Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
:
132S80~
This invention relates to N,N~-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfides, to a process for their
production, to processes for the production of their
dlsproportionation products, to the use of the tetrasulfides and
s of the disproportionation products as cross-linking agents or
vulcanization accelerators in rubber mixtures and to vulcanizable
rubber mixtures containing them.
.
N,N'-substituted bis-(2~4-diamino-s-trlazln-6-yl)-
disulfides are known and are described in DE-PS 1,669,954. They
are prepared from the corresponding N,N'-substituted 2,4-diamino-
6-mercaptotriazines by oxidation, for example with iodine, sodium
."3 hypochlorite or hydrogen peroxide. The most well known compound
` in this group is bis-(2-ethylamino-4-diethylaminotriazin-6-yl)-, 15 disulfide. Disulfides of this group may be used as accelerators
in rubber mixtures.
The present invention provides compounds which improve
the vulcanization behavior of rubber mixtures and which lmpart
better properties to their vulcanizates and to provide processes
, for the production of these compounds.
;'
The present invention provides compounds corresponding
to the following general formula:
.
'.'' .
, 30
:j
.,,
~,
,,. ~k
.`,' ~
'~
~, ~ - 1 -
'~1
,;~,
.;~ .
. . .
,.~. . .
132~805
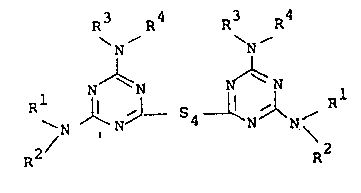
R R 4 R R
N~ N
S \ ~ N~L 54~
R2
in which
Rl and R2 are H; R2 is benzyl
R2, R3 and R4 are Cl-C8 alkyl, preferably Cl-C4 alkyl,
branched or unbranched, alkyl, C3-C8 cycloalkyl,
unsubstituted or substituted by 1 to 3 methyl groups,
2-hydroxyethyl, 3-hydroxypropyl, 2-hydroxypropyl or
15 R and R (together) represent C4-C6 alkylene, -(CH2-
` CHX)2Y where H is CH3, H and Y is O, S.
The present invention also relates to a process forthe production of these compounds. The process for
: producing the pure tetrasulfides with a linear S4-chain
between the two substituted triazine rings is characterized
in that an aqueous alkaline solution of the corresponding
. N,N'-substituted 2,4-diamino-6-mercaptotriazines is
~ reacted in a two-phase system with'an S2C12 solution in
`.~ an inert organic solvent, in which the reaction product is
` 25 insoluble or very sparingly soluble, at temperatures of
from -5C to -<+20DC and preferably at a temperature of
0 DC. It is of advantage to prepare an alkaline
aqueous solution of the mercaptotriazine which contains
at least the stoichiometric quantity of alkali hydroxide
required for the reaction and preferably an excess of
from 1 to 20 mole ~D~ based on the mercaptotriazine used.
To this solution is added a solvent in which the end
product of the reaction is insoluble or sparingly soluble,
preferably a C5-C10 alkane or a C5-C8 cycloalkane, option-
ally substituted by 1 to 3 methyl groups, and mixtures
;''
"
. ~ 2
., .
. 132~8~5
1 thereof. This mixture is vigorously stirred and cooled,preferably to +10C. A solution of S2Ci2 in the solvent
used is then added dropwise to this mixture with thorough
cooling. S2C12 is used at least in a ratio of 2 moles
mercaptotriazine to 1 mole S2C12, although this ratio
may also be 2~ 1.2, depending on the excess of alkali.
Under these conditions, S2C12 has solely a condensing
effect.
` The product formed is separated off by well known
methods and is advantageously dried in vacuo ~10 Torr) at
temperatures of up to +50C.
The present invention also relates to mixtures of
compounds corresponding to the following general formula
15R3 R4 R3 R
N N
N ~ N ~ N
~ N ~ N~ xN N (II)
20 R2/ R
in which Rl, R2, R3 and R4 are as defined hereinbefore and
Sx corresponds to an average statistical chain length with
x = 4.
These mixtures, which are also referred to hereinafter
as oligosulfides or disproportionates because they are
formed by disproportionation of compounds corresponding
to formula I, may be prepared by several methods.
The reaction conditions have to be controlled in
such a way that no free sulfur is formed.
One process is characterized in that the isolated
compound corresponding to formula I is heated beyond its
Z melting point, preferably by 20 to 50C.
In another process, the compounds corresponding to
~ - 3 -
,,
.
13258~
formula I are dissolved in an inert organic solvent and the
disproportionation reaction is carried out at temperatures
between 20~C (standing at room temperature) and the boiling
point of the solvent used.
One particularly elegant method comprises reacting an
aqueous alkaline solution of the corresponding N,N'-
substituted 2,4-diamino-6-mercaptotriazines in a two-phase
system with a solution of S2C12 in an inert organic solvent
which dissolves the tetrasulfide formed. The linear
tetrasulfide formed is then immediately disproportionated to
the mixture according to the invention which consists of
oligosulfides.
Suitable solvents are, in particular, chlorinated
` hydrocarbons, for example CH2C12 and CHC13; ethers, esters,
aromatic hydrocarbons and ketones are also suitable solvents
for the disproportionation reaction, they may be used
providing they form a two-phase mixture with water. The
reaction conditions for the preparation of the
disproportionates are otherwise identical with those under
;; which the compounds of formula I are produced.
~- 25
. ....................................................................... ~
' ~
132~8~5
The present invention also relates to the use of the
compounds of formula I and II in vulcanizable rubber mixtures
and to the rubber mixtures containing the compounds according
to the invention.
When used as crosslinking agents or vulcanization
accelerators, the tetrasulfides according to the invention or
their disproportionates have been found to be distinctly
superior to the standard compounds in use today.
A wide range of accelerators (J. van Alphen, Rubber
Chemicals (1977, pages 1-46)) is available to the rubber-
processing industry, preferably for sulfur vulcanization,
including for example benzthiazolyl sulfenamides,
~5 benzthiazolyl disulfide and 2-mercaptobenzthiazole
: or zinc salts thereof. In addition, there are a number
.
- 4a -
.
,. ~
~ ,; ,
132580r)
1 of special compounds, such as thiuram disulfides and
peroxides, which act as crosslinking agents even in the
absence of other additives of sulfur, but which are often
used in combination with sulfur as accelerators. The
particular application is determined by the particular
effect to be obtained.
Today, the most widely used accelerators in practice,
particularly among the elastomers accessible to accelerated
sulfur vulcanization, are the benzthiazolyl sulfenamides.
However, new production processes and new products and
also the ever-present need for rationalization have in
recent years brought about changes in the requirements
which accelerators have to satisfy compared with the past
to such an extent that, today, it can be difficult to
satisfy the quality demands imposed on the vulcanization
process and on the properties of the vulcanizates with the
accelerators or crosslinking agents currently available
for accelerated sulfur vulcanization.
