Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
t 32 t 454
-- 1 --
UNIFORM POLYMER PARTICLI~S
BACKGROUND OF T~IE INVENTION
The present invention relates to uniform
polymer particles, a process for preparing the uniform
polymer particles, an apparatus suitable for use in the
process and uniform polymer particles suitable for use in
a direct extracorporeal hemo-perfusion treatment. More
particularly, the present invention relates to uniEorm
polymer particles which can be widely used, Eor instance,
as a parent material for an ion exchange resinr as a
packing for a chromatography, as a support onto which an
enzyme is immobilized, as a support for an affinity
chromatography, as a material for a foamed article, and
the like; to a process for preparing the uniform polymer
particles; to an apparatus for forming uniform liquid
droplets by jetting a liquid with a high viscosity
containing a natural high molecular su~stance or a
synthetic high molecular substance from an orifice at a
constant flow rate with applying cyclic turbulences
having a constant frequency thereto; and to uniform
polymer particles suitable for use in a direct
extracorporeal hemo-perfusion treatment.
As methods for preparing polymer particles in a
spherical form, there have been hitherto known dispersion
methods and spray methods.
According to a dispersion method, polymer
particles are obtained by coagulating a dilute polymer
solution containing a pore-forming agent being dispersed
in the form of droplet in a dispersion medium containing
a surface active agent, through volatilizing the solvent
thereof [cf. Japanese Unexamined Patent Publication
(Tokkyo Kokai) No. 24430/1981], or through gradually
adding a coagulating agent for the droplets [cf. Japanese
Unexamined Patent Publication (Tokkyo Kokai) No.
159801/1982]. However, in the dispersion method/ no
polymer particles other than polymer particles with a
'; ', ~ -
s ~
: ,
,
~ ' ~
1 32 1 45~
-- 2
broad particle size distribution can be obtained. Also,
in the dispersion method, in order to remove the solvent,
the dispersion medium and the surface active agent from
the coagulated particles, it is necessary to wash the
coagulated particles not only with water but also with an
organic solvent.
There is known another dispersion method in
which polymer particles are obtained by polymerizing
polymerizable monomers after dispersing the monomers in a
1~ dispersion medium, the polymer particles thereby obtained
also has a broad particle size distribution. In
observing the particles by an electron microscope, there
can be found that minute spherical particles aggregate to
form a particle. When a suspension of the above-obtained
particles is stirred by a magnetic stirrer, minute
polymer chips occur in a large amount, which is presumed
to be due to the above-discribed structure of the
particles. Further, the particles obtained by the above
method have pores with a broa~ pore size distribution.
Especially, on the particle surface, the particles have
various size of openings.
According to a spray method, polymer particles
is obtained by spraying a polymer solution into a
coagurating liquid. Thus obtained polymer particles also
have a broad particle size distribution and the particles
have relatively large particle size [cf. ~apanese
Unexamined Patent Publication (Tokkyo Kokai) No.
129788/1977].
Polymer particles with a broad particle size
distribution require a further process of
classification. As generally known, by a classi~yin~
process, for instance, a sieving process, it is possible
to make not less than 8~ ~ by volume of the whole
particles have a particle size within the range of ~20 %
of the volume average particle size. In the sieving
process, it is also possible to remove particles with a
particle size of not less than the size of sieve opening
by the sieve. However, in order to pass all of the
, ~ .
.' .' ~ , ;
1 32 1 454
particles with a particle size of less than the size of
sieve opening, a long time sieving is necessary.
Further, taking the sieving process results in a very low
yeild. Therefore, it i5 not industrially practical to
take such a process. Particularly, it is practically
very diEficult or virtually impossible to remove all of
particles having a particle siæe of not more than 5 % of
the volume average particle size.
When very small particles or very large
particles are included in the polymer particles, various
problems arise in uses of the particles. For instance,
when the particles are used as a packing for
chromatography or an adsorbent, the very small particles
are mi~ed in the liquid to be treated, or cause a lar~e
pressure drop, and the very large particles cause a
lowering the separation efficiency or the adsorption
rate.
When particles having pores with a broad pore
size distribution, particularly openings on the particle
surface with a broad opening size distribution are used
as an adsorbent, such particles cause an inferior
physical selectivity.
Recently, there has been found a technique for
Eorming uniform liquid droplets, in which cyclic
turbulences are applied to a liquid jetted at a constant
flow rate (hereinafter such technique referred to as
"vibration method").
There has been already reported that uniform
particles aLe obtained by applying the above technique to
the dispersion method using polymerizable monomers [cf.
Japanese Unexamined Patent Publication (Tokkyo Kokai)
102905/1982]. However, the particles thus obtained have
the above-stated structure, i.e. minute particles
coagulate to form a particle, and therefore the particles
have defects that polymer chips easily occur and that the
particles have pores with a broad pore size
distribution.
There are reported some examples of that
1321~54
-- 4
polymer particles or capsules are prepared by applying
the vibration method [cf. Japanese Unexamined Patent
Publication ~Tokkyo Kokai) No. 129686/1977 and Japanese
Unexamined Patent Publication ~Tokkyo Kokai) No.
112333/1984]. ~owever, in an examplè disclosed in
Japanese Unexamined Patent Publication No. 129686/1977, a
very dilute polymer solution, i.e. a solution with a very
low viscosity are employed and micro-capsules are
prepared in another example disclosed in Japanese
Unexamined Patent Publication No~ 112833/1984, and
particles with a large particle size are prepared since
nozzles themselves are directly vibrated and therefore
the vibration frequency is restricted to a low
frequency. In both examples, it is necessary that a
multiple tubular nozzle is employed and more than one
kinds of solutions are simultaneously jetted with keeping
a delicate balance.
~ lso, in Japanese Examined Patent Publication
(Tokkyo Kokoku) No. 33134/1981, there is disclosed that
the vibration method is applied to a process for
preparing uniform particles of an inorganic compound. In
this or other publications, cyclic turbulences of flow
rate of the liquid jetted from an orifice of a nozzle are
applied by applying vibrational energy to the whole
nozzle part.
Generally, the force F (dyn~ required to
vibrate an object with a mass M (g) at a frequency f (Hz)
with an amplitude 2D (cm) is calculated by the following
equation:
F - M-(2~f)2-D
For instance, when the total vibrating mass of
nozzle and vibration transmission part is 1,000 g, the
frequency i5 10,000 Hz and the amplitude is 10 ~m, F is
about 2 x 109 dyn. In order to stably generate the above
vibration by an electromagnetic coil vibrator, there is
required an electric power as enomous as several decades
, .
.
'~
1 32 1 ~54
-- 5
KVA. Therefore, it is not economical to directly vibrate
the nozzles, particularly when a high frequency of
vibration is required.
In Japanese Unexamined Patent ~ublication
(Tokkyo Kokai) No. 83202/1986, it is disclosed that the
vibration method is applied to form uniform liquid
droplets of polymerizable monomers. In this case, such
enomous energy as in the previous case is not required
since the vibration is directly transmitted to
polymerizable monomers by piezoelectric vibrator which
itself constitutes a part of vessel containing the
monomers which are jetted through the orifices. However,
when employing a high viscosity liquid such as a polymer
solution, not a low viscosity liquid such as a
polymerizable monomer, it is supposed that a high
pressure is imposed on the piezoelectric vibrator and
therefore the vibrator is destroyed or the vibrator does
not generated a stable vibration. Also, there is the
possibility that the vibrator does not vibrate a stable
vibration when the li~uid is heated in order to reduce
the viscosity.
W. E. Yates and his co-worker developed an
apparatus for preparing uniform liquid droplets using a
magnetostrictive vibrator as a vibration generator. ~cf.
W. E. Yates and N. B. Akesson, Proceedings of the 1st
International Conference on Liquid Atomization and Spary
Systems (Proc. ICLASS), 181-185 and 459 to 460 (19-/8)].
