methyl, ethyl or
benzyl esters, the thioesters such as the methylthio
or ethylthioesters, the halides such as the acid
chloride, the anhydrides or imidazolides thereof.
The reaction of step (a) is conveniently carried
out in a solvent such as methylene chloride, chloroform,
carbon tetrachloride, ether, tetr~hydrofuran, dioxan,
henzene, toluene, acetonitrile or dimethyl formam;de,
132~7~
21 -
optionally in the presence of an acid-activating
agent or a dehydrating agent~ e.g. in the presence
of ethyl chloroformate, thionyl chloride, phosphorus
trichloride, phosphorus pentoxide, N,N'-dicyclohexyl-
carbodiimide, ~,N'-dicyclohexylcarbodiimide/N-hydroxy-
succinimide, N,N'-carbonyldiimidazole or N,N'-thionyl-
diimidazole or triphenylphosphine/carbon tetrachloride,
or an agent which activates the amino group, e.g.
phosphorus trichloride, and optionally in the presence
of an inorganic base such as sodium carbonate or
a tertiary organic base such as triethylamine or
pyridine, which may simultaneously be used as solvent,
at temperatures of between -25C and 250~, hut
preferably at temperatures of between 10C and
lS the boiling temperature of the solvent used. The
reaction may also be carried out without a solvent
and furthermore any water formed during the reaction
may be removed by azeotropic distillation, e.g.
by heating with toluene using a water separator,
or by adding a drying agent such as magnesium sulphate
or a molecular sieve.
If necessary, the subsequent spl;tting off in step
(a) of a protecting group is preferably carried
out by hydrolysis, conveniently either in the presence
of an acid such as hydrochloric, sulphuric, phosphoric
or trichloroacetic acid or in the presence of a
base such as sodium hydroxide or potassium hydroxide
in a suitable solvent such as water, methanol,
methanol/water, ethanol, ethanol/water, water/isopropanol
or water/dloxan at temperatures of between -10
and 120C, e.~. at temperatures of between ambient
temperature and the boiling temperature of the
react;on mixture.
i32~723
- 22 -
A tert.butyl group used as protecting group may
also be spl;t ofE thermally, optionally in an inert
solvent such as methylene chloride, chloroforrn,
henzene, toluene, tetrahydro~uran or dioxan and
preferably in the presence of a catalytic quantity
of an acid such as p-toluenesulphonic acid, sulphuric,
phosphoric or polyphosphoric acid.
Furthermore, a benzyl group used as protecting
group may also be split off hydrogenolytically
in the presence o~ a hydrogenation catalyst such
as palladium/charcoal in a suitable solvent such
as methanol, ethanol, ethanol/water, glacial acetic
acid, ethyl acetate, dioxan or dimethylforma~ide.
~xamples of hydrolysable groups in the compound
of formula IV used in step (b~ include functional
derivatives of the carboxy group and the unsubstituted
or substituted amides, estersl thioesters, ortho
esters, iminoethers, amidines or anhYdrides thereof r
the nitrile group, the tetrazolyl group, an optionally
substituted 1,3-oxazol-2-yl or 1,3-oxazolin-2-yl
group. Examples cf thermolytically cleavable groups
include esters with tertiary alcohols, e.g. the
tert.butyl ester. ~xamples of hydrogenolytically
cleavable groups ;nclude aralkyl groups, e.g. the
benzyl group, and examples of alcoholytically cleavable
groups include the cyano group.
~ydrolysis in step (b~ is conveniently carried
out either in the presence of an acid such as hydro-
chloric, sulphuric, phosphoric or trichloroacetic
acid or in the presence o~ a base such as sodium
hydroxide or potassium hydroxide in a suitable
solvent such as water, water/methanol, ethanol,
water~ethanol, water~isopropanol or water/dioxan
at temperatures of between -lO and 120C, e.q.
1 32!DrJ~23
- 23 -
at temperatures of between ambient temperatureand the boiling temperature of the reaction mixture,
and the alcoholysis of a cyano group is preferably
effected in an excess of the corresponding alcohol
such as methanol, ethanol or propanol and in the
presence of an acid such as hydrochloric acid at
elevated temperatures, e.g. at the boiling temperature
of the reaction mixture.
l~ A and/or L in a compound of formula IV represents
a nitrile or aminocarbonyl group, these groups
may be converted into a corresponding carboxy compound
by means of 100~ phosphoric acid at temperatures
of between 100 and 180C, preferably at temperatures
of between 120 and 160C, or with a nitrlte, e.g.
sodium nitrite, in the presence of an acid such
as sulphuric acid, the latter conveniently being
used as solvent as well, at temperatures of between
O and 50C.
If A and~or L in a compound of formula IV represents
the tert.butyloxycarbonyl group for example, the
tert.butyl group may also be split off thermally,
optionally in an inert solvent such as methylene
chloride, chloroform, benzene, toluene, tetrahydrofuran
or dioxan and preferably in the presence of a catalyt;c
quantity of an acid such as p-toluenesulphonic,
sulphuric, phosphoric or polyphosphoric acid, preferably
at the boiling temperature of the solvent used,
e.g. at temperatures of between 40 and lOO~C.
If A and/or ~ in a compound of formula IV represents
a benzyloxycarbonyl group for example, the benzyl
group may also be split off hvdrogenolytically
in the presence of a hydrogenation catalyst such
as palladium/charcoal in a suitable solvent such
as methanol, ethanol, methanol/water, ethanol~water,
132~ ~23
- 24 -
~lacial acetic acid, ethyl acetate, dioxan or dimethyl-
formamide, preferahly at temperatures o~ between
0 and 50C, e.g. at ambient temperature and under
a hydrogen pressure of from 1 to 5 bar. ~uring
hydrogenolysis, a compound containing halogen may
simultaneously be dehalogenated, any double or
triple bonds present may be hydrogenated and any
benzyloxycarbonyl group present may be converted
into a carboxy group.
Examples of hydroxy group protecting groups for
R5 include, for example, an alkyl, aralkyl or trialkylsilyl
group, e.g. a methyl, ethyl, propyl, allyl, benzyl
or trimethylsilyl group.
Depending on the protecting group used, the protecting
groups mentioned above may for example be split
off in step (c) either by hydrolysis or by hydrogenolysis,
optionally in a suitable solvent, at temperatures
of between -78 and 250C.
For examplel ether splitting may be carrie~ out
~0 in the presence of an acid such as hydrochloric,
hydrobromic or sulphuric acid, boron tribromide,
aluminium trichloride or pyridine hydrochloride,
conveniently in a suitable solvent such as methylene
chloride, glacial acetic acid or water or in mixtures
thereof at temperatures of between -78 and 250C.
The ether splitting may be carried out in the presence
of a proton acid conveniently at temperatures of
between 0 and 150~, preferably at temperatures
of between 50 and 150C or with a ~ewis acid preferably
in a solvent such as methylene chloride at temperatures
of between -78 and 20~.
For example, in the compound of formu]a V any protect;ng
group used such as a benzy] grouP may he split
7 2 ~
~7169-119
off hydrogenolytlcally ~ith hydrogen in the presence of a hydro-
genation catalys-t such as palladiumtcharcoal in a suitahle solvent
such as methanol, ethanol, ethanol/water, glacial acetic acld,
ethyl acetate, dioxan or dlmethylformami~e, preferably at ambient
temperature, for example, and under a hydrogen pressure of from 1
to 5 bar
The reaction of step (d) ls convenientl~ carrled out
with a correspon~lng hallde, sulphonic acid ester, sulphurlc acld
dlester or diazoalkane, e.g. with methyl lodide, dlmethyl sul-
phate, ethyl hromide, diethyl sulphate, propyl bromide, isopropylbromide, allyl bromide, ethyl p-toluenesulphonate, isopropyl-
methanesulphonate or diazomethane, optionally ln the presence of a
base such as sodium hydrlde, potassium carbonate, sodium hydrox-
lde, potassium tert.butoxlde or triethylamine ln a sultable sol-
vent such as acetone, dlethylether, tetrahydrofuran, dloxan or
dimethylformamlde at temperatures of bet~een 0 and 100C, pre-
ferably at temperatures of between 20 and 50C.
If in a compound of formula VI R3 represents a carboxy
group and/or W ~epresents a carboxy, carboxy-methyl, 2-carboxy-
~0 ethyl or 2-carboxy-ethenyl group, thls compound may slmultaneously
~e converted ln-to the correspondlng ester compound. A compound
thus obtalned ln step (d) is, lf necessary by cleavlng the ester
group, converted lnto the desired compound of formula I.
The cleavlng of the ester group may be carried out
hydrolytically, conveniently elther ln the presence of an acid
such as hydrochloric, sulphuric, phosphorlc Gr trichloroacetlc
acld or ln the presence oE a base such as sodium hydroxide or
potasslum
,, .
132~723
26 -
hydroxide in a suitable solvent such as water,
methanol, methanol/water, ethanol, ethanol/water,
water/isopropanol or water/dioxan at temperatures
of between -10 and 120C, e.g. at temperatures
of between ambient temperature and the boilinq
point of the reaction mixture.
The reaction of step (e) is conveniently carried
out in a suitable solvent such as dimethylsulphoxide
or dimethylformamide at temperatures of between
0 and 100C, preferably at temperatures of between
20 and 50C, or in a two-phase system such as methylene
chloride/water in the presence of a phase transfer
catalyst such as benzyl-tributyl-ammonium chloride
at temperatures of between 0 and lOO~C, preferably
at temperatures of between 20 and 50C.
The dehydration of step (f) may be carried out
with a water-cleaving agent such as phosphorus
pentoxide, phosphorus oxychloride, triphenylphosphine/
carbon ~etrachloride or p-toluenesulphonic acid
chloride, optionally in a solvent such as methylene
chloride, acetonitrile or pyridine at temperatures
of between 0 and 100C, preferably at temperatures
of between 20C and 80C.
The reaction of step (g) is convenienlly carried
out in a solvent such as diethylether, tetrahydrofuran,
L,2-dimethoxyethane, dioxan, dimethylformamide,
toluene or pyridine in the presence of a base as
condensation agent such as sodium carbonate, sodium
hydride, potassium tert.butoxide or pi.peridine
3C at temperatures of between 0 and 100C, Dreferably
at temperatures of between 20 and 80C.
The optional subsequent hydrolysis and/or decarboxylation
in step (g) is conveniently carr;ed out either
~:3 2 ~
- 27 -
in the presence of an acid such as hydrochloric,
sulphuric, phosphoric or trichloroacetic acid or
in the presence of a base such as sodium hydroxide
or potassium hydroxide in a su;table solvent such
as water, water/methanol, ethanol, water/ethanol,
water/isopropanol or water/dioxan at temperatures
of between -10C and 120C, e.g. at temperatures
of between ambient temperature and the boiling
temperature of the reaction mixture.
The reduction of step (h) is preferably carried
out in a suitable solvent such as methanol, ethanol,
isopropanol, ethyl acetate, dioxan, tetrahydrofuran,
dimethylformamide, benzene or benzene/ethanol with
hydrogen in the presence of a suitable hydrogenation
catalyst such as palladium/charcoal, Raney nickel
or tris-[triphenylphosphine]-rhodium(I)chloride
at temperatures of between 0 and 100C, but preferably
at temperatures of between 20C and 50C, under
a hydrogen pressure of from 1 to 5 bar or, if ~"
contains a cyano group, wi~h nascent hydrogen,
e.g. with magnesium/methanol, or with a copper
hydride complex, e.g. with the complex prepared
from copper bromide, sodium bis(2-methoxyethoxy)-
aluminium hydride and sec.butanol, at temperatures
of between -78 and 50C. Other qrouPs may be reduced
at the same time, e.g. a benzyloxy gro~p may be
reduced to the hydroxy group, an alkenyl or alkynyl
sroup may be reduced to the corresponding alkyl
group or a formyl group may be reduced to the hydroxy-
methyl group, or they may be repIaced by hydrogenatoms, e.g. a halogen atom may be replaced by a
hydrogen atom.
The reaction of step (i) is conveniently carried
out with a corresponding halide, anhydride, sulphonic
acid ester, sulph~ric acid diester or diazoalkane,
132~723
- 28 -
e.g. w;th methyl iodide, dimethyl sulphate, ethyl
iodide, diethyl sulphate, n-propyl iodide, isopropyl
bromide, acetyl chloride, acetic hydride, propionic
acid chloride, propionic acid anhydride, ethyl
p--toluenesulphonate or isopropylmethanesulphonate,
optionally in the presence of a base such as sodium
hydride, potassium carbonate, sodium hydroxide,
potassium tert.butoxide or triethylamine, or with
diazomethane, optionally in the presence of a I,ewis
acid, e.g. boron trifluoride, prererably in a
suitable solvent such as acetone, diethylether,
tetrahydrofuran, dioxan, pyridine or dimethylformamide
at temperatures of between 0 and 100C, preferably
at temperatures of between 20 and 50C, whilst
an anhydride used as the acylating agent may simul-
taneously also be used as solvent.