Another disadvantage of certain conventional acceler-
ators (for example certain sulfenamides, thiurams), which
` can now no longer be ignored, is that amines can be
released during the vulcanization process and, where they
are nitrosatable, lead to the for~ation of nitrosamines in
the vulcanizate which, if they are toxic, can be expected
permanently to restrict the potentional applications ofthese accelerators.
Another crucial disadvantage of the benzthiazolyl
accelerators, particularly benzthiazolyl sulfenamides,
is their increasingly pronounced tendency towards reversion
with increasing vulcanization temperature during the often
necessary overheating of the vulcanizates, particularly
wherc rubbers susceptible to reversion, such as natural
ruhber and polyisoprene and blends thereof with synthetic
rubbers, are used. ~lowever, the same also applies to
synthetic rubbers of all kinds if the reversion process is
132~0~
1 not masked by thermal crosslinking. The reversion rate
`~ increases so drastically, particularly with increasing
vulcanization temperature, that, firstly, there is a
significant reduction in the crosslinking density, even
with optimal vulcanization per se, secondly the vulcaniz-
ation optimum assumes the form of a peak rather than a
plateau, which makes it extremely difficult to reproduce
optimal vulcanization properties, and thirdly there is an
unavoidable reduction in the crosslinking density during
the often necessary overvulcanization, which leads to a
loss of uniformity of crosslinking in the vulcanizate,
particularly in the case of thick-walled rubber articles.
Commensurate with the increase in reversion, there is a
reduction in the performance of the vulcanizates which is
reflected, for example, in reduced 300% modulus and abrasion
resistance, etc.
The reversion effects mentioned can be remedied to a
certain extent by reducing the sulfur content and increasing
the accelerator content, i.e. by using so-called semi-
EV-systems (L. Bateman, 1963 "The Chemistry and Physics of
Rubber-like Substances", pages 522 et seq.). However,
; this reduction in reversion is achieved through a change
in the crosslinked structure (rati,o of -Sx-, -S-S-,
-S- bonds) in favor of monosulfidic crosslinking sites
1 25 which can adversely affect the vulcanizate properties.
However, this measure becomes more ineffective, the higher
the vulcanization temperature.
The disadvantages of the benzthiazole accelerators
limit their usefulness with increasing vulcanization
temperature and impose certain limits on the efforts of
the rubber industry to increase productivity by using
' higher vulcanization temperatures.
Surprisingly, the N,N'-substituted bis-(2,4-diamino-
s-triazin-6-yl)-tetrasulfides and their disproportionation
products, both in their use as accelerators in sulfur
132~8~5
1 vulcanization and in their use as crosslinkers, i.e.
without the additional use of sulfur, have proved to
be compounds which impart extremely high reversion stability
- even in the event of severe overheating - to the rubber
mixtures prepared with them, even at high vulcanization
temperatures, as reflected in the fact that, where they
are used, the otherwise usual reversion process occurs
to only a minor extent, if at all. The surprising fact
that the crosslinking sites formed by the N,N'-substituted
bis-~2,4-diamino-s-triazin-6-yl)-tetrasulfides according
to the invention and their disproportionates are extremely
reversion-stable and thermostable means that, where they
are used in rubber mixtures, even at high vulcanization
temperatures, the vulcanizates obtained on completion of
the crosslinking reaction show a high performance level
which they maintain even in the event of strong overheating.
These two effects taken together enable increases
in productivity to be achieved in the rubber-processing
industry without any loss of vulcanizate performance
by increasing the vulcanization temperature.
Commercial polysulfides,-such as Robac(R)P 25
(Robinson, dipentamethylene thiuram tetrasulfide) and
Tetron(R)A (Du Pont, dipentamethylene thiuram hexasulfide)
or dibenzthiazolyl tetrasulfide do not achieve even
remotely comparable reversion stability.
The use of the N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfides according to the invention and
their disproportionation products encompasses the rubber
mixtures known from the prior art based on natural rubber
(NR), isoprene rubber (IR), butadiene rubber (BR), styrene-
butadiene rubber (SBR), isobutylene-isoprene rubber (IIR),
ethylene-propylene terpolymer (EPDM), nitrile rubber (MBR),
halogen-containing rubbers and also epoxidized natural
rubbers (ENR) and blends thereof. The use of the N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfides
-- 7
132~8V5
1 according to the invention and their disproportionation
products is of particular significance in the case of
reversion-sensitive rubbers, such as for example natural
rubber, isoprene and butadiene rubbers and blends thereof
S with one another or with other r~bbers.
The N,N'-substituted bis-(2,4-diamino-s-triazin-6-
yl)-tetrasulfides according to the invention and their
disproportionation products are used as crosslinking agents
in rubber mixtures in a quantity of from 0.2 to 15 parts
by weight and preferably in a quantity of from 0.3 to 8
parts by weight, based on 100 parts of rubber.
In accelerated sulfur vulcanization, the N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfides
according to the invention or their disproportionation
! 15 products are used as accelerators in quantities of from
0.01 to 10 parts and preferably in quantities of from
0.1 to 5 parts, based on 100 parts of rubber, for sulfur
dosages of from 1 to 10 parts. A molar ratio of the
accelerators according to the invention to sulfur (S8) of
1:0.5-1.5 is preferred. In order to obtain a certain
range of variation of the vulcanization kinetics, it can
be useful to use two or more N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfildes or their dispro-
portionates in admixture with one another, the substitution
being made on a molar basis in order to keep to the
quantities and particularly to the preferred accelerator-
to-sulfur ratio mentioned above. The same also applies
where N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides or their disproportionates are used as
crosslinking agents without sulfur.
~ gain for kinetic reasons, it can be useful to use
the N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides or their disproportionation products in
admixture with conventional accelerators, such as for
example sulfenamides and thiurams. These measures are
132~8~
1 occasionally to the detriment of reversion stability by
comparison with the N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfide vulcanizates. However, they
do have a positive effect on reversion behavior where
conventional accelerators are partly replaced by N,N'-
substituted bis-t2,4-diamino-s-triazin-6-yl)-tetrasulfides
or their disproportionates.
A further significant effect on incubation time where
the compounds according to the invention are used in
rubber mixtures can be obtained by combination with
commercial vulcanization retarders, such as Santoguard(R)
PVI (N-~cyclohexylthio)-phthalimide) and Vulkalent(R)E (N-
phenyl-N-(trichloromethylsulfenyl)-benzenesulfonamide).
Vulkalent( )E~a product of Bayer AG, has proved to be
the most effective retarder. With increasing addition of
Vulkalent( )E, there is a linear increase in the incubation
time of mixtures containing N,N'-sustituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfides or their dispro-
portionates.