In the above apparatus, the vibration transmission part
is embedded in a fixing cylinder with a potting agent,
and at the end thereof i5 fixed to a vibrating rod. As
well known by a person skilled in the art, the vibration
transmission part is precisely designed so as to resonate
with the vibration generated by the magnetostrlctive
vibrator and so as to have a function of amplifying its
small amplitude of vibration. The vibrating rod is also
precisely designed so that the amplitude reaches its
maximum at the end of rod by resonating with the
vibration generated by magnetostrictive vibrator.
1 321 45~
-- 6
Therefore, when the vibration-transmission part is heated
or subjected to an external force, a constant vibration
can not be obtained, since the vibration transmission
part or the vibrating rod does not resonate with the
magnetos~rictive vibrator. In the apparatus of W. E.
Yates et al., it is not possible to keep the temperature
oE the vibration transmission part constant and to
restrict external force being applied only to the end of
vibrating rod. Therefore, it is presumed that it is not
1~ possible to form uniform liquid droplets from a high
temperature liquid or a high pressure liquid by the
apparatus of W. E. ~ates et al.
Various conditions for preparing uniform liquid
droplets by the vibration method have been studied in
detail as disclosed by T. Sakai [cf. T. Sakai, Proc.
ICLASS-'82, 37 to 45 (1982)~. It has been found that
when uniform liquid droplets are stably formed,
parameters including a viscosity and a surface tension of
the liquid, a flow rate of liquid jetted from the
orifice, a size of the orifice, and a frequency and a
displacement of cyclic turbulences are within a specific
range (hereinafter, such specific range referred to as
"synchronized condition"). As a general tendency, the
higher the liquid viscosity becomes and the smaller the
size of the orifice becomest the higher flow rate and
frequency of jetted liquid are required to be
synchronized. That is, in order to form liquid droplets
with a small diameter from a li~uid containing high
molecular substances, the aperture diameter of orifice
must be small, and the flow rate and frequency of jetted
liquid must be high. In addition, in many cases, such
liquid has a high temperature and a high pressure.
Consequently, it is difficult to form uniform liquid
droplets with a small particle size from such liquid by
applying the conventional methods.
In recent years, there has been tried an
extracorporeal hemo-perfusion treatment using a~
adsorbent, for various obstinate diseases and its effect
.
- . . ~
,
1 32 1 454
has been confirmed. ~s generally known, blood cells are
physiologically quite unstable outside of the body, and
therefore easily injured, for instance, there occurs a
decrease in their number because of their adhesion to the
matters with which blood cells contact, hemolysis or
coagulation. Therefore, in an extracorporeal hemo-
perfusion treatment, blood is separated into unstable
blood cells and relatively stable blood ]plasma by a
centrifugal separator or by a membrane for the separation
of blood plasma, and then the blood plasma containing
pathogenic substances is treated by an adsorbent~
Howeverj it is as a matter of course that if blood can be
treated directly by an adsorbent, not only the
extracorporeal hemo-perfusion treatment system can be
remarkably simplified but also physiological burden to
the patient can be alleviated.
As for the relationship between blood
compatibility and adsorbent characteristics, in other
words, whether an adsorbent injures blood or not, though
there are many points not explicated chemically~ there
has been physically explicated a point that in order not
to injure blood, an adsorbent should be smooth on its
surface and the pressure drop occured in using it should
be small.
A granular activated charcoal adsorbent has
been employed in a direct extracorporeal hemo-perfusion
system since decades ago lcE. T. M. S . Chang et al, Trans
Amer. Soc. Artif. Int. Organs 17, 246 (1971~]. At the
beginning, an adsorbent covered with a hydrophilic
polymer was employed for the purpose of avoiding the
effluence of activated charcoal and improving the
chemical blood compatibility. Recently, uncovered
activated charcoal with smooth surface has been employed
[cf. VO Bonomini and T. M. S~ Chang, "Hemoperfusion"
(1981), ISBN 3-8055-3421-3]. This fact shows that the
relationship between blood compatibility and chemical
characteristics of adsorbent is hard to estimate since
the chemical properties of the surface of the above two
1 321 454
-- 8
adsorbents are quite difEerent. The granular activated
charcoal has an relatively large average particle size of
0.5 to 3 mm. The granular activated charcoal adsorbent
is employed with the object of adsorbing blood plasma
components having a relatively low molecular weight.
Such components can be rapidly adsorbed even if the
granular activated charcoal has a large particle size.
However, pathogenic substances causing obstinate diseases
are, in many cases, substances having a high molecular
weight of several handreds of thousand to several
millions daltons. Since such substances having a high
molecular weight diffuse at a low rate, it is required
that particles for an adsorbent for such substances have
a particle size of not more than about 400 ~m, preferably
not more than 300 ~m so as to attain a practical
adsorption rate. However, there has not been hitherto
well known an example of carring out a direct
extracorporeàl hemo-perfusion treatment using granular
activated charcoal adsorbent having such a small particle
size. Though there is a report in which a direct
extracorporeal hemo-perfusion treatment is carried out
using a modified polyvinyl alcohol gel having particle
sizes of 74 to 210 ~m [cf. Ichikawa et al, Jinkozoki 12
(1), 116 ~1983)], there are not reported data showing
whether a hemolysis occurs or not and data of pressure
drop, these data being basic data relating to blood
compatibility.
As described above, in prior arts, there are
defects that the polymer particles have a broad particle
size distribution or that minute polymer chips occur due
to the particle structure, and further that in preparing
such polymer particles, such a complicated apparatus as a
multiple tubular nozzle is necessary and a delicate
operation is required.
In a conventional apparatus for forming liquid
dropletsl it is difficult to form uniform liquid droplets
having a small particle size from a high viscosity
liquid. Further, when the liquid has a high temperature
: ,
1 321 4~4
and a high pressure, it is still more difficult to form
uniform liquid droplets with a coventional apparatus.
An object of the present lnvention is to
provide uniform particles having a structure which does
not cause an occurence of minute polymer chips and a
process for preparing such particles.
A ~urther object o~ the present invention is to
provide an apparatus suitable for use in the above
process, which can form uniform liquid droplets from a
liquid even if the liquid has not only a high viscosity
but also a high temperature and a high pressure.
As described above, though there has not belen
explicated what chemical properties an adsorbent should
have so that a direct extracorporeal hemo-perfusion
treatment can be carried out with employing the
adsorbent, at least, the pressure drop occured in using
it should be small, and the surface of adsorbent should
be smooth. Further, there has not been clear what
properties of adsorbent suitable for evaluating blood
compatibility therewith since conventional adsorbents
have a broad particle size distribution. As described
above, there are many points not explicated chemically as
to the relationship between blood compatibility and
properties o~ adsorbent.
A still further object of the present invention
is to provide uniform polymer particles suitable for use
in a direct extracorporeal hemo-perfusion treatment with
a practical flow rate of blood to be treated without
causing problems such as hemolysis and increase in
pressure drop.
SUMMARY OF THE INVENTION
The present inventors have found that uniform
polymer particles in a spherical form, having a three
dimensional network structure and having a structure
which does not cause an occurrence of polymer chips can
be prepared without using a complicated apparatus such as
a multiple tubular nozzle when a polymer solution is
1 321 454
-- 10
jetted from an orifice into a gas atmosphere in the form
of uniform liquid droplets with electric charges with a
same sign at a constant flow rate while applying cyclic
turbulences having a constant fre~uency to the solution,
the droplets are let fly through the gas atmosphere, and
then the droplets are let into a coagulating liquid which
is a non-solvent of the polymer of the polymer solution,
is miscible with the solvent of the polymer solution, and
has a surface tension enough to spontaneously wet the
liquid droplets, the distance of the gas atmosphere
between the orifice and the surface of the coagulating
liquid being such that the droplets are not greatly
deformed by the collision with the coagulating liquid.