If in a compound of formula XIII W represents a
carboxy, carboxymethyl, 2-carboxy-ethyl or 2-carboxy-
ethenyl group and/or R4 represents a hydrogen atom,
this may simultaneously be converted into the correspon-
ding ester and/or ether compound.
An example of a reactive derivative of a compound
of formula XV used in step (k) is the imidazolide
thereof.
The reaction is of step (k) conveniently carried
out in the corresponding alcohol as solvent or
in a suitable solvent such as methylene chloride,
chloroform, ether, tetrahydrofuran, dioxan, dimethyl-
ormamide, benzene or toluene, optionally in the
presence of an acid-acti~ating agent or a dehydrating
agent, e.g. in the presence of hydrogen chloride,
sulphuric acid, ethyl chloroformate, thionyl chloride,
carbon tetrachloride/triphenylphosphine, carbonyldi-
imidazole or N,N'-dicyclohexylcarboAiimiAe or the
isourea ethers thereof, optionally in the presence
132~ ~23
- 29 -
of a reaction accelerator such as copper chlorideand optionally in the presence of an inorganic
base such as potass;.um carbonate or a tert.iary
organic base such as triethylamine, 1,8-diazabicyclo-
L5,4,0]undec-7-ene or pyridine, or by transesterification,
e.g. with a corresponding carbonic acid diester,
at temperatures of between ~20C and 100C, but
preferably at temperatures of between -10C and
the boiling temperature of the solvent used.
The optional subsequent hyd:rogenolysis in step
(k) may be carried out in the presence of a hydrogenation
catalyst such as palladium/charcoal in a suitable
solvent such as methanol, ethanol, ethanol/water,
glacial acet.ic acid, ethyl acetate, dioxan or dimethyl-
formamide.
If W in a compound of formula XV contains a carboxygroup, this may be converted during the reaction
of step (k) into the corresponding alkoxycarbonyl
group.
The reaction of step (1) may be carried out in
the presence of a strong acid which may simultaneously
serve as solvent, preferably in concentrated sulfuric
acid, at temperatures of between 0 and 150C, preferably
at t.emperatures of between 20 and 100C.
If in a compound oE general formula XVIII R4 represents
an allyl group, this may be split off during the
reaction or after the reaction by the addition
of water.
The reduction of step (m) is preferably carried
out with hydrogen in the presence of a hydrogenat;on
catalyst such as palladium/charcoal or Raney-nickel
in a suitable solvent such as methanol, ethanol,
'
~ 32~723
- 30 -
isopropanol, ethyl acetate, clloxan, tetrahydrofuran,
dimethylformamide, benzene or benzene/ethanol at
temperatures of between 0 and lOO~C, preferably
at temperatures of between 20 and 50C, and under
a hydrogen pressure of from 1 to 5 bar. ~hen a
suitable chiral hydrogenation catalyst is used,
such as a metal ligand compLex e.g. [(2S),(4S)-
l-tert.butoxycarbonyl-4~diphenylphosphino-2-diphenyl-
phosphinomethyl-pyrrolidine--rhodium-cyclooctadiene(1,5)]-
perchlorate, the addition of hydrogen occurs enantio-
selectively. Moreover, in the catalytic hydrogenation,
other groups may also be reduced, e.g. a benzyloxy
group may be reduced to the hydroxy group or a
formyl group may be reduced to the hydroxy methyl
group, or they may be replaced by hydrogen atoms,
e.g. a halogen atom may be replaced by a hydrogen
atom.
If according to the invention a racemic compound
of formu'a I is obtained wherein R3 has the meanings
given hereinbefore with the exception of the hydrogen
atom, this compound may be resolved into the enantiomers
thereof via the diastereomeric adducts, complexes,
salts or derivatives thereof.
The subsequent racemate splitting is preferably
carried out by column or ~PL chromatography by
forming diastereomeric adducts or complexes in
a chiral phase.
The compounds of formula I obtained according
to the invention may also be converted into the
salts thereof and, for pharmaceutical use, into
the physiologically acceptable salts thereof with
inorganic or organic acids or bases. Suitable
acids include, ~or example, hydrochloric, hydrobromic,
sulfuric, phosphoric, lactic, citrlc, tartaric,
-`- 13~7~
- 31 -
succinic, maleic, ~umaric, aspartic and glutamic
acid and suitable bases include sodium hydroxide,
potassium hydroxide, calcium hydroxide, cyclohexylamine,
ethanolamine, diethanolamine, triethanolamine,
S ethylenediamine, lysine and arginine.
The compounds of formulae tI to XIX used as starting
materials are known from the literature in some
cases or may be obtained by methods known ~ se.
Thus, for example, a compound of formula II may
be obtained by reducing a corresponding nitrile
with lithium aluminium hydride or with catalytically
activated hydrogen, by reacting a corres~onding
nitrile with a corresponding grignard or lithium
compound and subsequent lithium-alumini~m hydride
reduction or subsequent hydrolysis to form ~he
ketimine which is subsequently reduced with catalyti-
cally activated hydrogen, with a complex metal
hydride or with nascent hydrogen, by hydrolysis
or by hydrazinolysis of a corresponding phthalimido
compound, by reacting a corresponding ketone with
ammonium formate and subsequent hydrolysis or with
an ammonium salt in the presence of sodium cyanoboro-
hydride, by reduction of a corresponding oxime
with lithium aluminium hydride, with catalytically
activated or nascent hydrogen, by reduction of
a corresponding N-benzyl or N-l-phenylethyl Schiff's
base e.g. with a complex metal hydride in ether
or tetrahydrofuran at temperatures of between -78C
and the boilinq temperature of the solvent used
with subsequent splitting off of the benzyl or
l-phenylethyl group by catalytic hydrogenation,
by lithiation of a corresponding benzylideneimino-
benzyl compound, e.g. by means of lithium-diisopropyl-
amide at temperatures of between -7~ and 20C,
subsequent reaction with a corresponding halogen
132~72~
- 32 -
compound, e.g. with a corresponding bromoalkyl,
bromoalkenyl or bromoalkynyl compound, and subsequent
hydrolysis, by Ritter reaction of a corresponding
alcohol with potassium cyanide in sulfuric acid,
by Hofmann, Curtius, Lossen or Schmidt degradation
of a corresponding compound or by converting a
corresponding benzaldehyde into a corresponding
glycine derivative, e.g. using sodium cyanide~ammonium
carbonate in ethanol/water into a corresponding
hydantoin derivative, hydrolysis thereof, and,
if necessary, subsequent esterification and, if
necessary, subsequent reduction, e.g. with a complex
metal hydride in ether or tetrahydrofuran.
An amine of formula II thus obtained having a
chiral centre can be resolved into its enantiomers
by racemate splitting, e.g. by fractional crystalli-
sation of the diastereomeric salts with optically
active acids and subsequent decomposition o~ the
salts or by column or ~PL chromatograpy, optionally
in the form of the acyl derivative thereof, or
by forming dias~eromeric compounds, separating
them and subse~uently splitting them.
Moreover, an op~ically active amine of ~ormula
II may also be prepared by enantioselective reduction
of a corresponding ketimine using comple~ boron
or aluminium hydrides in which some of the hydride
hydrogen atoms have been replaced by optically
active alkoxide groups, or by means of hydrogen
in the presence o~ a suitable chiral hydrogenation
catalyst or anaIogously starting from a corresponding
N-benzyl or N~ phenethyl)-ketimine or from a
corresponding N-acyl-ketimine or enimide and optionally
subsequently splitt;ng off the benzyl, l-phenethyl
or acyl group.
132~723
- 33 -
Furthermore, an optically active amine of Eormula
~I may also be prepared by diastereoselective reduction
of a corresponding ketimine or hydrazone substituted
at the nitrogen atom with a chiral group, using
a complex or non-complex boron or aluminium hydride
in which some of the hydride hydrogens may optionally
be replaced by corresponding alkoxide, phenoxide
or alkyl groups or using hydrogen in the presence
of a suitable hydrogenation catalyst optionally
with subsequent splitting off of the chiral auxiliary
group by catalytic hydrogenolysis or hydrolysis.
Moreover, an optically active amine of formula
II may also be prepared by diastereo-selective
addition of a corresponding organometallic compound,
preferably a grignard or lithium compound, to a
corresponding aldimine substituted with a chiral
group at the nitrogen atom~ by subsequent hydrolysis
and optionally subsequent splitting off of the
chiral auxiliary group by catalytic hydrogenolysis
or hydrolysis.
The compounds of formulae IV, V, VI, VIII, IX,
X, XII, XIII and XV used as starting materials
may be obtained by reacting a corresponding amine
with a suitable carboxylic acid or a reactive derivati~e
thereof and, if necessary, subsequently splitting
off any protecting group used.
A compound of formula XVII used as starting material
may be obtained by reducing a corresponding carbonyl
compound, by reacting a corresponding carbonyl
compound with a corresponding grignard or lithium
reagent or by hydrolysis or alcoholysis of a corresponding
cyanohydrin and, if necessary, subsequent esterification.
A compound of ~ormula YIX used as starting material
may be obtained by acylating a corresponding ketimine
7 ~ 3
- 34 -
or the organometallic complex thereof with a corres-
ponding carboxylic acid or a reactive derivative
thereof, optionally with tautomerisation.
As already mentioned hereinbefore, the new compounds
of formula I and the physiologically active acceptable
~alts thereof have valuable pharmacological properties,
name~y an effect on the intermediate metabolism,
but particular a hypoglycaemic effect.
For example, the following compounds:O A = [2-ethoxy-4-~N-(1-(2-piperidino-phenyl)-1-
butyl)-aminocarbonylmethyl]-phenyl]-acetonitrile,
B = [2-ethoxy-4-[N-(~-phenyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-phenyl]acetonitrile,
C = ethyl 2-ethoxy-4-[N-(~-cyclohexylmethyl-2-
15piperidino-benzyl)-aminocarbonylmethyl]-benzoate,
D = 2-ethoxy-4-[N-(a-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid,
E = ethyl 2-ethoxy-4-[N-(a-ethoxycarbonyl-2-piper-
idinobenzyl)-aminocarbonylmethyl~-benzoate,
F = 2-ethoxy-4-(N-ta-ethoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate,
G = benzyl 2-ethoxy-4-[N-(a-methoxycarbonyl-2-
piperidino-benzyl)-aminocarbonylmethyl]-benzoate,
~ = 2-ethoxy-4-lN-(a-methoxycarbonyl-2-piperidino-
25benzyl)-aminocarbonylmethyl]benæoic acid,
= 2-ethoxy-4-[N-(a-isopropoxycarbonyl-2-piperidino-
benzyl~-aminocarbonylmethyl~-benzoic acid,
7 ~ 3
- 35 -
K = 2-ethoxy-4-1N-(~-cyclopropylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid,
L - 2-ethoxy-4-~N-(~-(tetrahydrofuran-2-yl-methyl)-
2-piperidino-benzyl~-aminocarbonylmethyl~-
benzoic acid,
M = 2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-
buten-l-yl)-aminocarbonylmethyl]-benzoic
acid,
N = 2-ethoxy-4-[~ (2-piperidino-phenyl)-3-
methyl-3-buten-1-yl)-clminocarbonylmethyl]-
benzoic acid~ and
o = 2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-
butyn-l-yl)-aminocarbonylmethyl~benzoic acid
were investigated for their hypoglycaemic properties
as follows:
~ypoglycaemic activity
The hypoglycaemic activity of the test substances
was investigated in female rats bred by the company
weighing from 180-220 g and kept without food for
24 hours before the start of the test. The test
substances were suspended in 1.5% methylcellulose
immediately before the start of the test and administered
by oesophageal tube.
slood samples were taken immediately before administration
of the test substance and 1, 2, 3 and 4 hours afterwards
from the retroorbital venus plexus ;n each case.
50 mlcrolltre batches were deproteinated with
0.5 ml of 0.33 ~ perchloric acid and centr;fuged.
Glucose in the supernatant was determined by the
132~3~23
- 36 -
hexokinase method using an analytical photometer.
Statistical evaluation was carried out using the
Student's t-test with p = 0.05 as the limit of
significance.
The following table contains the values found in
percentages relative to the control:
Sub- 10 mg/kg 1 mg/kg 0.5 mg/kg
stance 1 2 3 4h 1 2 3 4h 12
3 4h
_
A -50 -52 -43 -41 -29 -37 -35 -34
B -12 -10 -14 -14
C -16 -14 -17 -16
15 D -22 -47 -45 -45
E -40 -42 -32 -19
F -33 -17 n.s n.s.
G -40 -38 -36 -23
H -42 -35 -28 -18
20 I -36 -21 -18 n.s.
K -45 -45 -36 -36
L -46 -25 -13 -10
M -42 -39 -28 -35
N -44 -41 -31 -28
25 O -33 -18 -11 n.s.
(n.s. = statistically not significant)
In tests on the substances for their hypoglycaemic
activity, no toxic side effects were observed even
at a dosage of 10 mg/kg p.o.