Where N,N'-substituted bis-(2,4-diamino-s-triazin-6-
' yl)-tetrasulfides or their disproportionates are used as
crosslinking agents, it has proved to be best to keep the
molar ratio of N,N'-substituted bis-(2,4-diamino-s-triazin-
6~yl)-tetrasulfides or their disproportionates to Vulkalent
25 E at 1:0.5-1.5 and preferably at 1:0.8-1.2 parts to 100
parts of rubber.
Of greater importance is the use of retarders,
particularly Vulkalent E, in accelerated sulfur vulcaniz-
ation using N,N'-substituted bis-(2,4-diamino-s-triazin-
6-yl)-tetrasulfides and their dispr~portionates and also
mixtures thereof. The intended increase in the incub-
ation time of the crosslinking reaction is occasionally
accompanied by a slight reduction in the crosslinking
velocity, which can be arrested by increasing the temper-
ature, and by a slight reduction in reversion stability.
-
~32~8~
1 In this case, it has also been found to be advisable to
adjust the molar ratio of N,N'-substituted bis-~2,4-diamino-
s-triazin-6-yl)-tetrasulfides or their disproportionates
to Vulkalent E to 1:0.5-1.5 and preferably to 1:0.8-1.2,
5 the sulfur content being kept between 0.1 and 10 parts and
preferably between 0.5 and 8 parts, based on 100 parts of
rubber, depending on the type of mixture.
On the basis of these dosage guidelines, it is
possible to solve many of the vulcanization problems in
10 question without any significant deterioration in the
. properties of the vulcanizates which would go against the
essence of the invention.
Mixtures which contain only silica or carbon black/
silica blends with silica contents of more than 15 parts
:
~ 15 to 100 parts of rubber are very difficult or even impossible
i.; .
to crosslink with conventional sulfur vulcanization systems.
On the one hand, they particularly undergo reversion, on
the other hand they prevent adjustment of the crosslinking
density dictated by the requirements which the end products
3 20 have to satisfy. This is one of the reasons why silicas
are preferably used in soling material and, hitherto,
relatively infrequently in dynamically stressed products.
Surprisingly, it is possible with bis-(2,4-diamino-s-
~, triazin-6-yl)-tetrasulfides and their disproportionates,
25 both as accelerators and as crosslinking agents (without
sulfur), substantially to eliminate reversion in mixtures
containing black/white blends with silica contents of more
than 15 parts to 100 parts of rubber and also in silica-
filled mixtures and, at the same timé, to increase the
30 crosslinking density, which is reflected in a high modulus
level for silica mixtures.
The N,N'-substituted bis-(2,4-diamino-s-triazin-6-
yl)-tetrasulfides according to the invention and their
disproportionation products may also be used in combination
35 with oligosulfidic organosilanes, for example
-- 10
-- 132~80~
1 [(RO)3 - Si - (CH2)n ]2 Sx (RO)3-Si-(CH2)n-SH
with x = 2 - 6, R = Cl-C6 alkyl, cyclohexyl, n = 2 or 3
or
~ CH3 ~
(RO)3 Si ~ (CH2)n ~ 2Sx
with x = 2 - 6, preferably 3,
preferably bis-(3-triethoxysilylpropyl)-tetrasulfide
(Si 69, Degussa AG). N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfides and their disproportionates
may be used with advantage in sulfur-free organosilane
crosslinking instead of the accelerators described in
15 DE-PS 25 36 674 and in the sulfur vulcanization with
organosilanes described in DE-PS 22 55 577 and also
in the production of reversion-stable organosilane-con-
taining rubber mixtures by synthesis of equilibrium cure
j systems (DE-PS 28 48 559J.
' 20 This applies both to carbon-black-filled mixtures
and to mixtures containing carbon black/silica blends and
also to mixtures filled only with silicas. Mineral
fillers may also be added to the m~xtures mentioned with-
out any adverse effects. In this case, rubber/filler
bonds are formed in the presence of silica fillers or
carbon black/silica blends (Kautschuk & Gummi, Kunststoffe,
number 8, 1977, pages 516-523, number 10, 1979, pages
760-765 and number 4, 1981, pages 280-284) while rubber/
rubber bonds are formed in the presence of carbon blacks.
~he formation of rubber/rubber crosslinking sites in
the presence of carbon black or the formation of rubber/
filler crosslinking sites in the presence of silica or
the formation of both types of crosslinking sites in the
presence o~ carbon black/silica blends in any ratios is
also possible where N,N'-substituted bis-(2,4-diamino-s-
-- 11 --
1325~
1 triazin-6-yl)-tetrasulfides or their disproportionates
are used individually or in admixture with one another or
in admixture with conventional accelerators together with
conventional sulfur donors, such as for example Sulfasan(R)
R (morpholine disulfide).
The compounds according to the invention
are used in rubber mixtures which may
contain other typical components, such as for example:
- standard reinforcing systems, i.e. furnace blacks,
channel blacks, flame blacks, thermal blacks, acetylene
blacks, arc blacks, CC-blacks, etc.; synthetic fillers,
such as silicas, silicates, aluminium oxide hydrates,
calcium carbonates; natural fillers, such as clays,
siliceous chalks, chalks, talcums, etc. and silane-
modified fillers and blends thereof in quantities offrom 5 to 300 parts to 100 parts of rubber, silicas and
silicates being particularly preferred,
- zinc oxide and stearic acid as vuicanization promoters
in quantities of from 0.5 to 10 parts of rubber,
- typical antiagers, antiozonants, anti-fatigue agents,
such as for example IPPD, TMQ, etc. and also waxes as
; light stabilizers and blends thereof,
- plasticizers such as, for example, aromatic, naphthenic,
paraffinic, synthetic plasticizers and blends thereof,
- optionally, other silanes, such as y-chloropropyl tri-
alkoxysilanes, vinyl trialkoxy silanes and aminoalkyl
trialkoxysilanes and also blends thereof in a quantity
of from 0.1 to 15 parts and preferably in a quantity of
from 1 to 10 parts to 100 parts of fillers containing
silanol groups, such as silicas, silicates, clays etc.,
- optionally, dyes and processing aids in the usual
quantities.