The present inventors eagarly studied in order
to provide uniform polymer particles suitable for use in
direct extracorporeal hemo-perfusion treatment which do
not cause problems such as hemolysis and increase in
pressure drop even if their particle size is not more
than 400 ~m, and investigated particle properties
required for direct extracorporeal hemo-perfusion
treatment, with paying attention only to physical
properties. As a result, the present inventors have
found that a direct extracorporeal hemo-perfusion
treatment can be carried out with a practical flow rate
of blood to be treated without problems such as hemolysis
and increase in pressure drop by using uniform polymer
particles having a volume average particle size of 30 to
~00 ~m, wherein not less than 80 % by volume of the whole
particles have a particle size within the range of +20 %
of the volume average particle size, the content of
particles having a particle size of less than 74 ~m is
not more than 5 % by volume, the content of particles
having a particle size o less than 25 ~m is not more
than 0.1 % by volume, and said uniform polymer particles
do not include particles having a particle size of not
more than 5 % of the volume average size. It is also
found that such particles with a narrow particle size
distribution are prepared by a process hitherto unknown.
1 321 454
-- 11 --
According to the present invention, there is
provided uniform polymer particles in a spherical Eorm,
having a three dimensional network structure, wherein not
less~than 80 % by volume, preferably 90 ~ by volume of
the whole particles have a particle size within the range
of ~20 ~ of the volume average particle size, more
preferably within the range of +10 % of the volume
average particle size, and the polymer particles do not
include particles having a particle size of not more than
5 % of the volume average particle size.
Secondly, there is provided a process for
preparing uniform polymer particles in a spherical form
which comprises jetting a polymer solution in the form of
uniform liquid droplets with electric charges with a same
sign from an orifice into a gas atmosphere at a constant
flow rate while applying cyclic turbulences having a
constant frequency to the solutionl letting the droplets
fly through the gas atmosphere and then letting the
droplets into a coagulating liquid which is a non-solvent
of the polymer of the polymer solution, is miscible with
the solvent of the polymer solution, and has a surface
tension enough to spontaneously wet the liquid droplets,
the distance of the gas atmosphere between the orifice
and the surEace of the coagulating liquid being such that
the droplets are not greatly deformed by the collision
with the coagulating liquid.
Thirdly, there is provided an apparatus for
forming uniform liquid droplets, which comprises a
cylinder having an inlet of a liquid and at least one
orifice through which the liquid is sent ou~, a vibrating
rod inserted in the cylinder, a vibration generator
connected with the vibrating rod, and an O-ring for
sealing the clearance between the cylinder and the
vibrating rod.
~orthly, there are provided uniform polymer
particles in a spherical form, suitable for use in a
direct extracorporeal hemo-perfusion treatment having a
volume average particle size of 80 to 400 ~m, wherein not
::
:::,. .. : - ~ :.~ ..
: . .
1 321 ~5~
- 12
less than 80 % by volume oE the whole part;cles have a
particle size within the range of +20 % oE the volume
average particle size, the content o particles having a
particle size Oe less than 74 ~m is not more than 5 ~ by
volume, the content of particles having a particle size
of less than 25 ~m is not more than 0.1 % by volume, and
said uniform polymer particles do not include particles
having a particle size of not more than 5 % of the volume
average particle size.
BRIEF DESCRIPTION OF THE DRAWINGS
-
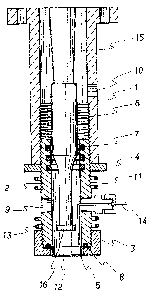
Fig. 1 is a schematic sectional view showing an
apparatus for forming uniform li~uid droplets oE the
present invention.
Fig. 2 is a sketch of the photograph oE uniform
liquid droplets obtained in ~xample 1.
DETAILED DESCRIPTION
The meanings of some specific terms or mesuring
process used in the present specification are explained
below.
The term "polymer particle~ in a spherical
form" means polymer particles in the form of a spheroid,
smooth on the surfacet which are applicable to various
uses.
The term "uniform particles" means particles
wherein not less than 80 % by volume of the whole
particles have an particle size within the range of +20 %
of, preperably within the range of +10 ~ of the volume
average particle size.
The term "volume average particle size" is the
value obtained by classifying particles by means of wet
sieve classification, in which water, an alcohol or the
like is employed as a dispersion mediuml using standard
sieves under Japanese Industrial Standards, collecting
particles captured in each sieve, measuring each total
volume of particles sedimented after allowing them to
stand for one day, and caluculating according to the
1 321 454
- 13
following equation:
DiVi
volume average particle size =
~vi
wherein Di is the size of sieve opening, and Vi is each
total volume of sedimented particles in particles
captured in each sieve having the size of sieve opening
of Di.
The term "particles with a particle size of
less than D ~m" means particles pass through a sieve with
the size of sieve opening of D ~m. The term "particles
with a particle size oE not less than D ~m" means
particles do not pass through a sieve with the size of
sieve opening of D ~m.
When not less than ~0 % by volume of the whole
particles have a particle size within the range of -~20 %
of the volume average particle size, such polymer
particles can be employed in various uses as they are
without a classification process. Further, not less than
90 % by volume of the whole particles have a particle
size within the range of -~20 % of the volume average
particle size, such polymer particles have advantages not
only a classification process is not needed but also, in
using as parent material for an ion exchange resin or as
a packing for a chromatography, they can be uniformly
packed and the pressure drop is small.
When uniform polymer particles have a volume
average particle size of 10 to 1,000 ~m, such uniform
polymer particles can be generally used in the above-
stated uses. When a uniform polymer particles have a
volume average particle size of 10 to 500 ~m, such
uniform polymer particles can be used as parent material
for an ion exchange resin with a high ion exchange rate,
as an adsorbent with a high adsorption rate and high
selectivity, or as a packing for a chromatography for an
ind`ustrial use. When uniform polymer particles have a
. .
t
~. :
,
1 321 45~
volume average particle size of 20 to 250 ~m, such
uniform polymer particles can be used as an adsorbent or
a packing for a chromatography for substances with a high
molecular weight with sharp fractions in addition to the
uses described above.
However, uniform polymer particLes having a
volume average particle size of less than 10 ~m are hard
to produce.
The presence of very small particles with a
particle size of less than 5 ~m, which contaminate a
treating liquid or cause an increase in pressure drop was
examined by a microscope or a Coulter Counter
(commercially available under the trade name "Coulter
Counter" made by Coulter Electronics Inc.)
The term "three dimensional network structure"
literally means a structure that fiber or porous sheet is
three dimensionally connected with each other, in
contrast to particles made by polymerizing polymerizable
monomers having a structure that minute particles
aggregate.
The term "direct extracorporeal hemo-perfusion
treatment" means that in an extracorporeal hemo-perfusion
treatment t blood is treated by an adsorbent directly
without separating the blood into blood cell and blood
plasma~
In order to obtain firm polymer particles which
do not cause an occurrence oE minute polymer chips, the
polymer concentration of polymer solution, though it i5
determined depending on the molecular weight of polymer,
should be not more than 5 ~ by weight. Such polymer
solution is a solution of polymer with relatively high
degree of polymerization, having a viscosity,though its
viscosity depends on the temperature at which the
measurement of viscosity is carried out, of not less than
about 10 cP, preferrably not less than about 50 cP. When
the solution has a viscosity of more than ~,000 cP, it is
hard to form liquid droplets having a particle size of
not more than 1,000 ~m by a vibration method. Therefore,
* Trade-mark
.
1 32 1 454
- 15
the viscosity of solution is preferably not more than
2,000 cP.
As previously mentioned, in order to form
uniform liquid droplets, the viscosity and surface
tension of polymer solution, the flow rate of li~uid, the
size of orifice, and the frequency and displacement of
cyclic turbulences, these parameters being mutually
related, should be adjusted in a speci~ic ran~e
(synchronized condition).