The new compounds are v;rtually non-toxic; for
example, after a single dose of 500 mg~kg p.o.
132~123
- 37 -
(suspension in 1~ methycellulose~ of substanees
D and F to 3 male and 3 female mice, none of the
animals died in the observation period of 14 days.
In view of their pharmacological properties, the
compounds of formula I prepared aceording to the
invention and the physiologically acceptable salts
thereof are suitable for the treatment of diabetes
mellitus. For this purpose, they may for example
be ineorporated in conventional galenic preparations
~0 sueh as tablets, eoated tablets, eapsules, powders
or suspensions, optionally eombined with other
aetive substanees. The single dose in adults is
eonveniently from 1 to 50 mg, preferably 2.5 to
20 mg, onee or twiee a day.
Thus, aeeording to a further aspeet of the invention,
we provide a pharmaeeutieal eomposition eomprising
a eompound o~ formula I as elaimed in any one of
elaims 1 to 8 or a physiologieally aeeeptable salt
thereof together with at least one pharmaeeutical
carrier or excipient.
According to a still further aspeet of the invention,
we provide a method of treatment of the human or
non-human animal body to combat diabetes mellitus
which comprises administering to said body a compound
of formula I or a physiologically acceptable salt
thereof.
Aecording to a yet further aspect the present invention
provides the use of a eompound of formula I or
a physiologically acceptable salt thereof for the
manufacture of a therapeutic agent for use in a
method of treatment of the human or non-human animal
body to combat diabetes mellitus.
132~723
- 38 -
The Examples wh;ch ~ollow are intended to illustrate
the invention in a non-limiting ~ashion. Percentages,
parts and ratios are by weight unless otherwise
speciEied:
132~723
- 39 -
Example 1
[2-Ethoxy-4-[N-(l-(2-piperidino-pheny~ -butyl)
amino~ lmethyl~-phenyl~-acetonitrile
To a solution of 2 g (4.5 mmol) of 2-ethoxy-4-[N-
(1-(2-piper;dino-phenyl)-1-butyl)-aminocarbonylmethyl]-
benzyl chloride [prepared from 2-ethoxy-4-rN-(l-
(2-piperidino-phenyl)-1-butyl)-aminocarbonylmethyl]-
benzyl alcohol with thionyl chloride in chloroform],
are addea 0.255 g (5.2 mmol) of sod;um cyanide,
dissolved in 2.2 ml of water, and 0.069 g (0.22 mmol)
of the phase transfer catalyst benzyl tributylammonium
chloride and the mixture is stirred for 5 days
at ambient temperature. Then a further 0.069 g
of the phase transfer catalyst are added, together
with a few small grains of potassium iodide and
0.2 g of sodium cyanide in 1 ml of water and the
mixture is stirred for a further 24 hours; then
the same amounts of these three components are
added again and the mixture is stirred for a further
12 hours. 40 ml of methylene chloride are added
and the mixture is extracted twice with water.
The methylene chloride phase is dried over sodium
sulphate/potassium carbonate, filtered and concentrated
by evaporation ln vacuo. The evaporation residue
is purified by column chromatography on silica
gel (toluenejethyl acetate = 5/1~.
Yield: 1.53 g (78~ of theory),
Melting point: 116-118C (methylene chloride/ether)
Calculated: C 74.79 H 8.14 N 9.69
30 Found: 74.86 8.19 9.42
~ 3 ~ ~ 7 2-~
- 40 -
Example 2
E 2-Ethoxy-4-[N-(~-phenyl-2-piperidino-benzyl)-
am;nocarbonylmethyl]-phenyl]-acetonitrile
To 0.32 g (6.5 mmol) of sodium cyanide in 40 ml
of dimethylsulphoxide, a solution of 2.6 9 (5.45 mmol)
of 2-ethoxy-4-[N-(~-phenyl-2-piperidlno-benzyl)-
aminocarbonylmethyl]-benzyl chloride in 10 ml
of dimethylsulphoxide is added dropwise at 50-60C.
The mixture is then stirred ~or 5 hours at 60C,
added to water and extracted with chloroform.
The extract is concentrated by evaporation in
vacuo. The residue is purified by column chromato-
graphy on silica gel (toluene/ethyl acetate
5/1).
Yield: 1.2 g (47% of theory),
Melting point: 145-148C
Calculated: C 77.05 ~ 7.11 N 8.99
Found: 76.92 7.05 8.78
Rxample 3
Ethyl [2-ethoxy-4-[N~ (2-piperidino-phenYl)-
l-butyl)-aminocarbonylmethylJ-phenyl]-acetate
Dry hydrogen chloride is introduced for 3 hours
into a stirred and boiling solution of 1.3 g (3 mmo]~
of [2-ethoxy-4-[N-tl-~2-piperidino-phenyl)-1-butyl)-
aminocarbonylmethyl]-phenyl~-acetonitrile in 30 ml
of absolute ethanol. The mixture is then evaporated
down in vacuo, 25 ml of water are added to the
evaporation residue and this is stirred for 15
minutes at 50C. The mixture is adjusted to a
p~ of 7 by the addition of solid sodium hydrogen
carbonate and is extracted three times with ethyl
acetate. The combined organic extracts are shaken
once with water, then dried over sodium sulphate/potassium
~32~723
- 41 -
carbonate, ~iltered and concentrated by evaporat;on
in vacuo. The evaporat;on residue is purified
by column chromatography on silica gel (chloroform/ethyl
acetate = 9/1).
Yiel~: 1.0 g (69~ of theory),
Melting point: 91-93C (petroleum ether)
Calculated: C 72.47 H 8.39 N 5.83
Found: 72.73 8.68 5.71
Example 4
Methyl E2-ethoxy-4-[N-(~-phenyl-2-piperi_lno-benzyl)-
aminocarbonylmethyll-phenyl~-acetatè
Dry hydrogen chloride is introduced for 4 hours
into a stirred and refluxed solution of 1.2 9 (2.57 mmol~
of [2-ethoxy-4~(N-(~-phenyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-phenyl]-acetonitrile in 20 ml
of methanol. The mixture is then concentrated
by evaporation, added to water and extracted with
chloroform. The extract is dried and filtered
and concentrated by evaporation in vacuo. The
evaporation residue is puriEied by column chromatography
on silica gel (toluene/ethyl acetate = 4/1).
Yield: 340 mg (26% of theory),
Melting point: 136-138C (acetonitrile/water)
Calculated: molecular peak m/e = 500
Found: molecular peak m/e = 500
Example 5
[2-~thoxy-4-EN-(1-(2-piperidino-phenyl)-1-butyl-
aminocarbonylmethyl]-phenyl]-acetic acid
2.8 ml of lN sodium hydroxide solution are added
to 0.67 g (1.4 mmol) of ethyl ~2-ethoxy-4-[N-(l-
(2-piperidino-phenyl)-1-butyl-aminocarbonylmethyl~-
~2~23
~ 42 -
phenyl]-acetate in 10 ml of ethanol and stirred
for 4 hours at ambient temperature. Then the mixture
is evaporated down in vacuo at 50C. Water and
a few drops of methanol are added to the evaporation
residue which is then adjusted to pH 6 with lN
acetic acid. It is cooled in ice, whereupon a
precipitate is formed. This is filtered off and
recrystallised from ethanol.
Yield: 0.47 g (74~ of theory),
Melting point: 158-159C (ethanol)
Calculated: C 71.65 F~ 8.02 N 6.19
Found: 71.35 8.30 6.21
Example 6
[2-Ethoxy-4-[N-(a-phenyl-2-piperidino~benzyl)-aminocarbonyl-
methyl]-~enyl]-acetic acid
Prepared analogously to Example 5 by alkaline saponification
of methyl [2-ethoxy-4-[N-(~-phenyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-phenyl]-acetate.
Yield: 45Q of theory,
Melting point: i46-148C
Calculated~ C 74.05 H 7.04 N 5.76
Found: 73.70 7.00 5.85
Example 7
Ethyl 2-ethoxy-4-~N-(1-(2-~_peridino-phenyl)-1-
butyl)-aminocarbonylmethyl]-cinnamate
A solution of 1.68 g (7.5 mmol) of ethyl diethyl-
phosphono-acetate in 3 ml of absolute dioxan is
slowly added dropwise, with vigoro~s stirring,
to a suspension of 0.327 g (7.5 mmol) of 55~ sodi~m
hydride (in oil) in 4 ml of absol~te dioxan. After
the reaction has ~ied d~wn the m;xt~re is heated
~32~3~
- 43 -
to 80C eor a further 45 minutes. It is then cooled
to ambient temperature, a solution of 2.11 g t5 mmol)
of 2-ethoxy-4-[~-(1-(2-p;peridino-phenyl)-1-butyl)-
aminocarbonylmethyl]-benzaldehyde [prepared from
the corresponding benzyl alcohol by oxidation with
pyridinium chlorochromate in chloroform] in 4 ml
of absolute dioxan is added dropwise thereto and
the mixture is heated for 2 hours at 50C. The
reaction mixture is poured onto ice/water and extracted
with chloroform. The organic extract is dried
and filtered and evaporated down in vacuo. The
evaporation residue is purified by column chromatography
on silica gel (chloroorm/ethyl acetate = 19/1).
Yield: 1.64 g (66.7~ of theory),
Melting point: 130-131C (ether)
Calculated: C 73.14 H 8.18 N 5.69
Found: 73.36 8.34 5.75
Example 8
2-Ethoxy~4-[N~ (2-piperidino-phenyl?-1-bUtyl)-
aminocarbonylmethyl]-cinnamic acid nitrile
Prepared analogously to Example 7 from 2-ethoxy-
4-[N-(1-(2-piperidino-phenyl)-1-butyl)-aminocarbonylmethyl]-
benzaldehyde with diethylphosphono-acetonitrile.
Yield: 61% of theory,
Melting point: 125-128C (petroleum ether)
Calculated: C 75.47 H 7.92 N 9.43
Found: 75.40 7.95 9.24
Example 9
Ethyl 2-ethoxy-4-[N-(~-phenyl-2-piperidino-benzyl)-amino-
carbon~1-methyl]-cinnamate
Under a n;trogen atmosphere, O.l9 ~ (8 mmol) o~
~32~2~
- 44 -
50% sodium hydrlde is added to a st;rred solution
of 1.8 g t8 mmol) of ethyl diethylphosphono-acetate
in 10 ml of absolute 1,2-dimethoxy-ethane, Then
a solution of 2 g (4.4 mmol) of 2-ethoxy-4-[N-(a-
phenyl-2-piperidino-benzyl)~aminocarbonylmethyl~-
benzaldehyde in 15 ml of absolute 1,2-dimethoxy-
ethane is added and the mixture is stirred for
30 minutes at ambient temperature. It is concentrated
by evaporation _ vacuo and the evaporation residue
is distributed between water and chloroform. The
chloroform extract is dried and filtered and concentrated
by evaporation in vacuo. The evaporation residue
is purified by column chromatography on silica
gel (toluene/ethyl acetate = 5/1).
Yield: 0.37 9 (17% of theory),
Melting point: 111-113C (cyclohexane)
Calculated: C 75.26 H 7.27 N 5.32
Found: 75.14 7.32 5.25
Example 10
2-Ethoxy-4-[N-(1-~2-piperidino~phenyl)-1-bUtYl)-
aminocarbonylmeth~-cinnamic acid
A solution of 0.49 q (1 mmol) of ethyl 2-ethoxy-
4-~N-(1-(2-piperidino-phenyl)-1-~utyl)-aminocarbonyl-
methyl~-cinnamate ;n 10 ml of ethanol is stirred
together with 2 ml of lN sodium hydroxide solution
for 3 days at ambien~ temperature. The mixture
is then concentrated by evaporation in vacuo, water
and a few drops of methanol are added to the evaporation
residue and this is then ad~usted to pH 6 w;th
lN acetic acid. The precipitate is filtered off,
dried and recrystallised from ethyl acetate.
Yield: 0.37 g (79.7~ of theory),
Meltlng po;nt: 175-177C (decomp.)
Calculated: C 72.39 ~ 7.81 N 6.03
~3~ ~J~
- ~5 -
Found: 72.50 7.88 6.06
Example 11
2-Ethoxy-4-[N-(~-~henyl-2-piperidino-benzyl)-aminocarbonYl-
methyl]-cinnamic acid
330 mg (0.62 mmol~ of ethyl 2-ethoxy-4-[N-(a-phenyl-
2-piperidino-benzyl)-aminocarbonylmethyl~-cinnamate
are dissolved in 10 ml of ethanol and, after the
addi~ion of 4 ml of 4N sodium hydroxide solution,
stirred for 3 hours at 50C. Then the mixture
is neutralised ~ith 4 ml of 4N hydrochloric acid,
diluted with water and filtered off from the precipitate.
It is then recrystallised from aqueous ethanol.