The range of application of the N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfides extends
to rubber mixtures of the
~ - 12 -
132~
1 type normally used in tire production and to technical
articles, such as for example mixtures for conveyor belts,
V-belts, molded articles, hoses with and without reinforae-
ment, rubber covers for rollers, linings, molded profiles,
: 5 freehand articles, films, shoe soles and uppers, cables,
solid rubber tires and vulcanizates thereof.The following products are mentioned as examples of
the compounds according to the invention:
A bis-(2-ethylamino-4-di-isopropylamino-s-triazin-
6-yl)-tetrasulfide
B bis-(2-n-butylamino-4-diethylamino-s-triazin-6-yl)-
tetrasulfide
C Bis-(2-isopropylamino-4-di-isopropylamino-s-triazin-
6-yl)-tetrasulfide
D bis-(2-ethylamino-4-di-isobutylamino-s-triazin-6-yl)-
. tetrasulfide
E bis-(2-ethylamino-4-di-n-propylamino-s-triazin-6-yl)-
tetrasulfide
F bis-(2-n-propylamino-4-diethylamino-s-triazin-6-yl)-
; 20 tetrasulfide
G bis-(2-n-propylamino-4-di-n-propylamino-s-triazin-6-yl)-
tetrasulfide
H bis-(2-n-butylamino-4-di-n-propylamino-s-triazin-6-
yl)-tetrasulfide
25 I bis-(2-ethylamino-4-di-n-butylamino-s-triazin-6-yl)-
tetrasulfide
K bis-~-isopropylamino-4-di-isopropylamino-s-triazin-6-
yl)-oligosulfide mixture
L bis-(2-cyclohexylamino-4-diethylamino-s-triazin-6-
yl)-oligosulfide mixture
M bis-(2-ethylamino-4-diethylamino-s-triazin-6-yl)-
oligosulfide mixture
O bis-(2-amino-4-diethylamino-s-triazin-6-yl)-oligo-
sulfide mixture
132~8~
1 EXAMPLE 1
454 g of 2-ethylamino-4-diethylamino-6-mercapto-
triazine are dissolved in soda lye prepared from 84 g
NaOH ~ 1.5 liters H2O.
The solution is poured into a 4-liter three-necked
flask. After the addition of 1.5 liters light petrol
(Bp. 80 - 110C). the mixture is cooled with vigorous
stirring to 0C.
A solution of 137 g of S2C12 in 100 ml petrol is then
run in over a period of 20 minutes during which the
temperature must not exceed +5C.
The tetrasulfide precipitates immediately. On
completion of the reaction, the reaction mixture is stirred
for 5 minutes, filtered under suction and washed.
` 15 The snow-white fine powder is dried in vacuo (12 Torr)
at 40 - 45C.
; Yield: 499.5 g, corresponding to 97.1% of the theoretical.
Mp.: 149 - 150C.
~ Analysis:
; 20 Bis-(2-ethylamino-4-diethylamino-s-triazin-6-yl)-tetrasulfide
Molecular weight 516, C18H32NloS4
Calculated: C 41.9 H 6.2 N 27.1 S 24.8
Found: 41.8 6.5 26.8' 24.8
Analysis by TLC and HPLC shows that the product contains
97.1% linear tetrasulfide.
EXAMPLE 2
56.6 g of 2-ethylamino-4-di-n-butylamino-6-mercapto-
triazine are dissolved in a solution of 8.8 g of NaOH in
250 ml water. 250 ml petrol are then added, after
which the mixture is cooled with thorough stirring to
+5~C. A solution of 13.5 g S2C12 in 30 ml petrol is
then run in. A white precipitate is immediately formed.
On completion of the reaction, the reaction mixture is
worked up in the same way as in Example 1. Yield: 56.g,
- 14 -
13258~5
1 corresponding to 89.2% of the theoretical.
26 48NloS4 (molecular weight 628)
Calculated: C 49.68 H 7.64 N 22.29 S 20.38
Found: C 49.59 7.59 22.18 20.40
HPLC analysis: purity >96~.
EXAMPLE 3
107.6 g of 2-i-propylamino-4-diisopropylamino-6-
mercaptotriazine are dissolved in soda lye prepared from
10 17.6 g NaOH in 600 ml H2O. 600 ml methylene chloride
are then added.
A solution of 27 g S2C12 in 50 ml CH2C12 is then run
in at 0 to 5C. On completion of the reaction, the
organic phase is separated off in a separation funnel,
dried and concentrated in vacuo. An amorphous powder
is obtained; softening point 90C. Yield: 112.5 g,
corresponding to 94~ of the theoretical.
y C24H44NloS4 (molecular weight 600)
Calculated: C 48 H 7.33 N 23.3 S 21.3
20 Found: 48.2 7.36 23.01 20.95
EXAMPLE 4
45.4 g of 2-ethylamino-4-diethylamino-6-mercapto-
triazine are dissolved in soda lye prepared from 8.8 g
NaOH and 200 ml water. 200 ml methylene chloride are
then added. The mixture is thoroughly stirred and cooled
to 0C. ]4 g S2C12 are then dissolved in 50 ml CH2C12
and the resulting solution run into the mercaptide solution.
The reaction product dissolves in CH2C12. On com-
pletion of the reaction, the phases are separated and the
CH2C12 solution is worked up, giving an amorphous powder
having a softening point of approx. 110C. Yield: 46.7 g,
corresponding to 90.5~ of the theoretical.
y 18H32NloS4 tmolecular weight 516)
Calculated: N 27.1 S 24.8
Found: 26.8 24.4
- 15 -
,
132~8~
According to analysis by TLC, the mixture obtained
contains 4 oligosulfides, but no free sulfur.
EXAMPLE 5
50 g of bis-~2-ethylamino-4-diethylamino-s-triazin-6-
yl)-tetrasulfide having a purity of 97.1% are placed in a
spherical flask and heated for 1 hour to 160C on an oil
bath. On cooling, the melt solidifies in amorphous form.
According to analysis by T~C, the product contains another
3 oligosulfides in addition approx. 50% of starting product.
EXAMPLE 6
, 70.25 g of 2-cyclohexylamino-4-diethylamino-6-mercapto-
triazine are dissolved in 11 g of NaOH and 250 ml of water.
15 250 ml of chloroform are then added, after which a solution
of 16.8 g of S2C12 in 30 ml of CHC13 is run in with
vigorous stirring. On completion of the reaction, the
phases are separated and the chloroform phase is worked up,
giving 71.9 g of a white amorphous powder, corresponding
to a yield of 92% of the theoretical.
y 26H44NloS4 (molecular weight 624)
Calculated: C 50 H 7.05 N 22.4 S 20.51
Found: 49.1 6.90 21.8 20
According to analysis by TLC, the product consists
of approx. 30% linear S4-product and 70% oligosulfides,
but contains no free sulfur.
\ - 16 -
132~83~
Test Standards:
The physical tests were carried out at room temperature
in accordance with the following standards:
Measured in
Tensile strength, DIN 53 504 Mpa
elongation at break and
modulus value on 6 mm
thick rings
Shore-A-hardness DIN 53 505
Firestone ball rebound AD 20 245
Reversion~ DE-PS 2 848 559
Incubation time t DIN 53 529 (mins.)