When the diameter of liquid droplets to be
formed is more than 1,000 ~m, the synchronized condition
can be also attained by directly vibrating the nozzle
[af. T. Sakai, Proc. ICLASS-1982, 37 (1982)]. However,
when the particle size of liquid droplets to be formed is
small, the frequency to be synchronized is so high that
an enomous ellergy is required to directly vibrate the
nozzle. Therefore, in this case it is preferable to
apply cyclic turbulences directly to the solution.
Especially, when the diameter of liquid droplets to be
formed is not more than 250 ~mr the frequency to be
synchronized reaches 3,000 to 40,000 ~z.
In Fig. lr the cylinder (2) is fixed on the
fixing cylinder (1). The nat (4) is tightened up to
ensure the fixation between the cylinder (2) and the
fixing cylinder (1). The cylinder (2) has an inlet of a
liquid and orifices (12). The cylinder (2) is further
equipped with the O-ring (7) on the position where the
vibrating rod (6) slidesr for sealing the clearance
between the cylinder (2) and the vibrating rod (6).
With the vibrating rod (6) was connected a
vibration transmission part (15) in order to transmit
vibration generated by a vibration generator (not shown
in Fig. 1) r for instance, a magnetostrictive vibratorr an
electrostrictive vibrator or a electromagnetic coil
vibrator to the vibrating rod (6).
When a magnetostrictive vibrator or an
electrostrictive vibrator is employed as a vibration
generator, the vibrator, the vibration transmission part
,
t3214~4
- 16
(15) and the vibrating rod (6) are organized 50 as to
form a resonator. When an electromagnetic coil vibrator
is employed as a vibration generator, the vibrating rod
(6) is directly connected with a vibrator of the
vibration generator. In the latter case in order to
reduce the weight of the vibrating rod (6), it is
preferred that the vibrating rod (6) is thin other than
the end (16) of vibrating rod ~6) which :Ls thick as shown
in Fig. 1.
In order to efficiently thansmit the
vibrational energy to the vibrating rod (6), it is
preferable to employ the O-ring (7) having a small
contact resistance for sealing the clearance between the
cylinder (2) and the vibrating rod (6).
The distance between the nozzle (5) having
orifices (12) and the end (16) of vibrating rod (6) can
be suitably controlled by a screw (11) of the cylinder
(2) and a nat (4) for fixing the cylinder.
The nozzle (5) is fixed to an end of the
cylinder (2j by a nat ~3) for fixlng the nozzle (5). The
clearance between the nozzle (5) and the cylinder (2) i5
sealed by the O-ring (8).
The liquid solution by a gear pump is
introduced into the cylinder (2) through an inlet of a
liquid (9) and is jetted from the orifices (12) while
applying a cyclic pressure change to the liquid by
reciprocating motlon of the vibrating rod (6) over the
nozzle (5). If necessary, a polymer solution in the
cyllnder (2) can be heated by a heater (13). The
temperature sensor (14) is therefore also used for
managing the temperature.
Flg. 1 shows a apparatus with a
magnetostrlctlve vibrator as a vlbrating generator. The
magnetostrictlve vibrator is used for the purpose of
generatlng vibration particularly with a high frequency,
for instance, supersonic vibration with a frequency of
about 20,000 to 40,000 ~z. When the apparatus for
forming liquld droplets of the present invention is used
1 321 454
- 17
and the above-mentioned vibration with a high frequency
is applied, uniform liquid droplets with a small particle
size are obtained. Herein7 the term "uniform liquid
droplets" means that the droplets are produced under the
S synchronized condition, in other words, that the droplets
are produced periodically with the same frequency with
the vibrating rod (6).
The frequency of vibration is preferably 1,000
to 40,000 Hz, more preferably 3,000 to 40,000 Hz. When
the frequency is less than 1,000 Hz, it is difficult to
form uniform liquid droplets. When the frequency is more
than 40,000 ~z, the distance between formed liquid
droplets becomes too small and the reconnection frequency
of liquid droplets becomes high, and consequently it
becomes hard to form uniform liquid droplets.
The clearance between the vibrating rod (6) and
the cylinder t2) is sealed by the O-ring (7) on a site
so-called node, which is well known by a person skilled
in the art, where the displacement is zero, and the
friction caused by a slide of the vibrating rod (61 on
the O-ring (7) can be almost neglected.
The distance between the end (16) of the
vibrating rod (6) and the nozzIe (5) is preferably 2 to
20 mm, more preferably 5 to 15 mm. In case such distance
is less than 2 mm~ when the frequency is as high as that
of supersonic waves, a cavitation occurs and there arises
a possibility of an erosion of the end (16~ of vibrating
rod (6) or the inside of the nozzle (5). In case such
distance is more than 20 mm, the turbulences given by the
vibrating rod (16) are attenuated before the turbulences
reach the inside of nozzle (5), the turbulences are not
transmitted to the liquid jetted from the orifice (12),
and it becomes difficult to form uniform liquid droplets.
The nozzle ~5) preferably has one or more than
one orifices, and each orifice is preferably a round
shape orifice having an aperture of not more than 500 ~m,
more preferably 10 to 250 ~m, still more preferably 20 to
100 ~m.
. . .
.~
~,;
1 32 1 454
- 18
When the orifice (12) have an aperture diameter
of more than 500 ~m~ uniform liquid drop:Lets having a
diameter of not more than 1,000 ~m, cannot be always
obtained. As the shape of the orifice (1~, there can be
exemplified round shape, slit shape, rectangle, multiple
tubular shape, and the like. Among themr round shape is
preferable since spherical liquid droplets usable in many
uses can be obtained.
If necessary, the apparatus may be equipped
with a heater (13) so that a liquid in the cylinder (2)
can be heated. The temperature sensor (14) may be used
for managing the temperature of heated liquid.
Particularly, when it is necessary to keep the liquid a
high temperature, the vibration transmission part (15~
should be cooled so that the synchronized condition is
maintained. The inlet/outlet of cool water (10) is used
for that purpose.
When the liquid in the cylinder (2) is heated
by the heater (13) in the present invention, it is
preferable to cool the vibration transmission part ~15)
and a part of the vibrating rod (161 from the joint with
the vibration transmission part (15) to the position
contacting the O-ring (7) by, for instance, letting cool
water flow there.
The process of forming very small liquid
droplets from a high temperature, high pressure, and high
viscosity liquid by the apparatus o the present
invention is explained belowO
A liquid sent from a gear pump at a constant
3Q f]ow rate is put into the cylinder (2) from the inlet of
the liquid (9) and jetted from orifices (12) while
applying a cyclic pressure change to the liquid by the
vibrating rod (6).
~s described above, as the size of orifice (12)
becomes small, the synchronized flow rate of the liquid
and frequency of the vibration become high and
consequently the pressure applied to the liquid becomes
high. The apparatus of the present invention have a
.
., ~ ,' ~
.: :
.
1 32 1 454
-- 19
pressure resistence of not less than several tens atm as
is presumed from the structure thereof. As the viscosity
of the liquid becomes high, the turbulences, i.e. the
pressure change applied to the liquid at the orifice (12)
by the vibrating rod (6) becomes large. The turbulences
and the pressure change can be controlled by adjusting
the distance between the nozzle (5) and the end (16) of
the vibrating rod (6). However, when the viscosity is
more than about 2,000 cP, in case using an orifice having
an aperture diameter of not more than 100 ~m, uniform
liquid droplets cannot be formed even if a large pressure
change is applied.
In order that the particles, which are obtained
by forming uniform liquid droplets, and coagulating the
droplets by contacting them with a coagulating liquid,
have a mechanical strength enough for using them in the
above-stated uses, the concentration of polymer in the
polymer solution should be at least about 5 % by
weight Such solution has a viscosity at least about 10
cP, though the viscosity depends on the temperature.
Therefore, in using the apparatus of the present
invention, a liquid with a viscosity of 10 to 2,000 cP,
preferably 50 to 2,000 cP is mainly employed.
