Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
r~
~3245~9
-1- PC-3148
ELECTROL_ E FLOWMETER
The present invention relates to the field of
electrowinning. More particularly, it relates to the field of
flowmeters used in measuring electrolyte flow rates.
: . .
BACKGROUND OF ART AND PROBLEM
Generally, an electrowinning tankhouse contains several
electrolytic cells. Each electrolytic cell contains several anodes
a~d cathodes for electrodepositing a metal from an electrolyte to the
cathodes. Electrowinning is generally performed in a continuous
operation where large volumes of electrolyte are passed through each
cell ln relation to the quantity of metal deposited. These
individual cells have different flow rates which maximi~e the
efficiency of electrowlnning while supplying sufficient surfactant
for acid mist control. The individual flow rates of electrolyte lnto
each cell are controlled by an adJustable valve. The problem wlth
~he valves is that they are adversely effected by scale which
deposits inside the valve. These valves become clogged with scale
32~09
-2- PC-3148
disrupting electrolyte flow rates into the individual cells.
Therefore, during electrowinning, it is desirable to periodically
measure flow rates of electrolyte into an electrolytic cell to
monitor changes in electrolyte flow rate. The problem with measuring
the flow rates is that conventional meters such as disk meters and
rotary-vane meters become clogged with scale. For example, when
electrowinning copper with lead anodes in aqueous sulfuric acid, lead
sulfate deposits on the flow meters. The lead sulfate adheres to the
flowmeters interfering with the accurate operation of the flowmeters.
As far as known, no one has discovered a flowmeter for use
in electrowinning, which effectively measures electrolyte flow rates
and remains accurate despite the accumulation of scale.
SUMMARY OF THE INVENTION
The present invention relates to a device and process for
measuring the flow rate of an electrolyte into an electrolytic cell
for electrowinning~ An electrolyte supply conduit transports
electrolyte to the electrolytic cell. A transparent tube extends
upwardly from the electrolyte supply conduit. The transparen~ tube
has a closed end and an open end. The open end is connected to the
supply conduit to permit electrolyte from the supply conduit to flow
freely to a level in the transparent tube. The level of electrolyte
in the transparent tube divides the transparent tube into a lower
portion containing electrolyte and an upper portion above the
electrolyte. An air supply conduit supplies air to the upper portion
of the ~ransparent tube. A level of electrolyte in the transparent
tube is proportional to the ~low rate of electrolyte to the
electrolytic cell.
Preferably, the transparent tube is marked between the
lower portion and the upper portion for recording a desired
electrolyte flow rate. The invention ~s ideally suited for aqueous
sulfurlc acid electrolytes which deposit a hard lead sulfate scale on
flowmeters.
,
,~ ~32~0~
-3- PC 3148
BRIEF DESCRIPTION OF THE DRAWING
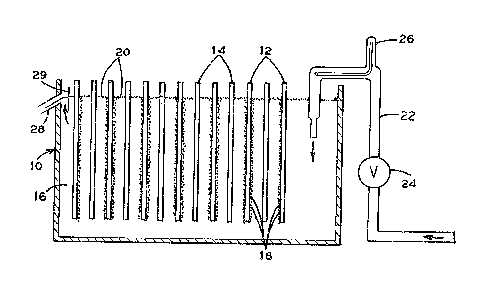
Figure 1 is a schematic view of an electrolytic cell and a
flowmeter which measures flow of electrolyte through an electrolyte
supply conduit into an electrolytlc cell.
Figure 2 is an enlarged schematic view of the electrolyte
flowmeter.
PARTICULAR DESCRIPTION OF THE INVENTION
Referring to Figure 1, the electrolytic cell 10 having
anodes 12 and cathodes 14 is used for electrowinning. The
electrowinning deposits metals, such as copper from an aqu~ous
sulfuric acid electrolyte 16 on the cathodes 14. The anodes 12
produce 2 bubbles 18 which rise to the surface. Bubbles 18 are
stabili~ed with a surfactant to form a protective foam layer 20. The
foam layer 20 prevents bubbles 18 from bursting at the electrolyte
surface to control the production of acid mist.
The electrolyte 16 enters the electrolytic cell 10 through
electrolyte supply conduit 22. Electrolyte in conduit 22 is supplied
from a constant head ~ank ~o supply electrolyte at a constant
pressure in conduit 22. Flow rate of electrolyte through the
electrolyte supply conduit 22 is controlled by ad~ustable valve 24.
The adjustable valve 24 is ad~us~0d to fine tune the electrolyte flow
into cell 10 to the rate which maximiæes electrowinning and properly
controls acid misting. Flowmeter 26 indicates changes in electrolyte
flow rate. The ad~ustable valve 24 is then ad~usted back to the
optimum electrolyte flow rate when a change of flow rate is indicatsd.
The electrolyte flows out overflow outlet 28 to maintain the electrolyte
16 in the electrolytic cell 10 at a constant level. Skimmer 29 prevents
foam 20 from directly exiting through over~low outlet 28.