Scorch time ASTM D 2084 (mins.)
. .
The names and abbreviations used in the Application
Examples have the following meanings:
: RSS: Ribbed Smoked Sheet (natural rubber)
CORAX(R) N220 Carbon black, BET surfa~e i2u m2jg
(Degussa)
Naftolen( ) ZD: Plasticizer of hydrcarbons
Ingraplast( )NS: Plasticizer of naphthenic hydrocarbons
Vulkanox(R) 301û NA: N-isopropyl-N'-phenyl-p-phenylenediamine
Vulkanox(R)HS: Poly-2,2,4-trimethyl-1,2-dihydroquinoline
Mesamoll(R): Alkylsulfonic acid ester of phenol
and cresol
Robac P 25: Dipentamethylene thiuram tetrasulfide
Tetrone A: Dipentamethylene thiuram hexasulfide
Protektor( )G35: Anti-ozonant wax
Vulkacit(R)MOZ: Benzthiazolyl-2-morpholinosulfenamide
Vulkacit(R)Mercapto: 2-mercaptobenzthiazole
Vulkacit(R) Thiuram: Tetramethyl thiuram monosulfide
Vulkacit(R) CZ: N-cyclohexyl-2-benzthiazole sulfenamide
Vulkalent(R)E: N-phenyl-N-(trichloromethylsulfenyl)-
- benzene sulfonamide
PVI: N-cyclohexylphthalimide
Ultrasil(R)VN3: Precipitated silica (Degussa)
Gran. Granulate
\ - 17 ~
132~8~
V143: Bis-(2-ethylamino-4-diethylamino-s-
triazin-6-yl)-disulfide
Vulcacit(R)NZ: Benzothiazyl-2-tert.-butyl sulfenamide
- 18 -
132~80~
EX~MPLE 7
Reversion stability of N220-filled NR (without sulfur)
crosslinked with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfides
1 2 3 4 '
RSS 1, ML4 = 70-80 100 100 100 100
CORAX N220 50 50 50 50
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ZD 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43 - - -
V143 - 1.29
Santoguard PVI - 0.4 - -
_ - 3.34
D - - - 4.10
Sulfur 1.5 1.5
Reversion:
max ~ D(max+60') 30.1 8.6 2.3 2.1
D - D
at 170C vulcanization
temperature
132~80~
Example 7 continued:
Reversion stability of N220-filled NR (without sulfur)
crosslinked with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-oligosulfides
6 7
RSS 1, ML4 = 70-80 100 100 100
CORAX N220 50 50 50
ZnO RS 5 5 5
Stearic acid 2 2 2
Naftolen ~D 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5
Protector G35
K 3.84
L - 3.62
M - - 4.0
Reversion:
Dmax D(max+60~ ) 2.5 3.1 2.3
Dmax min
at 170C vulcanization
temperature
When used as crosslinking agents (without sulfur), the
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-oligo-
sulfides according to the invention prove to be extremely
reversion-stable in carbon-black-filled NR mixtures
(mixtures 3-7) by comparison with a mixture containing
a semi-EV-system (mixture 1) or a bis-(2-ethylamino-4-
diethylamino-s-triazin-6-yl)-disulfide (V143) (mixture 2).
- 20 -
132~80~
EXAMPLE 8
Reversion stability of N220/silica-filled NR (without sulfur)
crosslinked with N,N'-substiteuted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfides
8 9 1011 12 13 14 15
RSS 1, ML 1~4 = 70-80 100 100 100 100 100 100 100 100
CORAX N220 25 25 25 25 25 25 25 25
Ultrasil VN3 Gran. 25 25 25 25 25 25 25 25
ZnO RS 5 5 5 5 5 5 5 5
Stearic acid, 2 2 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3 3 3
Vulkanox 4010NA2.5 2.5 2.52.5 2.5 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.51.5 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43
C - 3.48 - - - - - -
E - - 3.46
G - - - 3.62
I - - - - 3.78
K - - - - - 3.48 - -
L - - - - - - 3.62
M - - - - - - - 3.0
Sulfur 1.5
Reversion:
Dmax (max~60') (%) 47.1 1.8 2.4 3.3 6.2 2.5 3.1 4.7
max min
at 170C vulcanization
temperature
Silica-containing NR mixtures show particularly pronounced
reversion phenomena, even where semi-EV-systems are used
(mixture 8). With the N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetra- or -oligosulfides used as crosslinking
agents in accordance with the invention (mixtures 9-15),
an a~most reversionless state is achieved for otherwise the
same mixture composition.
~ - 21 -
132~8~
EXAMPLE 9
Reversion stability of N220-filled NR accelerated with
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
sulfides
16 17 18 19 20 21 22 23 24
RSS 1, MLl+4 = 70-80 100 100 lq0 100 100 100 100100 100
COI?AX N220 50 50 50 50 50 50 50 50 50
ZnO RS 5 5 5 5 5 5 5 5 5
Stearic acid 2 2 2 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5 2.5 2.5 2.5 2.5 2.52.5
Vulkanox HS ' 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.51.5
Protector G 35
Vulkacit MOZ 1.43 - - - - - - - -
B - 1.66
C -- -- 1.74
D - - - 1.76
E - - - - 1.73
F - - - - - 1.65
G - - - - - - 1.81
H - - - - - - - 1.82 -
- - - - - - - - 1.82
Sulfur 1.5 0.8 0.8 0.8 0.8 0.8 0.8 0.80.8
Reversion:
max (max+60')(%)31.9 0.4 0.0 0.0 1.3 1.3 2.0 1.22.1
max min
at 170C vulcanization
temperature
Vulcanizate data
at 170C, t 95%
Tensile strength23.0 22.624.1 21.320.8 19.922.1 22.023.2
Modulus 300~6 9.6 10.910.9 10.5 9.1 10.410.8 11.910.5
With equimolar accelerator dosage, the sulfur dosage can be
reduced in the case of the N,N'-substituted bis-(2,4-diamino-
s-triazin-6-yl)-tetra- or -oligosulfides. Inspite of this,
higher modulus-300% values are obtained. The mixtures pre-
-- 22
132~80~
EXAMPLE 9 continued:
Reversion stability of N220-filled NR accelerated with
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
sulfides
26 27
RSS 1, MLl+4 = 70-80 100 100 100
CORAX N220 50 50 50
ZnO RS 5 5 5
Stearic acid 2 2 2
Naftolen ZD, 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5
Protector G35
K 1.74
L - 1.81
M - - 1.5
Sulfur 0. 8 0 . 8 0 . 8
Reversion:
D -D
max (maX+60 )(96) 0.4 1.5 5.2
max min
at 170C vulcanization
temperature
Vulcanizate data at
170 C, t9596
Tensile strength22.9 24.2 22 . 2
Mbdulus 300~ 10.3 10.0 10.6
pared with N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetra- or -oligosulfides (mixtures 17-27) prove to be
extremely reversion-stable against a semi-EV-system
(mixture 16).