Even if the liquid has such high viscosity,
uniform li~uid droplets can be formed, when the diameter
of liquid droplets is more than about 1,000 ~m, by a
conventional apparatus in which an orifice is directly
vibrated. However, when the diameter of liquid droplets
is not more than 250 ~m, the frequency of turbulences to
be synchronized is not less than about 3,000 Hz. And
since, as described above, an enomous energy is requied
in order to directly vibrate the orifice, it is
economical to apply the turbulences to the liquid
directly as the apparatus of the present invention.
It is possible to confirm whether the diameter
of the liquid droplets is uniform or not in an usual
manner. That is to say, in case uniform li~uid droplets
are formed, liquid droplets ranging at regular intervals
1321454
- 20
can be seen by observing the droplets with a magnifying
glass or taking a photograph of the droplets while the
flashing cycle of the stroboscope is synchronized with
the liquid droplets generation cycle.
The uniform liquid droplets formed move at
random by air resistance with going away ~rom the nozzle
(5), and many liquid droplets collide and reconnect each
other. As reported by J. M. Schneider et al, the
reconnection of the liquid droplets can be prevented for
a arelatively long time by electrifying each of the
liquid droplets with electric charges with a same sign
[cf. Rev. Sci, Instr. 35, 1349 ~1964)].
In the process of the present invention, the
liquid droplets thus jetted into a gas atmosphere at a
constant flow rate are let into a coaguating liquid which
is a non-solvent of the polymer of the polylner solution,
is miscible with the solvent of the polymer solution, and
has a surface tension enough to spontaneously wet the
liquid droplets, after the droplets let fly such a
distance that the flying rate of droplets are lowered as
low as the droplets are not greatly deformed by the
collision with the coagulating liquid.
~ hen the surface tension of coagulating liquid
is more than that of the solvent of polymer solution and
therefore the liquid droplets are not spontaneously
wetted, the droplets ~loat on the surface of the
coagulating liquid for a long time even iE the specific
gravily of the liquid droplets is more than that of
coagulating liquid, and ~roplets later reached the
surface collide with the floatin~ droplets to form a
large reconnected droplets. On the other hand, when the
droplets reached the surface of coagulating liquid are
wetted with the coagulating liquid, since the
reconnection of droplets does not occur even if the
droplets collide with the droplets later reached the
surface, and the droplets later reached the surface are
also wetted with the coagulating liquid, each droplet
becomes a separate polymer particle. The coagulating
1 321 454
- 21
liquid is selected from ones having a surface tension
approximate to that of the solvent of the polymer
solution or preferably ones having a surface tension of
less than that of the solvent of the polymer solution~
~s disclosed in a preceding pat:ent application
by the present inventors [Japanese Unexamined Patent
Publication (Tokkyo Kokai) No. 191033/1987), when the
viscosity of the polymer solution is high and the
diameter of the liquid droplets is small/ since the
jetting rate of the polymer solution jetted from the
orifice, i.e~ the initial flying rate of the liquid
droplets, reaches several m/sec to decades m/sec, if
these droplets are immediately let into the coagulating
liquid, the droplets are broken or deformed to be a flat
shape by an impact of the collision. In order to avoid
such break or deformation, the jetted droplets should be
let into the coagulating liquid after reducing flying
rate of the droplets.
As described above, the reconnection of the
liquid droplets can be prevented for a relatively long
time by electrifying each of the liquid droplets with a
single sign. In addition, the present inventors have
found that the flying rate of the jetted liquid droplets
is rapidly decreased by electrifying each of the liquid
droplets with a single sign~ Particularly, when the
diameter of the liquid droplets is small, the
synchronized initial flying rate i~ high, and therefore
the liquid droplets without electric charges are
sometime~ deformed to be a flat shape even if letting the
droplets fly a distance of 2 m. However, in case such
liquid droplets have elecric charges with a same sign,
they enter the coagulating liquid without deformation
even if letting the droplets fly a distance of 30 cm.
It is important to minimize the distance
between the orifice and the surface of the coagulating
li~uid in preventing the reconnection of the liquid
droplets. Therefore, the distance between the orifice
and the surface of the coagulating liquid should be
~ . ~
. ~
1 321 454
- 22
minimized as far as the droplets are not deformed in such
extent that the droplets become not spheroidal.
When the liquid droplets have electric charges
with a same sign, liquid droplets are scattered from each
other because of their mutual repulsion. These droplets
are attracted to a matter having the opposite electric
charge or a conductors grounded. That i5 to say, the
droplets flown off tend to be attracted to the wall of
container Eor coagulating liquid or the electrodes for
providing electric charges. The droplets attracted to
such matters cause some obstraction, for instance,
ununiform particles are formed, the electric field
strength are reduced, or the like. However, the whole
liquid droplets can be attracted into the coagulating
liquid by using an electric conductive coagulating liquid
in a metallic container, and grounding the metallic
container.
The porosity of the particles is controlled by
various methods usually employed in dry-wet spinning
processes.
It is known that the pore size of pores on the
surface of particles prepared by the dry-wet spinning
process have a high uniformity whether they have a skin
layer or not.
Generally, when li~uid droplets are coagulated
by a coagulating liquid with a high coagulating activity,
porous particles with a thin fine surface layer, so-
called non-porous skin layer, are obtained. On the
contrary, when a coagulating liquid having a low
coagulating activity is employed, the particles have a
skin layer with a porous structure. When a coagulating
agent having a still lower activity is employed, the
particles do not have a skin layer and the surface of
particles also have a network structure.
Through a skin layer, substances having a size
of not more than a certain size pass, but substances
having a size of more than the certain size do not pass.
By controlling the pore size of the skin layer, the
:,,
. .
1 32 1 454
- 23
molecular weight of substance which pass through, ~hich
corresponds to the above stated certain size, can be
varied from decades to hundreds of thousand. Therefore,
an adsorbent showing an excellent selectivity to a
substance having such molecular weight can be obtained by
employing particles with a skin layer. Conventional
polymer particles prepared by polymerizing liquid
droplets of polymerizable monomers do not have such
advantage.
Particles which do not have a skin layer and
have a network structure over the particle have an
excellent selectivity to a substance having a molecular
weight of more than the aforementioned, for instance, to
a substance having a molecular weight of several
millions. Also, even if the particles have such porous
structure, minute polymer chips do not occur since the
polymer in the particle have a three dimensional network
structure formed by fiber or porous sheet.
Generally, the size of mesh of the three
dimensional network structure decreases as the
concentration of a polymer in the polymer solution
increases.
The porosity of the particles can be also
controlled by adding a bad solvent of the polymer to the
polymer solution or by adding an additives which can be
easily removed by extraction, for instance, a water-
soluble polymer such as polyethylene glycol, polyvinyl
pirrolidone or dextran to the polymer solution in an
amount such that the viscosity oE the polymer solution is
a viscosity in the range of constituting a synchronized
condition, that is, according to the experience of the
present inventors, the range of not more than 2,000 cP.
A porous particulate polymex in a spherical
form of the present invention, is thus obtained. In
order to improve the characteristics of the polymers
according to their uses, various post-treatment may be
conducted. Particularly, heat-treatment in a non-solvent
is effective to stabili~e the particle structure.
1 321 ~54
- - 2~ -
In the process of present invention, any
polymers being dissoluble in the solvent can be used.
Examples of the polymers which are especially use~ul in
the invention are mentioned below.
~ polystyrene is useful as a packing material
used Eor chromatography, which does not have defects such
that when using, a large pressure drop is caused or
minute particles contaminate the liquid to be treated,
and as an adsorbent causing only a small pressure drop
and no occurrance of minute polymer chips, and having
excellent selectivety.
A polymer which is available for crosslinking
and introduction of an ion exchange groupl such as a
styrene-butadiene copolymer or a styrene-chloromethylated
styrene copolymer, is suitably used as a parent material
for an ion exchange resin which causes only a small
pressure dropJ high ion exchange rate and no generation
of minute polymer chips.