Referring to Figure 2, the electrolyte 16 flows through
elec~rolyte supply conduit 22 and through electrolyte supply conduit
outlet 30 into the electrolytic cell. The electrolyte supply conduit
is preferably constructed out of a durable material such as polyvinyl
chloride (PVC). The electrolyte supply conduit outlet 30 has a fixed
diameter or fixed flow area. The flow rate is maintained relatively
, : ,
-4- PC-3148
constant by maintaining the flow area of the outlet 30 constant and
the pressure of the electrolyt~ in conduit 22 constant. To measure
changes in flow rates, flowmeter 26 is added to the conduit 22. A
transparent tube 32 extends upwardly from the electrolyte supply
conduit 22. The transparent tube has an open end 34 and a closed end
36. The open end 34 is connected to conduit 22 in a manner which
allows electrolyte 16 to flow into a lower portion 38 of the
transparent tube 32. The lower portion 38 is defined as the portion
of transparent tube 32 fllled with electrolyte. Above the lower
portion 38 is the upper portion 40. The upper portion 40 is defined
as that portion inside the transparent tube 32 above the lower
portion 38.
The upper portion is supplied with air from air supply
conduit 42. The air supply conduit 42 pierces electrolyte supply
conduit 22 proximate the electrolytic cell at air inlet 44. A
threaded connector provides a sealed means for securing the air
supply conduit 42 to the electrolyte supply conduit 22 with the air
supply conduit 22 open to the atmosphere. The air supply conduit 42
extends through conduit 22 and up lower portion 38 of transparent
20 tube 32 into the upper portion 40 of tube 32. The air supply conduit
42 is held in position by having one end fixed to the threaded
connector and by having the opposite end bent to press against the
far inside wall of the upper portion 40 of the transparent tube 32.
Air flows in the upper portion 40 of the tube 32 through air outlet
46. The level of electrolyte in the tube reaches a relatively
constant height when the pressure of electrolyte 16 through the open
end 34 equals the pressure of the atmosphere through the air supply
conduit 42 and out air outlet 46 plus the height of the electrolyte
16 in the tube 32. Alternatively, an air hole could be formed
through the upper portion 40 of the transparent tube 32. However,
the air hole would be a safety hazard and would eventually become
clogged with scale.
To utilize the invention, the valve 24 (Figure 1) on the
electrolyte supply conduit 22 is adjusted until the flow through the
fixed area outlet reaches the desired flow rate. The intersection
between the upper and lower portions 38 and 40 is marked to record
the optimum flow rate. Thereafter, when the flow rate varies, the
:: . ~
' ' ~ ,; : ,
32~L5~
-5- PC-3148
intersection of the upper and lower portions changes to indicate an
increased flow when the intersection has risen and a decreased flow
when the intersection has fallen (when the value 24 is located on the
opposite slde of the flowmeter 26, the intersection will rise when
flow decreases and fall when flow increases). The flow through the
conduit 22 is then appropriately adjusted until the intersection of
the upper and lower portions, 38 and 40 reaches the marked
inters~ction. This flowmeter facilitates simplified ad~ustment of
electrolyte flow rates to the flow rate of maxl~um efficiency. The
slight variations in atmospheric pressure have insignificant effect
on the accuracy of the flowmeter.
The invention, as constructed provided a substantially
constant flo~ of electrolyte at a flow rate of approximately 40
liters per minute tlpm). The electrolyte supply conduit 22 was
constructed with PVC piping having an internal diameter of
approximately 3.81 cm (1.5 in~. The electrolyte supply conduit
outlet 30 had an internal diameter of approximately 2.54 cm (1.0 in).
Air supply conduit 42 was constructed out of polypropylene and had an
internal diameter of approximately 0.32 cm (0.125 in). Transparent
tube 32 was constructed out of a clear plastic having an internal
diameter of approximately 1.27 cm (0.5 in). The above components are
rather economical and are easily replaced when broken.
The flowmeter has proven particularly effective when
electrowinning copper with lead anodes in an aqueous sulfuric acid
solution. When electrowinning with lead anodes, the lead anode
partially oxidizes and dissolves into the electrolyte. I,ead disolved
in the electrolyte deposits as lead sulfate on flowmeters forming a
hard scale, which causes rotating type flowmeters to lose accuracy.
The static pressure design of the invention resists the negative
effects of scale. Lead sulfate scale deposits on the outside of the
air supply conduit 42 immersed in electrolyte and on the inside walls
of the lower portion 38 of transparent tube 32. However, the scale
does not disrupt the accurate measuring of electrolyte flow rates
with flow meter 26.
Another unique feature of the invention is the placement of
air inlet 44, which facilitates the quick refill of the electrolytic
cell 10 (Figure 1). The electrolytic cell is periodically emptied
.
~32~9
-6- PC-3148
for repairing and cleaning purposes. After cleaning the cell, it is
desirable to fill the cell as quickly as possible to resume
electrowinning. To accomplish this valve 24 (Figure l) is completely
opened to increase the velocity of electrolyte into the cell. When
S the velocity is increased, electrolyte flows through air outlet 46,
through air supply tube 42 and out air inlet 44. The electrolyte
sprays out air lnlet 44 into the electrolytic cell. The location of
the alr inlet directs the electrolyte into the cell and helps prevent
workers from being sprayed with harmful electroly~es, such as
electrolytes containing sulfuric acid.
While in accordance with the provisions of the statute,
there is illustrated and described herein specific embodiments of the
invention. Those skilled in the art will understand that changes may
be made in the form of the invention covered by the claims and the
certain features of the invention may sometimes be used to advantage
without a corresponding use of the other features.
I,
.: :