- 23 -
1325~5
EXAMPLE 10
Effectiveness comparison between sulfenamide and N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide
acceleration in N220-filled NR for the same S8 dosage
28 29
RSS 1 ML(1+4) = 70-80 100 100
CORAX N220 50 50
ZnO RS 5 5
Stearic acid 2 2
Naftolen Zp 3 3
Protector G35
Vulkanox 4010NA 2.5 2.5
Vulkanox HS 1.5 1.5
Vulkacit MOZ 1.43
D - 1.76
Sulfur 1.5 1.5
Reversion:
max (max+60')(%) 31.9 5.1
max min
at 170 DC vulcanization
temperature
Vulcanizate data at
160C, t 95~
Modulus 300~, MPa 10.6 12.8
Shore-A-hardness 63 64
Example 9 compares MOZ with N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfides or their disproportion-
ation products at different sulfur concentrations. If,
by contrast, the sulfur concentration is kept constant for
an equimolar accelerator dosage, the difference in reversion
stays, whereas the modulus 300% value increases considerably.
` 1325gO~
EXAMPLE 11
Comparison of the reversion behavior of commercial oligo-
sulfides and dibenzthiazolyl tetrasulfides against N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-oligosulfide
in N220-filled NR
31 32 33
RSS 1, ML(1~4) = 70-80100100 100 100
CORAX N220 50 50 50 50
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ZD 3 3 3 3
Vulkanox 4010NA 2.52.5 2.5 2.5
Vulkanox HS 1.51.5 1.5 1.5
Protector ~35
Robac P25 1.12
Tetrone A - 1.3
Dibenzthiazolyl tetrasulfide - - 1.15
M - - - 1.5
Sulfur 0.80.8 0.8 0.8
Scorch time 170C 1.41.9 2.7 3.1
(t 10~), mins.
Reversion:
max (max+60') (~) 18.5 21.3 35.7 3.2
max min
at 170C vulcanization
temperature
Vulcanizate data at
170C, t 95%
Tensile strength 23.1 21.2 18.9 23.6
Modulus 300% 9.4 9.5 7.3 10.4
Commercial oligosulfides (mixtures 30 and 31) and
dibenzthiazolyl tetrasulfide (mixture 32) show several
times higher reversion in NR than an N,N'-subtituted
bis-(2,4-diamino-s-triazin-6-y')-oligosulfide (mixture 33).
~ - 25 -
1325~
EXAMPLE 12
Blend of N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide with sulfur donor in N220-filled NR
34 35
RSS 1, ML(1+4) = 70-80 100 100
CORAX N220 50 50
ZnO RS 5 5
Stearic acid 2 2
Naftolen ZD 3 3
ProtectorlG 35
Vulkanox 401ONA 2.5 2.5
Vulkanox HS 1.5 1.5
Vulkacit MOZ 1.43
Sulfasan R - 0.7
D - 1.76
Sulfur 1.5
Reversion:
max (max+60 )(~) 30.6 2.1
max min
at 170C vulcanization
temperature
Where N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide is used with Sulfasan R as sulfur donor (mixture
35), there is a distinct improvement in reversion over
conventional sulfenamide acceleration (mixture 34).
132~8~
EXAMPLE 13
Blend of commercial accelerators with N,N'-substituted bis-
(2,4-diamino-s-triazin-6-yl)-tetrasulfide in N220-filled NR
36 37 38 39 40 41 42 43 44
RSS 1, ML(1+4)=70-80 100 100 100 100 100 100 100 100 100
CORAX N220 50 50 50 50 50 50 50 50 50
ZnO RS 5 5 5 5 5 5 5 5 5
Stearic acid 2 2 2 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5 2.5 2.5 2.5 2.5 2.5 2.5
Vulkanox HSI 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43 0.71 1.5 - - - - - -
Vulkacit DM - - - 1.56 0.78 1.56 - - -
Vulkacit Merkapto - - - - - - 1.2 0.6 1.2
B - 0.8 1.58 - 0.8 1.58 - 0.8 1.58
Sulfur 1.5 1.5 - 1.5 1.5 - 1.5 1.5
Reversion:
Dmax (max+60 )(%) 28.7 16.8 5.7 31.2 16.1 4.1 28.7 14.9 4.5
Dmax Dmin
at 170C vulcanization
temperature
Vulcanizate data
at 170C
Mbdulus at 300%, t95% 11.3 11.9 6.8 9.0 11.4 7.0 7.8 11.3 5.9
t95%+80' 8.9 10.7 6.7 7.5 10.5 6.9 6.5 10.8 5.9
::~
The joint use of commercial accelerators with N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfides (mixtures 37,
40,41) produces a clear improvement in reversion behavior over
the sole use of the commercial accelerators (mixtures 36,39,42).
A furLher increase in reversion stability may be obtained by
elimination of the sulfur (mixtures 38,41,44).
; - 27 -
- 132580~
EXAMPLE 14
Blend of N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-oligosulfide in N220-filled NR
46 47 48
RSS 1, ML(1+4)=70-80100 100 100 100
CORAX N220 50 50 50 50
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ZD 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43
M 1.5 -0.75
B - - 1.660.83
Sulfur 1.5 0.8 0.8 0.8
Reversion:
max (max+60')(%) 30.1 2.3 0.4 1.2
max min
at 170C vulcanization
temperature
The use of N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides differing in their reversion stability in
combination with one another produces a set of values which
lies between the pure substances (mixture 48).
- 28 -
13278~
EXAMPLE 15
Reversion in B-accelerated rubber filled with
carbon black/silica blend
49 50
RSS 1, ML(1+4)=70-80100 100
CORRX N220 25 25
Ultrasil VN3 Gran. 25 25
ZnO RS 5 5
Stearic acid 2 2
Naftolen b 3 3
Protector G35
Vulkanox 4010NA 2.5 2.5
Vulkanox HS 1.5 1.5
Vulkacit MOZ 1.43
B - 1.67
Sulfur 1.5 0.8
Reversion:
max (max+60')(~) 46.7 10.8
max min
at 170C vulcanization
temperature
When the sulfenamide accelerator (mixture 49) is replaced
by N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide (mixture 50), there is a drastic reduction
in reversion.