A polyvinyl alcohol or ethylene-vinyl alcohol
copolymer is useEul in affinity chromatography as a
carrier having an active hydroxyl group, having excellent
selectivety, and causing only a small pressure drop.
Also, various kinds of natural high molecular
substances such as cellulose, silk, collagen, and their
derivatives are useful as a carrier for chromatography as
they are, or as a carrier for affinity chromatography.
Further, many other vinyl polymers and
condensation polymers can be used for the above-mentioned
uses.
The solvents of these polymer solutions are
found in manuals, e.g. JO Brandrup, "Polymer Handbook 2nd
edition", John Wiley and Sons, Inc. (1975), and the
like. However, as mentioned later, the preferable
coagulating liquid in the invention is an aqueous
solution, thereforel a water-miscible solvent is
desirable in order that it i5 miscible with the
coagulating liquid. Examples of the solvent which is
used in a number of polymer solutions are, for instance,
: . ........ ~ . :
.
1 32 1 454
N-methyl-2-pyrrolidone, dimethylformamide,
dimethylacetoamide, dimethyl sulfoxidel diacetone
alcohol, acetone, tetrahydrofuran, dioxane, and the like.
Also, it i5 possible to use these solvents as an
admixture, or mixed solvents of the above solvents with
other solvents such as ethanol, methanol, ethylene
glycol, propylene glycol and glycerol. E~urther, there
can be added a water soluble polymer such as polyethylene
glycol or polyvinyl pyrrolidone as an additive for the
purpose of controlling the porosity of the particles,
which can be extracted.
For example, as a solvent for cellulose, a
known solvent, e.g. a mixed solvent of dimethyl sulfoxide
and formaldehyde, cuprammonium solution, an aqueous
solution of calcium thiocyanate can be used.
Generally, the solvents are suitably selected
depending on the kinds of the polymers.
As mentioned above, it is desirable that the
surface tension of the coagulating liquid is approximate
to or less than that of the solvent of the polymer
solution, and that the coagulating liquid is electric
conductive. As the coagulating liquid, water with a
surface active agent, an aqueous solution of alcohol and
an aqueous solution of the above-mentioned solvent or a
mixture thereof are particularly preferable so as to
control the fine structure of the obtained polymer
particles.
Uniform polymer particles in a spherical form
suitable for use in the direct extracorporeal hemo-
perfusion treatment of the present invention is describedhereinafter.
The blood compatibility is judged according to
the following method. A column having an inner diameter
of 7 mm and a length of 100 mm i5 prepared and filters
made of pclyester, of which opening diameter is 20 ~m,
are fixed at both ends of the column in order to prevent
the particles from leaking out. Then, the particles are
dispersed in water of a volume of five times as much as
. . . ~ . ~ ::
: ~ ~
1 321 45~
- 26
the volume of the sedimented particles, and the particles
are packed into the column by transmitting the dispersion
into the column at a rate of 5 m~/min as observing so
that bubbles of gas are not mixed.
~he average linear velocity is determined at
1.3 cm/min in considera~ion that blood is circulated at
the ~low rate of at least 50mQ/min in actual treatment
system and a column used in practice has a sectional area
of about a hundred times of that of the column used in
this examination.
Bovine blood maintained at the temperature o~
37C is passed through the column at the above-mentioned
average linear velocity. After an hour, the difference
between the pressure at the inlet and the pressure at the
outlet of the column, i.e. the pressure drop is measured,
and it is con irmed whether hemolysis occurs or not to
judge the blood compatibility. That is, when no
hemolysis occurrs and the pressure drop i5 not more than
100 mmHg, the blood compatibility is judged good. The
judgement whether hemolysis occurred or not is conducted
by means that the blood run out of the column is
subjected to the centrifugation, it is decided that
hemolysis occurred when the plasma is extremely colored
and hemolysis did not occur when the plasma is not
colored.
As materials of the particles, cellulose
acetate and cellulose are employed for convenience sake
since there are a lot of points not explicated in the
relation between the blood compatibility and the chemical
properties of the particles as mentioned above, and
therefore, the materials cannot be defined.
Generally available particles, e.g. particles
used for chromatography have a broad particle size
distribution. By repeating classification, it becomes
possible to obtain the particles having a narrow particle
size distribution from them. Nevertheless, the yield is
low in this case, accordingly, the method is not
practical~
.
.
1321454
- ~7
Thereforel as described later the uniform
particles of cellulose acetate are prepared according to
the process of the present invention and the particles
are compared with the commercially available cellulose
particles used for chromatography on the blood
compatibility.
Herein, when the uniform particles are densely
packed into the column, the particle size of the
substances which can be passed through between the
particles in the column is about 15 % of the particle
size of the uniform particles. That is, when the
particles having the particle size of about 70 ~m are
packed into the column, there arises a fear that
erythrocyte sticks between the particles. If the volume
average particle size is less than 80 ~m, the pressure
drop becomes too large. Accordingly, it is required that
the particles have the volume average particle size of 80
to 400 ~m, preferably 80 to 300 ~m. When the volume
average particle size is less than 80 ~m, erythrocyte
sticks between particles and the pressure drop is over
100 mmHg, also when the volume average particle size is
more than 400 ~m, the ability of adsorbing pathogenic
substances having a high molecular wei~ht is small, both
of which are unpreferable. Further, it is necessary that
not less than 80 % by volume of the whole particles have
a particle size within the range of _20 % of the volume
average particle size. It is not preferable that the
particles have a particle size distribution broader than
the ab~ve-mentioned, i.e. not less than 80 % by volume o
the whole particles do not have a particle size within ~
the range of ~20 % of the volume average particle size in
such case, the increase of the pressure drop or the
hemolysis occurs when many particles have a particle size
of less than -20 % of the volume average particles, or
the ability of adsorption is lowered when many particles
have a particle size of more than +20 % of the volume
average particles. Still more, even though the volume
average particle size is not less than 80 ~m, the content
!
' ': ' , ', ' ' ' : .
1 321 454
- ~8
of the particles having a particle size of less than
74 ~m should be limited to not more than 5 ~ by volume of
the whole particles. When the content of them i5 more
than 5 ~ by volume, the pressure drop is over 100 mmHg,
S which is unpreferable.
When a direct extracorporeal hemo-per~usion
treatment is carried out, minute particles must not flow
into the blood. For the above reason, a filter with
openings as small as possible is attached to the outlet
for the blood of a column packed with particles.
However, it is also desired that the opening diameter of
the filter is at least 20 ~m so as not to injure
erythrocyte which is nearly 10 ~m in diameter.
Therefore, the content of the particles having a particle
lS size of less than 25 ~m must be not more than 0.1 % by
volume of the whole particles. When the column is packed
with the particles containing more than 0.1 % by volume
of the particles of which particle size is less than
25 ~m, the opening of the filter is clogged. However,
according to the process of the present invention, it can
be obtained the uniform polymer particles in a spherical
form containing only few particles having a particle size
of less than ~5 ~m, further, it is possible to prepare
the uniform polymer not containing the minute particles
of the above size at all.
The particles of the present invention, as a
matter of cource, have a strength so as not to be
deformed with the pressure drop of at least 100 mmHgr
which is confirmed from a result that the relation of the
pressure drop and the flow rate, when passing water
through the column packed with the particles, ke~ps a
linear relationship until the pressure drop is increased
to 100 mm~g.
The adsorbent use of the uniform polymer
particles of the present invention, having the above-
mentioned physical properties makes it possible to
promptly remove pathogenic substances having a high
molecular weight by a direct extracorporeal hemo-
.
" ~' ''~ ', ` . ' " '
, ~ :.~. . : . ,
~ ' , ,'' ' ~ ' .
1 321 454
- 29
perfusion treatment, which i5 of great value.
The present invention is more specifically
described and explained by means of the Eollowing
Examples in which all percents ant parts are by weight
unless otherwise noted. It is to be undlerstood that the
present invention is not limited to the Examples and
various changes and modifications may be made in the
invention without departing from the spirit and scope
thereof.