Yield: 210 mg (67~ of theory),
Melting point: 181C
Calculated: C 74.67 H 6.87 N 5.62
Fou-nd: 74.72 6.76 5.42
Example 12
Ethyl 3-[2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-
l-butyl)-aminocarbonYlmethyl]-phenyl]-propionate
A solution of 0.54 g (1.1 mmol) of ethyl 2-ethoxy-
4-[N-~l-t2-piperidino-phenyl)-1-butyl)-am;nocarbonylmethyl~-
cinnamate in 15 ml of ethanol is hydrogenated for
1 hour at ambient temperature and under 3 bars
of hydrogen on 0.1 g of 10~ palladium/charcoal.
The mixture is filtered, concentrated by evaporation
in vacuo and the evaporat;on residue is crystallised
from petroleum ether.
Yield: 0.30 g (55~ of theory~,
Melting point: 71-73C
Calculated: C 72.84 ~ 8.56 ~ 5.66
35 Found: 73.19 8.54 5.70
~2~7~
- 46 -
~xample 13
Eth~l 3-[2-ethoxy-4-[N-(a-phenyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-phenyl]-propionate
Prepared analogously to Example 12 by catalytic
hydrogenation of ethyl 2-ethoxy-4-[N-(~-phenyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-cinnamate
and subsequent purificatlon by column chromatography
on silica gel (cyclohexane/ethyl acetate/methanol
= 6/1/0.5).
Yield: 48% of theory,
Melting point: 130C (ethanol/water)
Calculated: C 74.97 ~ 7.63 N 5.30
Found: 74.65 7.61 5.15
Example 14
3-[2 Ethoxy-4-[N-(1-~2-piperidino-phenyl)-1-butyl)-
aminocarbonylmethyl]-phenyl~-~ro~ionic acid
Prepared analogously to Example 12 by catalytic
hydrogenation of 2-ethoxy-4-[N-(1-(2-piperidino-
phenyl)-l-butyl)-aminocarbonylmethyl]-cinnamic
acid.
Yield: 41% of theory,
Melting point: 112-114C
Calculated: C 72.07 H 8.21 N 6.00
Found: 72.30 8.42 6.19
Example 15
3-[2-Ethoxy-4-[N-~1-(2-piper.idino-phenyl.)-1-butyl)-
aminocarbonylmethyl~-phenyl]~propionitrile
Prepared analogously to Example 12 by catalytic
hydrogenation of 2-ethoxy-4-[N~ (2-p;per;dino-
~ ~ 2 ~ 3
- 47 -
phenyl)-l-butyl~-aminocarbonylmethyl~-cinnamic
acid nitrile.
Yield: 34% of theory,
Melting point: 102-103C (petroleum ether~
Calculated: molecular peak m/e = 447
Found: molecular peak m/e = 447
Example 16
3-[2-Ethoxy-4~[N-(1-(2-piperidlno-phenyl)-1-butyl)-
aminocarbonylmethyl]-phenyl]-propionic acid
Prepared analogously to Example 5 by alkaline saponifi-
cation of ethyl 3-[2-ethoxy-4-[N~(1-(2-piperidino-
phenyl)-l-butyl)-aminocarbonylmethyl]-phenyl]-propionate
and subsequent purification by column chromatography
(chloroform/methanol = 9/1).
Yield: 80~ of theory,
Melting point: 112-115C (petroleum ether~
Calculated: C 72.07 H 8.21 N 6.00
Found: 72.40 8.21 6.03
Example 17
3-~2-Ethoxy-4-~N-(~-phenyl-2-piperidino-ben~yl)-
aminocarbonylmethyl~-phenyl]-pro~ionic acid
Prepared analogously to Example 5 by alkaline saponifi-
cation of ethyl 3-[2-ethoxy-4-[N-(~-phenyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-propi~nate.
Yield~ 70~ of theory,
Melting point: 74C
Calculated: C 74.37 ~ 7.25 ~ 5.60
Eound: 74.29 7.31 5.27
l32a723
- ~8 -
Example 18
3-[2-Ethoxy-4-[N-(1-(2-piperidino-pheny~ butyl)-
aminocarbonylmethyl]-phenyl~-propionitri.le
At ambient temperature, 45.8 mg (0.24 mmol) of
p-toluenesulphonic acid chloride are added to a
mixture of S6 mg (0.12 mmol) of 3-[2-ethoxy-4-[N-
(1-(2-piperidi.no-phenyl)-1-butyl)-aminocarbonylmethyl]-
phenyl]-propionic acid amide, melting point 153-155C
[prepared from the corresponding propionic acld
by reacting with carbonyldiimidazole and then with
ammonia in tetrahydrofuran] and 0.044 ml of absolute
pyridine. The mixture is stirred for 45 minutes
at 20C and for 2 hours at 50 to 60C. After cooling,
water is added, the mixture is made alkaline with
concentrated ammonia and extracted three times
with chloroform. The combined chloroform extracts
are washed with water, dried over sodium sulphate,
filtered an~ concentrated by evaporation in vacuo.
The evaporation residue is purified by column chromato-
graDhy on silica gel (chloroform/ethyl acetate
= 9/])-
Yield: 11 mg (20~ of theory),
Calculated: molecular peak m/e = 447
Found: molecular peak m/e = 447
Example 19
Ethyl 2-ethoxy-4-[N-(a-cyclohexylmethyl-2-Piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
To a solution of l.l3 g (3.96 mmol~ of a-cyclohexylmethyl-
2-piperidino-benzylamine ;n 11 ml of acetonitr;le
are added, successivelyr 1 g (3.96 mmol) of 3-ethoxy-
4-ethoxycarbonyl-phenyl acetic acid, 1.25 g (4.76 mmol)
of tr;phenylphosph;ne, 1..1.1 ml (7.92 mmol) of triethyl-
1~2~7~
- 4~ -
amine and 0.38 ml (3.96 mmol) of carbon tetrachloride
and the mixture is stirred for 15 hours at ambient
temperature. It is then concentrated by evaporation
in vacuo and partitioned between ethyl acetate
and water. The organic extract is dried and filtered
and concentrated by evaporation 1n vacuo. The
evaporation residue is purified by column chromatography
on silica gel (toluene/acetone = 10/1).
Yield: 1.4 g (68~ of theory),
Melting point: 95-97C (petroleum ether/cyclohexane =1/1)
Calculated: C 73.81 ~l 8.52 N 5.38
Found: 73.98 8.49 5.61
Example 20
Ethyl 2-ethoxy-4-[N-(~-benzyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoate
Prepared analogously to Example 19 from a-benzyl-
2-piperidino-benæylamine and 3-ethoxy-4-ethoxycarbonyl-
phenylacetic acid.
Yield: 72% of theory,
Melting point: 102-105C (petroleum ethee)
Calculated: C 74.68 H 7.44 N 5.44
Found: 74.73 7.68 5.39
Example 21
2-Ethoxy-4-[N~ cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoic acid
1.15 g (2.21 mmol~ of ethyl 2-ethoxy-4-[N-(~-cyclohexyl-
methyl-2-piperidino-benzyl)-aminocarbonylmethyl]-
benzoate in 12 ml of ethanol are stirred together
with 3.3 ml of lN sodium hydroxide solut;on for
2 hours at 50C. Then 3.3 ml of lN hydrochloric
acid are added and the mixt~re is cooled in ice.
132~723
- 50 -
The precipitate formed is filtered off, washed
with a little ice cold ethanol and dried in vacuo
at 100C.
Yield: 0.9 g (82~ of theory),
Melting point: 153-156~C
Calculated: ~ 73.14 H 8.18 N 5.69
Found: 73.30 8.175.66
Example 22
2-Ethoxy-4-[N-(a-benzyl-2-p~eeridino-benzyl)-aminocarbonyl
ethYl~-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethoxy-4-rN-(a-benzyl-
2 piperidino-benzyl)-aminocarbonylmethyl]-benzoate.
Yield: 90~ of theory,
Melting point: 100-105C
Calculated: C 74.05 H 7.04 N 5.76
Found: 73.77 7.iO5.50
Ex~ple 23
Ethyl ?-ethoxy-4-[N-(-ethoxycarbonyl-2-piperidino-
benzyl)-aminocarbonYlmethyl]-benzoate
To a mixture of 2 g ~5096 mmol) of (2-piperidino-
phenyl)-glycine-ethyl ester-dihydrochloride in
12 ml of acetonitrile are added successively 1.52 g
(5.96 mmolj of 3-ethoxy-4-ethoxycarbonyl-phenyl-
acetic acid, 1.77 g (6.75 mmol) of triphenylphosphine,
2.45 ml (17.9 mmol) of triethylamine and 0.57 ml
(5.96 mmol) of carbon tetrachloride and the mixture
is stirred overnight at ambient temperature. It
is then concentrated by evaporation 3 n vacuo and
partitioned between chloroform and water. The
organic extract is dr;ed and filtered and concentrated
~32~723
- 51 -
by evaporation in vacuo. The evaporation residue
-
is purified by column chromatography on silica
gel (toluene/acetone = 4/1).
Yield: 1.2 g (41% o~ theory),
Melting point: 100-103C (ether)
Calculated: C 67.72 H 7.31 N 5.64
Found: 67.87 7.46 5.61
Example 24
Benzyl 2-ethoxy-4-[N-(a-ethoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylme~hyl]-benzoate
Prepared analogously to Example 23 from (2-piperidino-
phenyl)-glycine-ethyl ester-dihydrochloride and
3-ethoxy-4-benzyloxycarbonyl-phenyl acetic acid.
Yield: 30~ of theory,
Melting point: 90-93C
Calculated: molecular peak m/e = 558
Found: molecular peak m/e = 558
Example 25
Benzyl 2-ethoxy-4-[N-(a-methoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylmethYl]-benzoate
Prepared analogously to Example 23 from (2-piperidino-
phenyl)-glycine-methyl ester-dihYdrochloride and
3-ethoxy-4-benzyloxycarbonyl-phenyl acetic acid.
Yield: 40~ of theory,
Melting point: 100-102C (ether)
Calculated: C 70.57 H 6.66 N 5.14
Found: 70.46 6.67 5.14
132~72~
- 52 -
_ample 26
Benzyl 2-ethoxy-4-~N-(~-propoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
Prepared analogously to Example 23 from (2-piperidino-
phenyl~-glycine-n-propylester-dihydrochloride and
3-ethoxy-4-benzyloxycarbonyl-phenylacetic acid.
Yield: 65% of theory,
Melting point: 100-102C (petroleum ether)
Calculated: C 71.31 H 7.04 N 4.89
Found: 71.62 7.01 4.97
Example 27
Benzyl 2-ethoxy-4-rN-(~-isopropoxycarbon~1-2~piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
Prepared analogously to Example 23 from (2-piperidino-
phenyl)-glycine-isopropylester-dihydrochloride
and 3-ethoxy-4-benzyloxycarbonyl-phenylacetic acid.
Yield: 52~ of theory,
Melting point: 85-88C (acetone/petroleum ether)
Calculated: C 71.31 ~ 7.04 N 4.89
Found: 71.64 7.10 4.77
Example 28
Ethyl 4-[N-(~-ethoxycarbonyl-2-piper;dino-benzyl)-
aminocarbonylmethyl]-2-hydroxy-benzoate
Prepared analogously to Example 23 from (2-piperidino-
phenyl)~glycine-ethylester-dihydrochloride and
4-ethoxycarbonyl-3-hydroxy-phenylacetic acid.
Yield: 55~ o~ theory,
Melting point: 107-110C (petroleum ether)
Calculated: C 66.65 ~ 6.88 N 5.98
~32~72~
- 53 -
Found: 66.60 6.86 6.03
Rxample 29
Ethyl 2-ethoxy-4-[N-(a-hydroxymethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
2.52 g (10 mmol) of 3-ethoxy-4-ethoxycarbonyl-phenylacetic
acid and 1.62 g (10 mmol) of ~,N'-carbonyldiimidazole
are heated to 70C for 45 minutes in 15 ml of absolute
~etrahydrofur~n. A solution of 2.07 g ~9.4 mmol)
of 2-hydroxy-1-(2-piperidino-phenyl)-1-ethylamine
[prepared by reducing (2-piperidino-phenyl)-glycine-
ethylester with lithium aluminium hydride in ether]
in 7 ml of absolute tetrahydrofuran is added thereto
and the mixture is refluxed for 1 hour. After
standing overnight it is diluted with 50 ml of
ethyl acetate and shaken twice with 30 ml of water.
The organic phase is dried and filtered and concentrated
by evaporation in vacuo. The evaporation residue
is purified by column chromatography on silica
gel (chloroform/methanol = 19/1).
Yield: 2.4 g (56% of theory),
Melting point: 127-128C ~acetone)
Calculated: C 68.70 ~ 7.54 N 6.16
Found: 68.80 7.58 6.15
Example 30
Benzyl 2-ethoxy-4- EN- (~-hYdroxymethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
Prepared analogously to Example 29 from 3-ethoxy
4-benzyloxycarbonyl-phenylacetic acid and 2-hydroxy-
1-(2-piperidino-phenyl)-1-ethylamine.