- 29 -
132~80~
EXAMPLE 16
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
and-oligosulfides in N220-filled SBR
51 52 53 54 55
SBR 1500 100 100 100100 100
CORAX N220 50 50 50 50 50
ZnO RS 5 5 5 5 5
Stearic acid 2 2 2 2 2
Naftolen ZD 3 3 3 3 3
Protector G35
Vulkanox 4010NA 2.5 2.5 2.52.5 2.5
Vulkanox HS 1.5 1.5 1.51.5 1.5
Protector G35
Vulkacit MOZ 1.43
B - 1.66
F - - 1.58
K - - - 1.5
M - - - - 1.5
Sulfur 1.51.5 1.51.5 1.5
Reversion:
max (max+60')(~) 12.9 11.3 11.5 9.2 12.5
max min
at 170C vulcanization
temperature
Vulcanizate data
at 170C, t 95%
Tensile strength 20.6 21.3 20.8 20.5 lS.9
Modulus 300~ 10.0 11. 6 12.2 12.1 12.3
Elongation at break 460 44,0 420 420 390
Shore hardness 61 63 63 63 64
For the same sulfur concentration, N,N'-substituted bis-
(2,4-diamino-s-triazin-6-yl)-tetra and -oligosulfides
(mixtures 52-55) show a distinctly higher modulus value
and slightly improved reversion properties in SBR 1500
compared with conventional acceleration (mixture 51).
,.
- 30 -
132~
EXAMPLE 17
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
and -oligosulfides in N220-filled isoprene rubber
56 57 58 59
Polyisoprene 3,4 100 100 100 100
CORAX N220 50 50 50 50
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ZD 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5 2.5
. Vulkanox HS 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43
B - 1.76
L - - 1.6
i M - - - 1.5
` Sulfur 1.5 0.8 0.8 0.8
Reversion:
max (max+60')(%)21.0 2.1 0.0 0.0
max min
at 170C vulcanization
temperature
Vulcanizate data at 170C
Modulus 300% t95% 7.9 6.6 6.3 6.4
t95~+80'6.2 6.8 6.3 7.1
In polyisoprene rubber, the use of N,N'-substituted bis-
(2,4-diamino-s-triazin-6-yl)-tetra- and oligosulfides
(mixtures 57-59) again produces a distinct improvement in
reversion over conventional accelerators (mixture 56).
This improvement is also reflected in the stability of
the vulcanizate data in the event of distinct overheating.
- 31 -
132~8~5
EXAMPLE 18
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
and oligosulfides in N220-filled EPDM
61 62
Buna AP 451 100 100 100
CORAX N220 50 50 50
ZnO RS 5 5 5
Stearic acid 2 2 2
Ingraplast NS 10 10 10
Vulkacit Thiuram
Vulkacit Mercapto 0.5
B - 2.93
M - - 2.5
Sulfur
Reversion:
max (max+60') (~) 3 3 0 0 0 0
max min
at 160C vulcanization
temperature
;
EPDM mixtures which are already hi~hly reversion-stable
show a further reduction in reversion to zero if conventional
accelerator mixtures (mixture 60) are replaced by N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra- and
oligosulfides (mixtures 61 and 62).
132~
EXAMPLE 19
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
and oligosulfides in N220-filled NBR
63 64 65
Perbunan N3307NS 100100 100
CORAX N220 60 60 60
ZnO RS 5 5 5
Stearic acid
Paraffin solid
Mesamoll ' 10 10 10
Vulkanox HS 1.5 1.5 1.5
Vulkacit CZ 1.5 1.5 1.5
: D - 1.76
M - - 1.5
Sulfur 1.2 1.2 1.2
Reversion:
Dmax D(max+60')
D -D (%) 8.0 5.4 5.6
` max min
at 170C vulcanization
temperature
Vulcanizate data at 170C, t95%
Modulus 300% 11.8 14.0 14.6
Shore hardness 6'8 69 69
For the same sulfur content, replacement by N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetra- and oligosulfides
(mixtures 64 and 65) in NBR produces a distinct increase
in the modulus value at 300% elongation and a reduction
in reversion compared with the reference mixture (mixture 63).
- 33 -
132~80~
EXAMPLE 20
Reversion stability of N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-oligosulfides in N220-filled BR
66 67 68
Buna CB 10 .100 100 100
CORAX N220 60 60 60
ZnO RS 3 3 3
Stearic acid 2 2 2
Naftolen ZD 15 15 15
Protector~G35
Vulkanox 4010NA 1.5 1.5 1.5
Vulkacit NZ 1.5
M - 1.5
D - - 1.76
Sulfur 1.5 1.5 1.5
Reversion:
max (max+60')(%) 30.3 20.8 19.1
max min
at 170~C vulcanization temperature
Vulcanizate data at 170C
Modulus 300% t 95% 8.6 8.9 8.2
t 95%+75' 5J6 6.8 6.6
By virtue of the lower reversion of the M- and D-accelerated
BR mixtures (mixtures 67 and 68), the reduction in the
modulus 300~ value in the event of strong overheating is
far smaller than in the reference mixture (mixture 66).
- 34 -
13258~
EXAMPLE 21
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
and oligosulfides in N220-filled NR/BR blend
69 70 71 72
RSS 1, ML(1+4)=70-80 70 70 70 70
Buna CB 10 30 30 30 30
CORAX N220 50 50 50 50
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ZD 3 3 3 3
Vulkanox 4010NA 2.5 2.52.5 2.5
Vulkanox HS 1.5 1.51.5 1.5
Protector G35
Vulkacit MOZ 1.43
C - 1.72
D - - 1.76
M - - - 1.5
Sulfur 1.5 0.8 0.8 0.8
Reversion:
max (max+60')(~) 32.0 15.8 20.6 23.8
max mln
at 170C vulcanization
temperature
.
In a blend of NR and polybutadiene, mixtures 70 and 72
show a distinct reduction in reversion where N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra- and
oligosulfides are used instead of a semi-EV-system
(mixture 69).
132~805
EXAMPL _
Crosslinking of 50% epoxidized N 220-filled natural
rubber (ENR 50) with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetra- and oligosulfide
73 74 75
ENR 50 100 100100
CORAX N220 50 50 50
ZnO RS 5 5 5
Stearic acid 2 2 2
Vulkanox HS 2 2 2
Vulkacit MOZ 2.4
Vulkacit Thiuram 1.6
C - 4.6
M - - 4.0
Sulfur 0.3 0.3 0.3
Reversion:
max (max+60')(%) 6.7 0.0 0.0
max min
at 150C vulcanization
temperature
Vulcanizate data at 150C, t 95%
Tensile strength 18.7 23.9 24.6
Modulus 300% 18.0 18.4 18.9
Tear propagation strength 12 14 12
Shore hardness 75 76 78
For the same modulus value at 300~ elongation and considerably
increased tear resistance, the replacement of a conventional
accelerator (mixture 73) by N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetra and oligosulfides (mixtures
74 and 75) produces an additional improvement in reversion.