Example l
Cellulose acetate (degree of acetylation: 55 %)
was dissolved in a mixture of N-methyl-2-pyrrolidone and
propylene glycol mixed at a weight ratio of 4 : 6 to
prepare a polymer solution having a cellulose acetate
concentration of 5 %. The polymer solution had a
viscosity of 78 cP at 90C. The surface tension of the
mixed solvent was 38 dyn/cm, which is the arithmetical
mean value of the surace tension of the solvent at 25C.
By using the apparatus shown in Fig. 1, the
polymer solution was jetted through the nozzle (5) having
the orifice (12) of two having a diameter of 50 ~m,
aligned at an interval of 5 mm. At that time, the
parameters were adjusted to attain the synchronized
conditiont i.e. the distance between the end (16) of the
vibrating rod (6) and the nozzle (5) was 15 mm, the
temperature of the polymer solution was maintained at
90C, the frequency of the vibrating rod (6) connected
with the magnetostrictive vibrator was 25 ~Hz and the
flow rate of the jetted liquid was 18 m/sec, to give
uniform liquid droplets.
~ t the position of about 2 mm from the lower
ace of the nozzle (5), parallel-plate electrodes having
a width of 20 mm and a distance between the plates of lO
mm was set paraliel to the orifices (12), and a voltage
of 500 V was given to the droplets between the cylinder
and the parallel-plate electrodes. The cylinder was
grounded.
:. : :. . .
1 32 1 ~54
- 30
A coagulating liquid was put into a grounded
cylindrical container made of stainless steel having a
diameter of about 40 cm, and the container was put right
under the nozzle (5) so as to make the distance between
the nozzle and the surface o the coagulating liquid 40
cm. As the coagulating liquid, a 40 % ethanol aqueous
solution under room temperature was employed. The
surface tension of the coagulating liquid was 32 dyn/cm
at 25C.
The obtained particles were perfectly
spherical, and from which any minute polymer chips did
not occur even though the particles were stirred with a
magnetic stirrer for about 3 hours.
The above particles were suspended in water and
classified by passing through wet sieves of which opening
size were 44 ~m, 63 ~m, 74 ~m, 88 ~m, 105 ~m, 125 ~m and
149 ~m, respectively. Particles which gathered on each
sieve were suspended in water and thè volume of
sedimented particles was measured after allowing them to
stand for a day. The volume average particle size of the
obtained particles was 111 ~m and not less than 97 ~ by
volume of the whole particles had a particle size within
the range of ~20 % of the volume average particle size.
There could not be found a particle with a particle sizé
of less than 44 ~m. Further, particle with a particle
size of not more than 5 ~ of the volume average particle
size or with a particle size of less than 5 ~m was not
recognized by using COULTER COUNTER~ (made by COULTER
ELECTRONICS, INC.)
Example 2
The particles obtained in Example 1 were fully
washed with methanol and dried under vacuum at room
temperature. After vapor deposition of gold on them, the
surface and section oE the particles were observed with a
scanning electron microscope. It was observed that the
particles had no skin layer and had a lot of pores of
about 0.5 ~m on the surface, also on the section, had
.: .
1 32 1 454
~ 31
pores which were nearly as large as and as many as those
on the suracel which showed that a three dimensional
network structure was extended all over the particle.
Example 3
Cellulose acetate (degree of acetylation: 61.5
%) was dissolved in a mixture o~ dimethyL sulfoxide and
propylene glycol mixed at a weight ratio of 6 : 4 to
prepare a polymer solution having a concentration oE 5 ~.
Then, uniform polymer particles were obtained in the same
manner as in Example 1 e~cept that a 0.2 % aqueous
solution of a detergent for home use (commercially
available under the trade name "Runa Mild" made by Kao
Corporation) was used as the coagulating liquid. The
viscosity of the polymer solution was 52 cP at 90C. The
surface tension of the mixture solvent of the polymer
solution was 39 dyn/cm, which is the arithmetical mean
values of the surface tension of the solvents at 25C.
The surface tension of the coagulating liquid was 20
dyn/cm at 25C.
The particles was heated at 120C for 30
minutes with immersing the particles in water. ~y means
of this treatment, the particles were uniformly shrinked
about 20 % in diameter.
The obtained particles were perfectly
spherical, and from which any minute polymer chips did
not occur even though the particles were stirred with the
magnetic stirrer for about 3 hours.
The surface and section of the particles were
observed with the scanning electron microscope in the
same manner as in Example 2. There was skin layer having
a thickness of about 0.1 ~m on the surface of the
particle, on which no pore was observed even though the
observation was carried out at a magnification of 20,000
X. Except the surface, the three dimensional network
structure was observed.
The classification was carried out with wet
sieves in the same manner as in Example 1. As a result
.
. : ..
. ,,
. ~: ;, : : :
.
13~1454
- 32
of the measurement of the particle size distributionl the
obtained particles had the volume average particle size
of 103 ~m and not less than 97 ~ by volume o the whole
particles had a particle size within the range of ~20 %
of the volume average particle si~e. There could not be
found a particle having a particle size of less than
44 ~m.
Example 4
Polystyrene was dissolved in N-methyl-2-
pyrrolidone to prepare a polymer solution having a
concentration of 15 %. The polymer solution had a
viscosity of 250 cP at 90C and a surface tension of the
solvent of the solution 41 dyn/cm at 25C. ~niform
polymer particles was obtained from the above solution in
the same manner as in Example 3.
The obtained particles were perfectly
sph~rical, and from which any minute polymer chips did
not occur even though the particles were stirred with the
magnetic stirrer for about 3 hours.
The classification was carried out with wet
sieves in the same manner as in Example 1. As a result
of the measurement of the particle size distribution, the
obtained particles had a volume average particle size of
116 ~m and not less than 97 % by volume of the whole
particles had a particle size within the range of ~20 ~
of the volume average particle size. Particles with the
particle size of less than 44 ~m could not be found.
Example 5
Cellulose was dissolved in a 60 % aqueous
solution of calcium thiocyanate to prepare a polymer
solution having a cellulose concentration of 4 %. The
solution had a viscosity of 550 cP at 100C and a surface
tension of the solvent of the solution 73 dyn/cm at
25C. ~y using the above solution, uniform polymer
particles were obtained in the same manner as in Example
1 except that a 50 ~ ethanol solution was used as the
,~ .:. . .
. .~ .
~ : "
1 321 45~
- 33
coagulating liquid, and, the apparatus shown in Fig. 1
was set so that the distance between the end (16) of the
vibrating rod (6) and the nozzle (5) was 5 ~n and the
temperature of the polymer solution was lOO~C. The
S surface tension of the coagulating liquid was 30 dyn/cm
at 25C.
The obtained particleq were perfectly spherical
and from which any minute polymer chips did not occur
even though the particles were stirred with the magnetic
stirrer for about 3 hours.
The classification was carried out with wet
sieves in the same manner as in Example 1. As a result
of the measurement of the particle size distribution, the
obtained particles had the volume average particle size
of 112 ~m and not less than 97 % by volume of the whole
particles had a particle size within the range of ~20 ~
of the volume average particle size. There could not be
found a particle having a particle size of less than
44 ~m.
In Exmaples 1 to 5, there was used the nozzle
which had the orifice (12) with a aperture diameter of
50 ~m. However, by changing the aperture diameter and
adjusting the parameters to attain the synchronized
condition for giving unifoxm liquid droplets, droplets
having a diameter of not more than 1,000 ~m, not more
than 500 ~m or not more than 250 ~m, respectively, and
having a narxow particle size distribution can be
suitably obtained in compliance with the uses of the
polymer paxticles.
Fxample 6
Cellulose acetate (degree of acetylation: 61.5
%) was dissolved in N-methyl-2-pyrrolidone to prepare a
polymer solution having a cellulose acetate concentration
of 7 %. The solution had a viscosity of 350 cP at 64C
and a surface tension of the solvent of the solution 41
dyn/cm at 25C.