Yield: 52% of theory,
Melting point: 89-91C (acetone/ether)
-` 132~723
- 54 -
Calculated: C 72.07 H 7.02 N 5.42
Found: 72.10 7.155.29
~xample 31
2-Ethoxy-4-[N-(~-carboxy-2-piperidino-benzyl)-aminocarbonyl-
methyl]-benzoic acid
0.45 g (0.9 mmol) of ethyl 2-ethoxy-4-[N-(~-ethoxycarbonyl-
2~piperidino-benzyl)-aminocarbonylmethyl]-benzoate
in 5 ml of ethanol is stirred together with 2.7 ml
of lN sodium hydroxide solution for 2 hours at
50C. Then 2.7 ml of lN hydrochloric acid are
added and the mixture is concentrated by evaporation
in vacuo. The evaporation residue is partitioned
between water and chloroform. The combined chloroform
extracts are shaken once with water, then the organic
phase is driedl filtered and evaporated down in
vacuo. The evaporation residue is crystallised
with ether.
Yield: 0.27 g (69~ of theory),
Melting point: 222-225C (decomp.~
Calculated: C 65.44 H 6.41 N 6.36
Found: 65.58 6.59 6.28
Example 32
4-lN-(~-Carboxy-2-piperidino-benzyl)-aminocarbon~lmethyl]-
2-hydroxy-benzoic acid
Prepared analogously to Example 31 by alkaline
saponification of ethyl 4-[N-(~-ethoxycarbonyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-2-hydroxy-
benzoate.
Yield: 82% of theory,
Melting point: 220-228C
Calculate~: C 64.07 H 5.87 N 6.79
~2~2:~
- 55 -
Found: 63.84 5.95 7.l3
Exam~e 33
2-Ethoxy~4-[N~ hydroxymethyl-2~piperidino-benzyl)
aminocarbonylmethyl~-benzoic acid
Prepared analogously to Example 31 by alkaline
saponification of ethyl 2-ethoxy-4-[N-(~-hydroxymethyl-
2-piperidino-benzyl~-aminocarbonylmethyl¦-benzoate
and purification by column chromatography on silica
gel (chloroform/ethanol = 95/5).
Yield: 66~ of theory,
Melting point: 80-81C (decomp., sintering from
75o~)
Calculated: molecular peak m/e = 426
Found: molecular peak m/e = 426
Example 34
Ethyl 2-ethoxy-4-~N-(~-carboxy-2-piperidino-benZyl)-
aminocarbonylmethyl]-benzoate
0.45 g (0.9 mmol~ of ethyl 2-ethoxy-4-[N-(~-ethoxycarbonyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
in 5 ml of ethanol are stirred together with
0.90 ml of lN sodium hydroxide solution for 4 hours
at ambien-t temperature. Then 0.90 ml of lN hydrochloric
acid are added and the mixture is evaporated down
in vacuoO The residue is partitioned between water
and chloroform, the chloroform solution is dried
and filtered and concentrated by evaporation in
vacuo. The evaporation residue is purified by
column chromatography on silica gel (chloroform/ethanol
= 5/1~-
Yield: 0.23 g (54~ of theory),
Melting poin~: 177-180C (ether)
-` ~32~72~
- 56 -
Calculated: C 66.65 H 6.88 N 5.98
Found: 66.65 7.11 5.79
Example 35
Ethyl 4 [N-(~-carboxy-2-piperidino-benzyl~-aminocarbonyl-
methyl]-2-hydroxy-benzoate
Prepared analogously to Example 34 by alkaline
saponi~ication of ethyl 4-[N-(~-ethoxycarbonyl
2-piperidino-benzyl)-aminocarbonylmethyl]-2-hydroxy-
benzoate.
Yield: 58~ of theory,
Melting point: 156-159C (ether)
Calculated: C 65.44 H 6.41 N 6.36
Found: 65.66 6~38 6.33
Example 36
Benzyl 2-ethoxy-4-[N-(a-carboxy-2-piperidino-benzyl)-
aminocarbonYlmethyl]-benzoate
Prepared analogously to Example 34 by alkaline
saponification of benzyl 2-ethoxy-4-[N-(~-methoxycarbonyl
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
in dioxan.
Yield: 48% of theory,
Melting point: 140-142C
Calculated: C 70.17 H 6.46 N 5.28
Found. 70.21 6.50 5.31
Example 37
Ethyl 2-ethoxy-4-[N-(~-acetoxymethyl-2-piperidino-
benzyl~-aminocarbonyLmethYl~-benzoate
To a solution of 0.227 q (0.5 mmol~ of ethyl 2-
132~723
- 57 -
ethoxy-4-[N-(~-hydroxymethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoate and 0.126 ml (0.9 mmol)
of absolute triethylamine in 3 ml of absolute chloroform,
a solution of 0.063 ml (0.9 mmol) of acetyl chloride
in 1 ml of absolute chloroform is added dropwise.
After 4 days' stirring at ambient temperature the
mixture is diluted with chloroform, washed with
dilute aqueous sodium bicarbonate solution, the
chloroform solution is dried and filtered and evaporatea
down in vacuo. rhe evaporation residue is purif-ied
by column chromatography on silica yel (chlo~oform/acetone
= 4/1)-
Yield: 0.17 g (68~ of theory),
Melting point: 107-109C (ether/petroleum ether~
Calculated: C 67.72 H 7.31 N 5.64
Found: 67.70 7.48 5.74
Example 38
Benzyl 2-ethoxy-4-rN-(~-acetoxymethyl-2-piperidino-
benzyl)-aminocarbonylme~hyl]-benzoate
Prepared analogously to Example 37 from benzyl
2-ethoxy-4-~N~ hydroxymethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoate with acetyl chloride.
Yield: 93~ of theory,
Calculate~: molecular peak m/e = 558
Found: molecular peak m/e = 558
Example 39
Benzyl 2-ethoxy-4~[N-(~-pro~ionYloxYmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
Prepared analogously to Example 37 from benzyl
2-ethoxy-4-[N-(~-hydroxymethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoate with propionyl chloride.
~32~23
~ 58
Yield: 85~ of theory,
Melting point: 73-74C
Calculated: C 71.31 ~J 7.04 N 4.89
Found: 71.20 7.10 4.61
s
Example 40
2-Ethoxy-4-[N-(~-ethoxycarbonyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoic acid
A soluti-on of 0.140 9 (0.25 mmol) of benzyl 2-ethoxy-
4-[N~ ethoxycarbonyl~2-piperidino-benzyl)~aminocarbQ~yl-
methyl]-benzoate in 1.4 ml of ethanol is hydrogen~a-ted
with O . 03 g of 10% palladium/charcoal ~or 4.5 hours
at 50C under 5 bar of hydrogen. The mixture is
lS filtered, evaporated down in vacuo and the evaporation
residue is purified by column chromatography on
silica gel (chloroform/methanol = 10/1).
Yield: 0.041 g (35~ of theory)~
Melting point: 115-118C lpetroleum etherJ
Calc~lated: molecular peak m/e = 468
Found: molecular peak m/e = 468
Example 41
2-Ethoxy-4-[N- ~-me~hoxycarbonyl-2-~iperidino-benzyl)-
aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 40 by cataly~ic
hydrogenation of benzyl 2-ethoxy-4-[N-~-methoxyc~rbonyl-
2-piperidino-benzyl)-aminocarbonylme~hyl~-benzoate
in methanol.
Yield: 69-~ of theory,
Melting point: 147-I50C tdecomp.) (ether)
Calculated: C 66.06 ~ 6.65 N 6.16
Founa: 66.28 6.56 5.90
132~723
- 59 -
Example 42
2-Ethoxy-4-[N-(a-n-propoxycarbonyl-2-piperidino-
benzyl)-aminocarbonYlmethyl]-benzoic_acid
Prepared analogously to Example 40 by catalytic
hydrogenation of benzyl 2-ethoxy-4-~N-(~-n-propoxycarbonyl-
2-piperidino-benzyl)-aminocarbonylmethyl~-benzoate
in n-propanol.
Yield: 74% of theory,
Melting point: 122-125C (lether/petroleum ether = 1/1)
Calculatea: C 67~20 ~ 7~10 ~ 5O80
Found: 67.39 7.24 5.78
2-Ethox~-4-~N-(a-isopropox~car~onyl-2-piperidi~o-
benzyl -aminocarbonylme-thyl~-benzoic ac-id
Prepared analog~usly to Exam~le 4Q by cataIytic
hydrogenation cf benzyl 2-ethoxy-4-[N-(a-isopropoxycarbonyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
in isopropanol.
Yield: 72% of theory,
MeltIng point: 149-151C (acetone/petroleum ether)
C~lculated: C 67.20 ~ 7.10 N 5.80
Found: 67.50 6.99 5.78
~xample 44
~-EthGxy-4-[N-t~-aceto~ymethyl-?-pi~eriaino benzyl)-
aminocarbony methyl]-benzoic acid
Prepared ~nalogously to Example 40 by catalytic
hydrogenation of benæyl 2-ethoxy-4-~N-(~-acetoxymethyl-
2-piperidino-benzyI~-aminocarbonylmethyl]-benzoate
in ethanol.
132~72~
- 60 -
Yield: 42% of theoryr
Calculated: molecular peak m/e = 468
Found: molecular peak m/e = 468
Example 45
2-Et xy-4-EN-(~-propionylox~_ethyl-2-piperidino-
benzyl?-aminocarbonylmethyl]-benzoic acid
Prepared analogously to Ex~mple 4Q by catalytic
hydrogenation of benzyl 2-ethoxy-4-~N-(a-propionyloxymethyl-
2-piperidino-benzyl)-aminocarbonylmet~yl~-benzoate
in ethanol.
Yield: 53% of theory,
Melting point: 64-67C (ethanol/water)
Calculated: molecular peak m/e = 482
Found: molecular peak m/e - 482
Example_46
2-Hydroxy-4-EN-(~ ropox~carbonx--2-pi~e~idin
benzyl)-aminoc _bonylmethyl]-benzoic acid
To a stirred solution of 0.2-0 g (0.414 mmol) of
2-etho~y-4-E~ isopropoxycarbonyl-2-plperidino-
benzyl)-aminocarbonylmethyl]-ben~oic acid in S ml
of 1,2-di_h-loroethane, 0.04 ml (0.414 mmol) of
boron tribromide are added at -20-C with the exclusion
of moisture. The mixture is allowed to come up
to ambient temperature and is then stirred for
2 hours. It is poured into isopropanol, the mixture
is concentrated by evaporation in vacuo, water
is added and the mixture is e~tracted with chloroform.
The organic extract is dried and filtered and evaporatea
down _ vacuo. The evapora-tion resi~ue is purified
by column chromatography on silica gel (chloroform/meth-
anol/glacial acetic acid = 5/1/0.01).
~ 32~723
- 61 -
Yield: 0.14 g (74~ of theory~,
Melting point: 190~200C (ether)
Calculated: molecular peak m/e = 454
Found: molecular peak m/e = 454
Example 47
thyl 2-ethoxy-4-[N-(~-ethoxymethyl-2-piperidino-
benzyl)-aminoca _onylmethyl]-benzoate
To 0.0~1 9 ~1.4 mmol) of sodium hydride ~55% in
oiI) in 6.4 ml of absolute tetrahydrofuran is added,
with stirring at ambient temperature, 0.64 g (1~4 mmol)
of ethyI 2-ethoxy-4-rN-(a-hydroxymethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate. ~he mixture
is stirred for 1 hour, then 0.113 ml (1.4 mmol)
of ethy~ iodide are added and the mixture is stirred
for a further 16 hours at ambient temperature.
Then 2 ml of ethanol are added and the mixture
i~ eYaporated down in vacuo. The evaporation residue
is partitioned between chloroform and water. The
organic phase is washed twice with water, dried,
filtered and concentrated by evaporation in vacuo.
The evaporation residue is purified by column chro~ato-
graphy on silica gel (chloroform/acetone = 17/3).
~ield: 0.05 9 (7~ of theory),
Melting point: 85-87C (petroleum ether)
Calculated: molecular peak m/e = 482
Found: molecular peak m/e - 482
3-0 Example 48
2-Ethoxy-4-[N-(~-ethoxymethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 31 by alkaline
saponification of ethyl 2-ethoxy-4-[N-(~-ethoxymethyl-
132~23
~ 62 -
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate.
Yield: 47~ of theory,
Calculated: molecular peak m/e = 454
Found: molecular peak m/e = 454
Example 49
4-[N-(a-Carboxy-2-piperidino-benzyl) aminocarbonylmethyl]-
2-hydroxy-benzoic acid
Prepared ~nalogously to Example 46 by reacting
2-ethoxy-4-~N-r~-isoprGpoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoic acid with
2.5 equivalents of boron tribromide in methylene
chloride.