By using the apparatus shown in Fig. 1, the
,
- -:,
: -' ~ ' : j
.
1 321 454
- 34
polymer solution was jetted through the orifice (12)
having a round shape with a diameter of 50 ~m at a flow
rate of 2500 cm/sec.
In order to obtain uni~orm liquid droplets,
each parameter was adjusted to attain the synchronized
condition, i.e. the distance between the end (16) o the
vibrating rod (6) and the nozzle (5) was 15 mm, the
temperature of the polymer solution was maintained at
64C, the frequency of the vibrating rod (6) which was
connected with the magnetostrictive vibrator (which was
not shown in Fig. 1) via the vibration transmission part
(15) were 25 KHz and the amplitude of vibration of the
top (16) of the vibrating rod was 5 ~m. When the
pressure of the polymer solution in the cylinder (2) was
15.9 Kg/cm3, uniform liquid droplets were stably
formed.
It was confirmed that the uniform liquid
droplets were formed actually by taking a photograph of
the droplets at the position of 5 cm downward from the
nozzle with turning on and off a stroboscope ~"MSX-lA"
type, made by Kabushiki Kaisha Sugawara Kenkyusho~. As
shown in Fig. 2 which is a sketch of the photograph, it
was observed that the droplets ~17) having a uniform
diameter of about 155 ~m were in a line at regular
intervals.
~ Examele 7
Cellulose acetate (degree o acetylation: 55 ~)
was dissolved in dimethyl sulfoxide to prepare a polymer
solution having a cellulose acetate concentration of 16
%. The solution had a viscosity of 380 cP at 130C and a
surface tension of the solvent of the solution 43 dyn/cm
at 25C.
By using the apparatus shown in Fig. 1, the
polymer solution was jetted from the orifice ~12) having
a round shape with a diameter of 50 ~m at a flow rate of
2,800 cm/sec. Uniform liquid droplets having a diameter
of about 115 ~m were obtained stably when the parameters
* Trade-mark
,~
,
.
- : ~ : : . , - :
: ~ : .. : :
,
~ ' : ' : '
:
1 321 454
- 35
were adjusted to attain the synchronized condition, i.e.
the distance between the end (16) of the vibrating rod
(6) and the nozzle (5) was 5 ~n, the temperature of the
polymer solution was maintained at 130C, the frequency
of the vibrating rod (6) was 25 KHz and the amplitude o~
vibration of the top ~16) of the vibrating rod was
5 ~m. At that time, the pressure of the polymer solution
in the cylinder (2) was 16.2 Kg/cm2.
Example 8
Polystyrene was dissolved in N-methyl-2-
pyrrolidone to prepare a polymer soIution having a
polystyrene concentration of 15 ~. The viscosity of the
solution was 250 cP at 90C.
By using the apparatus shown in Fig. 1, the
polymer solution was jetted from the round shape orifice
(12) with an aperture diameter of 35 ~m at a flow rate of
2000 cm/sec. The parameters were adjusted to attain the
synchronized condition, i.e. the distance between the end
(16) of the vibrating rod (6) and the nozzle (5) was 15
mm, the temperature of the polymer solution was
maintained at 90C, the frequency of the vibrating rod
(6) was 25 KHz and the amplitude of vibration of the end
(16) of the vibrating rod was about 5 ~m. The pressure
of the polymer solution in the cylinder (2) was 29 Kg/cm2
at that time, and uniform liquid droplets having a
diameter of about 115 ~m were obtained stably.
From the above-mentioned results, it is
recognized that minute uniform liquid droplets can be
obtained from a liquid having a high temperature, a high
pressure and a high viscosity by using the apparatus of
the present invention, which is impossible with an
apparatus conventionally used.
Example 9
Cellulose acetate (degree of acetylation: 61.5
%) was dissolved in a mixture of dimethyl sulfoxide and
propylene glycol mixed in a weight ratio of 6 : 4 to
: ~ :
- :; . :
.
1321~54
- 36
prepare a polymer solution having a cellulose acetate
concentration of 5 %. The solution was heated to 90C
and jetted through a nozzle with a orifice haviny an
aperture diameter of 50 ~m at a flow rate of 18 m/sec,
while applying turbulences with a constant frequency of
25 KHz to give uniform liquid droplets. In order to give
electric charge to the liquid droplets, parallel-plate
electrodes having a width of 20 mm and a distance between
the plates of 10 mm was set at the position of about 2 mm
from the lower face oE the nozzle and a voltage of 500 V
was given to the droplets between the nozzle and the
electrodes. A grounded container made of stainless steel
in which a 40 % ethanol solution was contained was set
about 50 cm right under the nozzle.
When the minute droplets with electric charge,
uniformly jetted one by one were letted into the aqueous
solution of ethanol, they were coagulated to be spherical
particles. The obtained particles were washed with water
and heated at 120C for 30 minutes with immersing them in
water.
The particles were suspended in water and
classified by using wet sieves having the size of sieve
opening 44 ~m, 63 ~m, 74 ~m, 83 ~m, 105 ~m, 125 ~m and
149 ~m, respectively. The effluent solution which was
passed through the sieve of 44 ~m and each particle
caught on each sieve was suspended in water. After
allowing to stand them for a day, the volume of
sedimented particles was measured and the volume average
particle size and the particle size distribution were
calculated. The volume average particle size was 100 ~m,
the content of particles having a particle size of less
than 74 ~m was not more than 1 % by volume, and particles
with the particle size of less than 44 ~m were not
recognized. Further, not less than 97 % by volume of the
whole particles had a particle size within the range of
+20 % of the volume average particle size.
The particles were suspended in water of a
volume of about five times as much as the volume of
1 321 454
- 37
sedimented particles and the suspension was passed
through a column with a inner diameter of 7 mm and a
length of 100 mm, equipped with a filter made of
polyester, of which opening diameter was 20 ~m, at the
outlet of it at a flow rate of 5 mQ/min to pack the
particles in the column. When the column was filled with
the particles, it was stopped to transmit the suspension
and the inlet of the column was also covered with a cap
attached with a filter of which opening cliameter was
20 ~m.
Bovine blood containillg 1 part by volume of 3.1
% tris buffer solution of sodium citrate as an
anticoagulant, based on 9 parts by volume of blood was
rnaintained at the temperature of 37C, and the blood was
sent into the above-mentioned column at a flow rate of
0.5 mQ/ min. Though the pressure drop was gradually
risen with the passage of time, it was 85 mmHg after 1
hour had passed. Also, there occurred no hemolysis.
Comparative_Example 1
The procedure of Example 1 was repeated except
that commercially available hard cellulose particles used
in chromatography were classified with sieves of which
opening size were 25 ~m, 44 ~m, 63 ~m, 74 ~m, 88 ~m,
105 ~m, 125 ~m and 149 ~m, respectively. The content of
the particles having particle size of less than 25 ~m was
0.3 % by volume and the content o the particles having
particle size of less than 74 ~m was 7.7 % by volume.
The volume average particle size of the particles was
95 ~m and at most 66 % by volume of the whole particles
had a particle size within the range of +20 % of the
volume average particle size.
A column was made with the above particles and
bovine blood was passed through the column in the same
manner as in Example 9. Since the pressure drop reached
172 mmRg after 30 minutes, the hemo-perfusion was
stopped. It was confirmed that hemolysis occurred in
effluent blood Erom the column.
: . .~ : :
,. . . ,
,'
. ; :
1321454
- 38
From the results mentioned above, it was
confirmed that when the direct extracorporeal hemo-
perfusion was conducted by employing the particles oE the
present inventionr the pressure drop was not increased to
more than 100 mmHg even after 1 hour of operation, which
showed the particles of the present invention were
excellent in blood compatibility.
In addition to the ingredients used in the
~xamples, other ingredients can be used ln the Examples
as set forth in the specification to obtain substantially
the same results.
.,. - ~ , . ,
: ~ .