Yield: 70~ of theory,
Melting point: 22~-230C (water)
Calculated: C 64.07 H 5.87 N 6~79
Found: 64.21 5~9~ 6.81
Example 5~
3-~2-E h~y-4- rN-~ ?-piperidino-phenyl)~ utyl)-
aminocar~nylmethyl}-phenyl]-propionitrile
0.11 g ~ mmol~ of magnesium chips are added
~5 to 0~05 g ~0.11 mmol) of 2-ethoxy-4-tN-~ 2-piperidin
phenyl)-l--butyl)-aminocarbonylmethyl~-cinnamic
acid nitrile in 1.1 ml of methanol and the mixture
is stirred for 45 minutes at 25C and for 1 hour
at 0C. It is then cooled to 0C and mixed with
4.5 ml of lN hydrochloric acid. It is diluted
with water, filtered over kieselguhr and extracted
with chloroform. The chloroform extract is washed
with aqueous sodiu~ bicarbonate solution, dried
and filtered and concentr-ated by evaporation n
vacuo. The evaporation residue is purified by
,
~32~723
- 63 -
column chromatography on silica gel (chloroform/ethyl
acetate = 9/1).
Yield: 0.015 g (30% of theory),
Melting point: 102-104C (petroleum ether)
Calculated: molecular peak m/e = 447
Found: molecular peak m/e = 447
Example S1
12-Ethox~-4-rN~ (2-piperidino-phenyl)-3-meth~l-
l-butyl)~aminocarbonylme h~l]-phenyl]-acetonitrile-
Prepared analogously to Example 1 from 2-ethoxy-
4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-
aminocarbonylmethyl]-benzyl chloride with sodium
cyanide.
Yield: 58% of theory,
Melting point: 135-136C
Calculated: C 75.13 H 8.33 N 9.39
Found: 75.-12 8.18 9~18
Example 52
EthYl 2-ethoxy-4-[N~ cyclopropylmethyl-2-piPeridino-
benzyl)-a~inocarbony~methyl]-henzoate
Prepared analogously to Example 1~- from ~-cyclopropylmethyl-
2-piperidino-benzylamine and 3-ethoxy-4-etho~ycarbonyl-
phe~ylacetic acid.
Yield: 29.5% of theory,
Melting point: 126-127C
Calculated: C 72.77 ~ 8.00 N 5.85
Found: 72.85 7.74 5.84
i320r,23
- 64 -
Example 53
2-Ethoxy-4-[N-(~-cyclo~ro~ylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoic acid he~ihydrate
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethQxy-4-[N-(~-cyciopropylmethyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate.
Yield: 88~ of theory,
Melting point: 103-104C
10 Calc. (x 0 5 ~2) C 70.55 H 7~68 N 6.10
Found: 70.67 7.67 6.37
Example 54
Ethyl 2-ethoxy-4-~fN~ cYclobutylmethYl-2-piperidino-
benzyl ? -aminocarbonylmethyll-benæoate
Prepared analogously to Example 19 from a-cyclobutylmethyl-
2-piperidino-benzylamine and 3-etho~y 4 ethoxycarb~nyl-
phenyl acetic acid.
20 Yield: 14~ o theory,
Meltin~ point: 116-11~C
Calculatea: C 73.14 ~ 8.18 ~J 5.69
Found: 73.I4 8.325.64
Ex_ ple 55
2-Ethoxy-4-[~ cyclobutylmethyl-2-PiPeridino;
benzyl)~aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification o ethyl 2-ethoxy-4-[N-(~-cyclobutylmethyl-
2-piperi~ino-benzyl)-aminocarbonylmethyl]-benzoate~
Yield: 33% of theory,
Melting point: 140-142C
Calculated: C 72.39 H 7.81 N 6.03
-` 13~723
65 -
Found: 72.15 7.79 5.97
Example 56
Ethyl 2-ethoxy-4-[N-(~-c,yclopentylmethyl-2-piperidino-
benzyl)-aminocarbonylmethYl]-benzoate
Prepared analogously to Example 19 from ~-cyclopentyl-
methyl-2-piperidino-benzylamine and 3-ethoxy-4-
ethoxycarbonyl-phenyl-acetic acid.
10 Yield: 36.8~ of theory,
Melting point: I20-121C
Calculated: C 73~49 H 8.36 N 5.53
Found: 73.31 8.55 5.3
Example 57
2-Ethoxy-4- r~- ~ eridino-
benzyl)-aminocarbony ~ cid
Prepared analogou~ly to E~ample 21 by ~Ikaline
saponification of ethyL 2-ethoxy-4-~N-(~-cyclopentyl-
methyl-2-piperidino-~enzyl)-aminocarbonylmethyl]-
benzoate.
Yield- 75~ of theory,
Meltiny poInt: 85-88C
Calculated: C 72.77 ~ 8.00 N 5.85
Found: 72.5Q 8.026.03
Example 58
EthYl 2-ethoxy-4-[N-(2-piperidino-~-(tetrahydrofuran-
2-yl-meth~l)-ben~yl)-aminoca~bonylmethyll-benzoate
Prepared analogously to Example 19 from 2-piperidino-
~-(tetrahydrofuran--2-yl-methyl~-benzylamine and
3-ethoxy-4-ethoxycarbonyl-phenylacetic a-cid.
- ~32~ ~23
- 66 -
Yield: 38~ of theory,
Melting point: 111-113C
Calculated: C 70.84 H 7.93 N 5.5l
Found: 70.76 7.73 5.51
s
Example 59
2-Ethoxy-4-[N-(2-piperidino-~-(tetrahydrofuran~
2-Yl-methYl)-benzyl)-aminocarbonylmethyl]-benzoic
acid
Prepared analogously to Exa~mple 21 by alkaline
saponification of ethyl 2-ethoxy-4-[N-(2~piperidino-
~-~tetrahydrofuran-2-yl-methyl)-benzyl1-aminocarbonyl-
methyl]-benzoate.
Yield: 75% of theory,
Melting point: 121-123C
Calculated: C 69.98 H 7.55 ~ 5.83
Foun~: 69.90 7.78 5.71
Example 60
EthYl 2-ethoxy-4-[N-(~-cycloheptylmeth~l-2-piperidIn
benzyl)-aminocarbonylmethyl]-ben2oate
Prepared analogously to Example 19 from ~-cy-cloheptyl-
methyl-benzylamine and 3-ethoxy 4--ethoxycarbonyl-
phenylacetic acid.
Yield: 48~ of theory,
Melting point: 96-98C
Calculated: C 74.12 ~ 8.67 N 5.24
30 Found: 74.40 8.87 5.39
-
132~723
- 67 -
Example 61
2-Ethoxy-4-[N-(-cyclohepty~methyl-2-Pi~eridin
benzyl)-aminocarbonylmethYl]-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethoxy-4-rN-(a-cycloheptylmethyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate.
Yield: 83% of theory,
Melting point: 127-130C
10 Calculated: C 73.49 ~ 8.36N 5.53
Found: 73054 8.62 5.47
Example 62
Ethyl 2-ethoxy-4-[N-(a-cyclohexylmethy~-2~ eridin
benzyl)-3minocarbon~1methyl]-benzoate
a) Ethyl 2-ethoxy-4-rN-(-(cyclohexyl-methylidene)-
2-piperidino-benzylj-aminocarbonylmethyl]-benzoate
Prepared a-nalogously to Example 19 from ~-cyclohexyl-
methyl-(2-piperidino-phenyl)-ketimine and 3-ethoxy-
4-ethoxycarbonyl-phenylacetic acid.
Yield: ~5% o-f theory,
Melting point: 85-88C
Calculated:. C 74.10 ~ 8.16 N 5.10
25 ~ound: 74.37 8.005.45
According to the 80 MHz- ~-NMR spectrum (CDC13)
there is a mixture of E/Z = 1/2. ~Olefinic H:
(E) D 6.26~ (Z) 5.42 ppm].
1~2~723
- 68 -
b) ~thyl 2-ethoxy-4-[N-(a-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
Prepared analogously to Example 12 by catalytic
hydrogenation of ethyl 2-ethoxy-4-[N-(a-(cyclohexyl-
methylidene)-2-piperidino-benzyl)-aminocarbonylmethyl]-
benzoate
Yield: 61~ of theory,
Melting point: 95-97C
Calculated- C 73.81 H 8.52 N 5.38
Found: 73.92 8.74 5.29
Example 63
2-Ethoxy-4-[N-(a-cyclohexylmethY1 2-pi~_ridino-
benzy~ aminocarbonylmeth~ benzoic acid
a1 2-Ethoxy-4-[N-(~-(cyclohexyl-methylidene)-2-
piperidino-benzyl)-aminocarbonylmethyl]-benzoic
acid
Prepared analogously to Example ~1 by alkaline
sapon~ficati~n of ethyl 2-ethoxy-4-tN-t-(cyclohexyl-
methylidene)-2-piperidino-benzyl)-aminocRrbonylmethyl~-
benzoate.
- Yield: 87% of theory,
~elting point: 95-100C
Calculated: C 73~44 ~ 7.81 N 5071
Found: 73.38 7.73 5.75
b) 2-Ethoxy-4-rN-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 12 by catalytic
hydrogenation of 2-ethoxy-4-[N-(a-(cyclohexyl-methylidene)-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoic
acid.
1 32~723
- 69 -
Yield: 52% of theory,
Melting point: 154-156C
Calculated: C 73.14 H 8.18 N 5.69
Found: 73.31 8.25 5~71
Example 64
EthX~_2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-
buten-l-yl)-aminocarbo ~lmethyl]-benzoate
Prepared analogously to Example 19 from 1-(2-piperidino-
phenyl)-3-buten-1-yl-amine and 3-ethoxy-4-ethoxycarbonyl-
phenylacetic acid.
Yield: 32.7% of theory,
Melting point: 110-112C
Calculated: C 72.39 H 7.81 N 6.03
Found: 72.10 7.665.94
Example 65
2-Ethoxy-4-~N-(1-(2-piperidino-phenyl)-3-buten-
l-yl?-aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethoxy-4-[N-(1-(2-piperidino-
phenyl)-3-buten-1-yl)-aminocarbonylmethyl]-benzoate.
25 Yield: 68~ of theory,
Melting point: 92-95C
Calculated: C 71.53 H 7.39 N 6.42
Found: 71.27 7.426~42
Example 66
thyl_2-ethoxy-4-[N-(1-(2 piperidino-phenyl)-3-
meth,yl-3-buten-1-yl)-aminocarbonylmethyl]-benzoate
Prepared analogously to Example 19 from 3-methyl-
~32~
- 70 -
1-(2-piperidino~phenyl)-3-buten-1-yl) amine and
3-ethoxy-4-ethoxycarbonyl-phenylacetic acid.
Yield: 33.6% of theory,
Melting point: 126-128~C
Calculated: C 72.77 ~ 8 00 N 5.85
Found: 72.82 8.22 5.78
Example 67
2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-
3-buten-1-yl) aminocarbonYlrnethyl]-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification o~ ethyl 2-ethoxy-4-[N~ (2-piperidino-
phenyl)-3-methyl-3-buten-1-yl)-aminocarbonylmethyl]-
benzoate.
Yield: 74% of theory,
Melting point: 64-66C
Calculated: C 71.97 ~ 7.61 N 6.22
Found: 7I.70 7.50 5.98
Example 68
Ethyl 2-ethoxy-4-~N~ ?-~îperidino-~he-nyl)-3-
meth~l-2-buten-l-yl)-aminocarbon~lmethYl]-ben2oate
~5 rwith 25~ of ethYl 2-ethoxy-4-r~-(1-(2-piperidino-phenyl)-
3-methyl-I-~u~yl)-aminocarbo~yl~ethyl]-benzoate~
Prepared analogously to Example 19 from 3-methyl-
1-~2-piperidino-phenyl)-2-buten-1-yl-amine [containing
25~ of 3-methyl-1-~2-piperidino-phenyl)-1-butylamine~
and 3-ethoxy-4-etnoxycarbonyI-phenylacetic acid.
Yield: 37~ of theory,
Melting point: 141-142~C
Calculated. C 72.77 H 8.00 N 5.85
Found: 72.607.77 5.73
1321~72~
- 71 -
The mixing ratio of 75/25 is obtained from the
corresponding ratio of intensities of the particularly
characteristic signals in the 400 MHz-lH-NMR spectrum
(CDC13). The position of the signals is: 3-methyl-
2-buten-1-yl compound: olefinic H: 5.25 (d),
-CH3: 1.64 (s) and 1.77 (s), benzylic ~ CH- 6.00
(t), benzylic -CH2-: 3.52 ppm (s) 3-methyl-1-butyl
compound: -CH3: 0.90 (d), benzylic ~CH- 5.35
(m), benzylic -CH2-: 3.54 ppm (s).
Exa~ple 69
2-Ethox,y-4-[N-(1-(2-piperidino-phenQl)-3-meth
2-buten-1-yl)-aminocarbonylmethyl]-ben~oic acid
[containin~_25% of 2-ethox~-4-~N-(1~(2-piperidino-
phenyl -methyl-l-butyl)-aminocarbonylmethyl]-
benzoic acid]
Prepared analogously to Example 21 by alkaline
sapon-ification of the corresponding ethyl ester
mixture from Example 68.
Yield: 91~ of theory,
Melting point: 154-1;6C
Calculated: C 71.97~ 7.61 ~ 6.2-2
Found: 71.80 7.57 5.98
Example 70
~,
Ethyl 2-ethoxy-4-1N-~1-(2~piperidinQ-phenyl)-3-
bu~ yl)-aminocarbonylmethyl¦-benzoate
Prepared analogously to Example 19 from 1-(2-piperi~ino-
phenyl~-3-butyn-1-yl-a~ine and 3-ethoxy-4-ethoxycarbonyl-
phenylacetic acid.
Yield: 52% of theory,
. Melting point: 86-90C
Calculated: C 72.70 ~ 7.41 N 6.06
~ ' '
~:
1~2~3
- 72 -
Eound: 72.60 7.40 6.04
Example 71
2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-butyn-
l-yl)-aminocarbonylmethyl]-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethoxy-4-[N-(1-(2-piperidino-
phenyl)-3-butyn-1-yl)-aminocarbonylmethyl]-benzoate~
10 Yield: 68~ of theory,
Melting point: 6-6-69C
Calculated: C 71~87 H 6.96 N 6.45
Found: 71.60 6.95 6.38
Example 72
Ethyl 2-ethoxy-4-lN-(1-~2~piperidino-phenyl)-4
penten-l-~l)-a~ ethyl]-benzoate
Prepared analogousl~ to Example 19 from 1-12-piperidino-
phenyl)-4-penten-1-yl-amine and 3-ethoxy-4-ethoxycarbonyl-
phenylacetic acid.
Yield; 58~ of theory,
Melting point: 117-120C
Calculated: C 72.77 H 8.00 N 5.85
25 Found: 72.73 7.976.~7
)
Exam~le 73
2-Ethoxy-4-~N-[1-12-pi~eridino-phenYl)-4-penten-
l-yl)-aminocarbonylmethyl~-benzoic acid
Prepared analogously to Example 21 by alkaline
saponification of ethyl 2-ethoxy-4-~N-tl-(2-piperidino-
phenyl)-4-penten-1-yl) aminocarbonylmethyl]-benzoate.
Yield: 61~ of theory,
- 132~723
- 73 -
Meltinq point: 82-85C
Calculated: C 71.97 H 7.61 N 6.22
Found: 71.97 7.59 5.9
Example 74
EthYl 2-ethoxy-4-~N-tl-(2-piperidino-a-(tetrahydropyran-
2-yl-methyl)-benzyl)-aminocarbonylmethyl]-benzoate
Prepared analogously to Example 19 from 2-piperidino-
~-(tetrahydropyran 2-yl-methyl)-benzylamine and
3-ethoxy-4-ethoxycarbonyl-phenylacetic acid.
Yield: 29% of theory,
Melting point: 82-85C
C-alculated: C 71.24 H 8.10 N 5.36
15 Found: 71.28 7.96 5.29
Example 75
2-Ethoxy-4-[N-(1-(2-piperidino-a-(tetrahydro~yran
2-yl-methY~)-ben~yl)-aminocarbonyl~ethyl]-benzoic
acid
Prepared analogously to Exa~ple 21 by alkaline
saponification of ethyl 2-ethoxy-4-r~-(1-(2-piperidino-
-(tetrahydropyran-2-yl-methyl)-benzyl)-a~inocarbonyl-
2-5 methyl~-benzoate.
Yield: 85% of theory,
Melting point: 140-142G (~inters from 70C,
partial softenIng at 105C)
Calculated: C 70.42 ~ 7.74 N 5.Q6
30 Found: 70.15 7.885.40
132~72~
- 74 -
Example 76
Ethyl 2-ethoxy-4-[N-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonYlmethYl]-benzoate
At 23-25C, a solution of 2.35 g (10 mmol) of
ethyl 2-ethoxy-4-cyanomethyl-benzoate and 2.88 g
(10 mmol) of ~-cyclohexylmethyl-2-piperidino-benzyl
alcohol in 15 ml of o-dichlorobenzene is added
dropwise to a mixture of 15 ml of concentrated
sulphuric ~cid and 15 ml of o-dichlorobenzene.
The mixture is stirred for 2 hours at ambient
temperatureO The o-dichlorobenzene phase is then
separated off and the residue is added to ice.
After being made alkaline with soda solution,
it is extracted with chloroform. The extracts
are dried over sodium sulphate and concentrated
by evaporation. The residue is purified by column
chromatography on silica gel (toluene/acet~ne
= 10/1).
Yield: i.l g (~1% of theory),
Melting point: 95 97C
Calculated: C 73.81 ~ 8.52 N 5.38
Found: 73.95 8.64 5.42
ExamPle 77
Benzyl 2-ethoxy-4-rN-(~-methoxycarbonyl-2-~Ip~ridin
benzyl)-aminocarbonylmethYl]-benzoate
0.28 g (2 mmol) of potassium carbonate are added
33 to a solution of 1.06 g (2 mmol) of benzyl 2-ethoxy-
4-[N-(~-carboxy-2-piperidino-~enzyl)-aminocarbonylmethyl~-
benzoate in 8 ml of anhydrous dimethyl formamide.
The mixture is stirred for 10 minutes at ambient
temperature, then 0.125 ml (2 mmo1) of methyl
` ~32~7%~
- 75 -
iodide are added and the resulting mixture is
stirred overnight at ambient temperature. It
is filtered and the filtrate is concentrated by
evaporation to dryness in vacuo. The evaporation
residue is partitioned between aqueous sodium
bicarbonate solution (pH = 9) and methylene chloride.
The organic phase is dried over sodium sulphate,
filtered and concentrated by evaporation 1n vacuo.
The evaporation residue is purified by column
chromatography on silica gel (toluene/acetone
= 4/1) and crystallised from ether/petroleum ether.
Yield: 0.56 q t51~ of theory),
Melting point: 100-102C
Calculated: C 70.57 H 6.66 N 5.14
15 Found: 70.&9 6.71 5029
13~7~3
- 76 ~
Analogously to the preceding Examples, the following
compounds are obtained:
[2-Methoxy-4-[N-(1-(2-piperidino-phenyl)~l-butyl)-
aminocarbonylmethyl]-phenyl]-acetonitrile
[2-Propoxy-4-[N-(1-(2-piperidino-phenyl)-1-butyl)-
aminocarbonylmethyl]-phenyl~-acetonitrile
[2-Ethoxy-4-[N-(1-(4-fluoro-2-piperidino-phenyl)-
l-butyl)-aminocarbonylmethyl]-phenyl]-acetonitrile
[2-Ethoxy-4-EN (1-(4-methoxy-2-piperidino-phenyl)-
l-butyl)-aminocarbonylmethyl~-phenyl]-acetonitrile
[2-Ethoxy-4-[N-(1-(3-methyl-2-piperidino-phenyl)
l-butyl)-aminocarbonylmethyl]-phenyl~-acetonitrile
[2-Ethoxy-4-[N-(1-(4-methyl-2-piperidino-phenyl~-
l-butyl)-aminocarbonylmethyl]-phenyl]-acetonitrile
[2-Ethoxy-4-[N-(1-(6-methyl-2-piperidino~phenyl)-
l-butyl)-aminocarbonylmethylJ-phenyl]-acetonitrile
[2-Ethoxy-4-rN~ (2-piperidino-phenyl)-3 but-en-
l-yl)-aminocarbonylmethyl~-phenyl]-acetonitri~e
: [2-~thoxy-4-[N-(1-(2-piperidino-phenyl)-2-buten-
l-yl)-aminocarbonylmethyl]-phenyl]-acetonI~rile
~2-Ethoxy-4-[~ -cycloprQpyl~ethyl-2-piperidIno-
benzyl)-aminocarbonylmethvl}-phenyl~-acetonitrile
~2-Ethoxy~4-[N-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-phenyl~-acetollitr-ile
[2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-1-ethyl)-
aminocarbonylmethyl]-phenyl]-acetonitrile
32~ ~2~
- 77 -
[2-Ethoxy-4-~N-(1-(2-piperidino-phenyl)-1-propyl)-
aminocarbonylmethyl]-phenyl~-acetonitrile
~2-Ethoxy-4-[N-(1-(2-pyrrolidino-phenyl)-1-butyl)-
aminocarbonylmethyl]-phenyl]-acetonitrile
[2-Ethoxy-4-[N-(1-(2-hexamethyleneimino-phenyl)-
l-butyl)-aminocarbonylmethyl]-phenyl]-acetonitrile
[2-Ethoxy-4-[~ (2-(4-methyl-piperidino)-phenyl)-
I-butyl)-aminocarbonylmethyl~-phenyl]-acetonitriIe
Ethyl 2-ethoxy-4-[N-(~-cyclopropylmethyl-2-pyrrolidino-
benzyl)-aminocarbonylmethyl~-benzoate
2-Ethoxy-4-rN-(a-cyclopropylmethyl-2-pyrrolidino-
benzyl)-aminocarbonylmethyl~-benzoic acid
Ethyl 2-ethoxy-4-[N-(~-cyclopropylmethyl-2-hexamethy-
leneimino-benzyl~-aminocarbonylmethyl~-benzoate
2-Ethoxy-4-1N-(a-cyclopropylmethyl-2-hexamethyleneimino-
benzyl~-aminocarbonylmethyl~-benzoic acid
Eth~I 2-ethoxy-4-[~ -cyclopropylmethyl-2-(4-
meth~l-piperidino)-benzyl)~aminocarbonylmethyl]-
benzoate .
2-Ethoxy-4-[~ -cyclopropylmethyl-2-~4-methyl-
piperi~ino)-benzyl)-a~inocarbonylmethyl~-benzoic
acid
Ethyl 4-[~ -cyclopropylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-methoxy-benzoate
4-[N-(~-Cyclopropylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-methoxy-~enzoic acid
132~723
- 78 -
Ethyl 2-ethoxy-4-rN-(a-cyclohexylmethyl-2-pyrrolidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-(a cyclohexylmethyl-2-pyrrolidino-
benzyl)-aminocarbonylmethylJ-benzoic acid
Ethyl 2-ethoxy-4-1N-~-cyclohexylmethyl-2-hexamethy-
leneimino-benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-~N-(~-cyclohexylrnethyl-2-hexamethyleneimino-
benzyl)-aminocarbonylmethyll-benzoic acid
Ethyl 2-ethoxy-4-lN-(a-cyclohexylmethyl-2-(4-methyl-
piperidino)-benzyl)-aminocarbonylmethylJ-benzoate
2-Ethoxy-4-[N-(~-cyclohexylmethyl-2-(4-methyl-
piperidino)-benzyl)-aminocarbonylmethyl~-benzoic
acid
Ethyl 2-ethoxy-4~[N-(~-cyclohexylmethyl-2-(3,5-
dimethyl-piperidino)-benzyl)-aminocarbony~methyl]-
benzoate
2-Etho~y-4-~N-(~-cyclohexylmetbyl-2-(3,5-dimethyl-
piperidino~-henzyl3-aminocarbonylmethyl~ benzoic
acid
Ethyl 2-ethoxy-4-[N-(a-cyclohexylmethyl-2-~3,3-
dimethyl-piperidino)-benzyl)-am~inocarbonylmethyl]-
benzoate
2-Ethoxy-4-[N~ cycloh~exylmethyl-2-~3,3-dimethyl-
piperidino1-benzyl)-aminocarbonylmethyl~-benzoic
acid
Ethyl 4-[N-(-cyclohexylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-hydroxy-benzoate
132~723
_ 79 w
4-[N- (~-Cyclohexylmethyl-2-piperidino~benzyl)-
aminocarbonylmethyl]-2-hydroxy-benzoic acid
Ethyl 4-[N~ (~-Cyclohexylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-methoxy-benzoate
S 4-[N- (~-Cyclohexylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-methoxy-benzoic acid
Ethyl 2-allyloxy-4-[N-t cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Allyloxy-4-~N-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Ethyl 4-~[N-(a-cyclohexylmethyl-2-piperidino-benæyl)-
aminocarbonylmethyl]-2-n-propoxy-benzoate
4-EN- (~-Cyclohexylmethyl-2-piperidino-benzyl)-
aminocarbonylmethyl]-2-n-propoxy-benzoic acid
Ethyl 2-ethoxy-4-[N-(~-cyclohexylmethyl-4-fluoro-
2-piperiaino-benzyl)-aminocarbonylmethyl]-benzoate
2-~thoxy-~ cyclohexylmethyl-4-fluoro-2-piperi~ino-
berzyl)-a~inocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-[N-(a-cyclohexylmethyl-3-chloro-
2~ 2-piperidino-~enzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-(~-cyclohexylmethyl-3-chloro-2-piperid-ino-
benzyl~-aminocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-rN-(~-cyclohexylmethyl-6-chloro-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-t~-cyclohexylmethyl-6-chloro-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
1'~2~723
- 80 -
Ethyl 2-ethoxy-4-LN-(~-cyclohexylmethyl-3-methyl-
2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-EN-(~-cyclohexylmethyl-3-methyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
Ethyl 2-ethoxy-4-[N-(~-cyclohexylmethyl-4-methyl-
2-piperidino-benzyl)-aminocarbonylmethyl~-benzoate
2-Ethoxy 4-~N-(-cyclohexylmethyl-4-methyl-2-piperidino-
benzyl)-aminocarbonylmethyl.]-benzoic acid
Ethyl 2-ethoxy-4-[N-(-cyclohexylmethyl-6-methyl-
10 2-piperidino-benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-ta-cyclohexylmethyl-6-methyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benæoic acid
Ethyl 2-ethoxy-4-[N-ta-cyclohexylmethyl-4-methoxy-
2-piperidino-ben~ylJ-aminocarbQnylmethyl~-benzoate
2-Ethoxy-4-[N-ta-cyclohexylmethyl-4-methGxy-2-
piperidino-benzyl) aminocarbonylmethyl]-benzoic
acid
Ethyl 2-ethox~-4-E~ -~ethoxymethyl-2-piperidino-
~enzyl)-aminocarhonylmethyll-benzoate
2-Ethoxy 4-EN~ methoxymethyl-2-piperidino benzyl)
aminocarbonylmethyll-benzoic acid
Ethyl 2-ethoxy-4-[~-(a-n-propoxymethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-~-n-propo~ymethyl-2-piperiaino-
benzyl)-aminoc-arbonylmethyl]-~enzoic acid
1~2~723
- 81 -
Ethyl 2-ethoxy-4-[N-(a-isopropoxymethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-(a-isopropoxymethyl-2--piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-rN-(~ methoxy-ethyl~-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
2-Ethaxy-4-rN-(~ methoxy ethyl)-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-tN-(~-(2-methoxy-ethyl)-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N-(a-(2-methoxy-ethyl)-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-rN-(a-(2-ethoxy-ethyl)-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
2 Ethoxy-4-[N-(~-(Z-ethoxy-ethyl)-2-piperidino-
benzyl)-amino~arbonylmethyl]-benzoic acid
Ethyl 2-etboxy-4-rN~ (2-cyclohexyl-ethyl)-2-
piperidino-benzyl)-~minocarbonylmethyl]-be:nzoate
2-Ethoxy-4-rN-(~-(2-cyclohexyl-ethyl)-2-piperidino-
benzyl~-aminocarbonylmethyl]-benzoic acid
Ethyl 2-ethoxy-4-~N-(-(Z-phenyl-e~hyl)-2-piperidino-
benzyl~-aminocarbonylm~ethyll-benzQate
2-Ethoxy-4-~N-(~-(2-phenyl-ethyl)-2-piperidino-
benzyl)-aminocarbonylrnethyl~-benzoic acid
Ethyl [2-ethoxy-4-[N-(1-(2-piperidino~phenyl)-3-
buten-l-yl)-aminocarbonylmethyl3-phenyl]-acetate
132~2~
- 82 -
[2-Ethoxy-4-[N-tl-~2-piperidino-phenyl)-3-buten-
l-yl)-aminocarbonylmethyl]-phenyl]-acetic acid
Ethyl [2-ethoxy-4-~N-(l-(2-piperidino-phenyl)-
2-buten-l-yl)-aminocarbonylmethyl]-phenyl]-acetate
~2-Ethoxy-4-[N-(l-(2-piperidino-phenyl)-2-buten-
l-yl)-aminocarbonylmethyl]-phenyl]-acetic acid
Ethyl [2-ethoxy-4-~N-~a-cyclopropylmethyl-2-piperidino
benzyl)-aminocarbonylmethyl]-phenyl]-acetate
[2-Ethoxy-4-lN-(~-cyclopropylmethyl-2-piperidino-
benzyl) aminocarbonylmethyl]-phenyl]-acetic acid
Ethyl t2-ethoxy-4-[~ -cyclohexylmethyl-2-piperidino-
benzyl)-amino~arbonylmethylJ-phenyl]-acetate
[2-Ethoxy-4-[N-(-cyclohexylmethyl-2-piperidino-
benzyl)-aminoca~b~nylmethyl~-phenyl]-acetic ~cid
Ethyl t2-ethoxy-4-[N~ (2-piperidino-phenyl)-
l-ethyl)-aminocarbonylmethyl]-phenyl]-acetate
[2-Ethoxy-4~ 2-piperidino-phenyl)-l-ethyl)-
aminocarbonylmethyl~-phenyl~-acetic acid
Ethyl [2-ethoxy-4-EN-(i-~2-piperidino-phenyl)-
l-propyl)-aminocarbonylmethyl~-phenyl~-acetate
[2-Ethoxy-4- 1N- (1- ( 2-piperidino-phenyl)~l-propyl)--
am-inocarbonylmethyl~-phenyl]-acetic acid
Ethyl 2-ethoxy-4-[N-[l-(2-piperidino-phenyl)-3-
buten-l-yl]-aminocarbonylmethyl]-cinnamate
2-Ethoxy-4-[N-~1-(2-piperidino-phenyl)-3-buten-
l-yl]-aminocarbonyl.methyl]-cinnamic acid
~L32~
- 83 -
Ethyl 2-ethoxy-4-[N-rl-(2-piperidino-phenyl)-2-
buten-l-yl]-aminocarbonylmethyl]-cinnamate
2-Ethoxy-4-[N-[l-(2-piperidino-phenyl)-2-buten-
l-yl]-aminocarbonylmethyl]-cinnamic acid
Ethyl 2-ethoxy-4-[N-(~-cyclopropylmethyl-2-piperidino-
benzyl1-aminocarbonylmethyl]-cinnamate
2-Ethoxy-4-[N-(~-cyclopropy:Lmethyl-2-piperidinQ-
benzyl)-aminocarbonylmethyl~-cinnamic acid
Ethyl 2-ethoxy-4-[N-(a-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-cinnamate
2-Ethoxy-4-[N-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-cinnamic acid
Ethyl 2-ethoxy-4-[N-(l-(2-piperidino-phenyl)-l-
ethyl)-aminocarbonylmethyl]-cinnamate
l~ 2-Ethoxy 4-[N-(l-(2-piperidino-phenyl)-l-ethyl~-
aminocarbonylmethyl]-cinnamic acid
Ethyl 2-ethoxy-4-[N- f l-(2-piperidino-phenyl~
propyl)-aminocarbonylmethyl]-sinna~ate
2-Ethoxy-4-[N-rl-(2-piperid-ino-phenyI)-l-propyl)-
aminocarbonylmet~yl] cinnamic acid
Ethyl 3-~2-ethoxy-4-rN-~-cyclohexylmethyl-Z-piperidino-
benzyl)-aminocarbonylmethyl]-phenyl]~propionate
3 [2-Ethoxy-4-[N-~a-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-phe-nyl~-propionic
acid
- 84 - ~ 32~72~
Ethyl 3-[2-ethoxy-4-tN-(1-(2-piperidino-phenyl)-
l-ethyl)-aminocarbonylmethyl]-phenyl]-propionate
3-[2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-1-ethyl)-
aminocarbonylmethyl]-phenyl]-propionic acid
Ethyl 3-[2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-
l-propyl)-aminocarbonylmethyl]-phenyl]-propionate
3-~2-Ethoxy-4-[N-(1-(2-piperidino-phenyl~-1-propyl1
aminocarbonylmethyl]-phenyl]-propionic acid
Ethyl 3-ethoxy-4-[N-f~-cyclopropylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl~-benzoate
3-Ethoxy-4-tN-(~-cyclopropylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Eth~l 3-ethoxy-4-tN-(~-cyclohexylmethyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoate
3-Ethoxy-4~ -cyclohexylmethyl-2-piperidino-
benzyl~-aminocarbonylmethyl~-benzoic acid
Benzyl 3-~thoxy-4-rN-~-met~oxycarbonyl-Z-piperidino-
benzyl)-aminocarb~nylmethyl]-benzoate
3 Ethoxy-4-[N-~-methoxycarbonyl-2-piperidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
: Benzyl 2-ethoxy~4-tN-~-methoxycarbonyl-2-pyrrolidino-
benzyl)-aminocarbonylmethyl]-benzoate
2-Ethoxy-4-rN-(~--~ethoxycarbonyl-2-pyrrolidino-
benzyl)-aminocarbonylmethyl]-benzoic acid
Benzyl 2-ethoxy-4 EN- (2-hexamethyleneimino-~-methoxy-
carbonyl-benzyl)-aminocarbonylmethyl]-benzoate
- 85 - 1 32~ 723
2-Ethoxy-4-[N-(2- hexamethyleneimino-~-methoxycarbonyl-
benzyl)-aminocarbonylmethyl]-benzoic acid
Benzyl 2-ethoxy-4-[N-(~-methoxycarbonyl-2-(4-methyl-
piperidino)-benzyl) aminocarbonylmethyl]-benzoate
2-Ethoxy-4-[N~ methoxycarbonyl-2-(4-methyl-piperidino)-
benzyl~-aminocarbonylmethyl]-benzoic acid
- 86 - 1 32 07 23
Example A
Tablets containing 5 mg of 2-ethoxy 4-[N-(a-cyclo-
hexylmethyl-2-piperidino-benzyl)-aminocarbonylmethyl]-
5 benzoic acid
Composition:
1 tablet contains:
Active substance (1)5.0 mg
Corn starch (2~62.0 mg
10 Lactose (3~48.0 mg
Polyvinylpyrrolidone (4)4.0 mg
Magnesium stearate (5)1.0 mq
120.0 mg
Preparation
Components 1, 2, 3 and ~ are mixed together andmoistened with water. The moist mixture is press-ed
through a screen with a 1.5 mm mesh size and d-ried
at about 45C. The dry granulate is passed thro~gh
a 1.0 mm me~h screen and mixed with component 5.
The finished mixture is compressed in a tablet
press with dies 7 mm in diameter, provi*ed with
a dividing notc~, to form tablets.
Weight of tablet: 120 mg
xample B
Coated tablets contain ng 2.5 mg of 2-ethd~y-4-
[~ -c~clohe~ylmethyl-2-piperidino benzyl)-amin~car~onyl-
methy~-benzoic acid
30 1 tablet core contains:
Active substance (1)2.5 mg
Potato starch (2)44.0 mg
Lactose (3)30.0 mg
- 87 - 132~7~
Polyvinylpyrrolidone (4) 3.0 mg
Magnesium stearate(5) 0.5 mq
80.0 mg
Preparation
S Components 1, 2, 3 and 4 are thoroughly mixed and
moistened with water. The moist mass is forced
through a screen with a 1 mm mesh size, dried at
about 45C and the granules are then passed through
the same screen again. Aftler component 5 has been
added, tablet cores ~ mm in diameter are pressed
out in a ta~let making machine. The tablet cores
thus produced are coated in known manner with a
coating consisting essentially of sugar and talc.
The finished coated tablets are polished with wax.
Weight of coated tabIet: 120 mg
Example C
Tablets containing 10 mg o~ 2-ethoxy-4-[N-(a~cyclo-
hexylmethy_-2-piperidino-benzYl)-aminocarbonylmethylJ
~enzoic acid
Composition:
1 tablet c~ntains:
Active substance10.0 mg
25 Powdered lactose70.0 mg
Corn starch31.0 mg
Polyvinylpyrrolidone 8.0 mg
Magnesium stearate 1.0 mq
120.0 mg
30 Preparation:
The mixture of active substance, lactose and corn
starch is moistened with a 20~ solution of polyvinyl-
pyrrolidone in water. The moist mass is granulated
,.
~ 32~723
- ~8 -
through a screen with a mesh size of 1.5 mm and
dried at 45C. The dried granulate J.S passed through
a screen with a mesh size of 1 mm and homogeneously
mixed with magnesium stearate.
Weight of tablet: 120 mg
Die: 7 mm diameter with dividing notch
Example D
Coated tablets containing 5 mq of 2~ethoxy-4-lN-
(~-cy~ exylmethyl-2-piperidino-benzyl)-aminocarbon
45 methYl]-benzo-ic acid
1 tablet core contains:
Active substance 5.0 mg
Secondary calcium phosphate 70.0 mg
Corn starch 50.0 mg
50 Polyvinylpyrrolidone4.0 mg
Magnesium stearate_1.0 mg
1~0.O mg
Preparation:
A mixture of active substancer calcium pho~phate
and corn starch is moistened with a 15% solution
of polyvinylpyrrolîdone in water. ~he moist mass
is passed through a screen with a mesh size of
1 mm, dried at 45C and then passed through t~e
same screen again. After mixing with the specified
quantity of magnesium steara-te, tablet cores are
compressed therefrom.
Weight of core: 130 mg
nie: 7 mm in diameter
A coating of sugar and talc is applied in a known
manner to the tablet cores thus produced. The
finished coated tablets are polished with wax.
Weight of coated tablet: 180 mg