~ - 36 -
1325~05
EXAMPLE 23
Effect of Vulkalent E in sulfur-free NR crosslinking
of N220- and N220/filica-filled NR with N,N'-substituted
, bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfides
76 77 78 79 80 81
RSS 1, ML(1+4)=70-80100 100100100 100 100
CORAX N220 50 50 50 50 50 50
Ultrasil VN 3 Gran. - - - 25 25 25
ZnO RS 5 5 5 5 5 5
Stearic ac,id 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3
Vulkanox 4010NA 2.52.5 2.52.52 5 2.5
Vulkanox HS 1.51.5 1.51.51.5 1.5
Protector G35
Vulkacit MOZ 1.43 - - 1.43
C - 3.79 3.79 - 2.84 2.84
Vulkalent E - - 3.2 - - 2.4
Sulfur 1.5 - - 1.5
Reversion:
Dmax D(max+60 )(%) 32.5 4.1 4.5 47.8 5.4 5.5
Dmin max
at 170C vulcanization
temperature
tI 170C, mins. 4.2 2.8 4.6 4.7 3.0 4.7
:
In N220- and N220/silica-filled NR -rosslinked with C, the
addition of Vulkalent E produces the desired extension of
the incubation time without any deterioration in reversion.
132~80~
EXAMPLE 24
Retarding effect of Vulkalent E on N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetra- and oligosulfide-
vulcanized N220-filled NR mixtures
82 83 84 85 86
RSS 1, ML(1+4)=70-80100 100 100 100 100
CORAX N220 50 50 50 50 50
ZnO RS 5 5 5 5 5
Stearic acid 2 2 2 2 2
Naftolen ZD 3 3 3 3 3
Vulkanox 9010NA 2.5 2.5 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43
B -1.66 1.66 - -
M - - -1.55 1.55
Sulfur 1.50.8 0.80.8 0.8
Vulkalent E - - 1.2 - 1.2
tI (mins.) at 170C3.83.0 4.42.9 4.1
Vulcanizate data
at 170C, t95~
Modulus value 300~ 10.6 10.2 11.5 10.6 11.0
The addition of Vulkalent E in B- and M-accelerated
mixtures ~mixtures 84 and 86) produces an increase in
the incubation time tI beyond the level of the mixture
conventionally accelerated with MOZ (mixture 82).
.
- 38 -
132580~
EXAMPLE 25
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides with addition of Vulkalent E in N220-filled
NR as a function of temperature
87 88 89
RSS 1, ML(1+4)=70-80100100 100
CORAX N220 50 50 50
ZnO RS 5 5 5
Stearic acid 2 2 2
Naftolen ZD 3 3 3
Protector G35
Vulkanox 4010NA 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5
Vulkacit MOZ 1.43 - -
B -1.66 1.66
Vulkalent E - - 1.2
Sulfur 1.5 0.8 0.8
Reversion:
max (max+60')
max min
at test temperature
145C 7.9 0.0 0.0
1 160C 25.6 0'0 1.9
170C 30.9 0.4 3.6
180C 39.1 2.6 5.5
Example 25 shows a pronounced insensitivity to temperature
of reversion with pure N,N'-substituted bis-~2,4-diamino-s-
triazin-6-yl)-tetrasulfide acceleration (mixture 88) and
also with addition of Vulkalent E (mixture 89) compared
with conventional acceleration (mixture 87).
- 39 -
13258~5
EXAMPLE 26
Effect of Vulkalent E on N,N'-substituted bis-(2,4-diamino-
s-triazin-6-yl)-tetrasulfide- and oligosulfide-crosslinked
NR filled with N220/silica
91 92 93 94
RSS 1, ML(1+4)=70-80100100 100100 100
Ultrasil VN 3 Gran.25 25 25 25 25
CORAX N220 25 25 25 25 25
ZnO RS 5 5 5 5 5
Stearic acid 2 2 2 2 2
Naftolen ZD 3 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.52.5 2.5
Vulkanox HS 1.5 1.5 1.51.5 1.5
Protector G35
Vulkacit MOZ 1.43
D - 3.5 3.5
M -- - 3.0 3.0
Vulkalent E -- 1.2 - 1.2
Sulfur 1.50.8 0.8 0.8 0.8
Scorch at 130C (mins.)29.5 15.0 28.5 16.5 29.0
Scorch at 170C (mins.)4.5 3.4 4.4 3.7 4.8
Where N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides and oligosulfides (mixtures 92 and 94) are
used for acceleration, scorch behavior comparable with
MOZ can be obtained with Vulkalent E.
- 40 -
. .
:. ,
132~8~5
EXAMPLE 27
Use of N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide in combination with Si 69 with and without
Vulkalent E in N220/VN3-filled NR
9596 97 98 99
RSS 1, ML(1+4)=70-80 100100100100100
CORAX N220 2525 25 25 25
Ultrasil VN3 Gran. 2525 25 25 25
Si 69 3.75 3.75 3-75 3-75 3-75
ZnO RS , 55 5 5 5
Stearic acid 22 2 2 2
Naftolen ZD 33 3 3 3
Vulkanox 4010NA 2.52.52.52.52.5
Vulkanox HS 1.51.51.51.51.5
Protector G35
Vulkacit MOZ 1.43 - - - -
B -2.22.2 1.66 1.66
Vullcalent E - - 1.6 - 1.2
Sulfur 1.5 - - 0.8 0.8
Reversion: -
D -D 19.6 0.0 0.0 0.0 0.0
max min
at 170C vulcanization
temperature
tI at 170~C (mins.) 4.64.25.7 4.2 5.2
Where N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfides are used together with Si 69, reversion-
free mixtures are obtained both with and without sulfur
(mixtures 96,98) as against conventional acceleration with
silane (mixture 95). In this case, too, the addition of
Vulkalent E lengthens the scorch time without recurrence
of reversion (mixtures 97 and 99).
- 41 -
1325~
EXAMPLE 28
Crosslinking of N220/silica-filled NR with N,N'-substituted
bis-(2,4-diamino-s-triaizn-6-yl)-tetrasulfide without sulfur
RSS 1 100 100 100100 100 100 100
CORAX N220 25 25 25 25 25 25 25
Ultrasil VN3 Gran.25 25 25 25 25 25 25
ZnO RS 5 5 5 5 5 5 5
Stearic acid 2 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.52.5 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.51.5 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43
D - 2 3 4 5 6 7
Sulfur 1.5 - ~ - - - -
Reversion:
max (max+60 )(~)
D -D . 44.7 3.2 2.01.3 3.1 2.8 2.6
max mln
at 170C vulcanization
temperature
Vulcanizate data
(t 95~) at 170C
Modulus 300~ 4.9 2.6 4.45.8 6.8 7.8 8.8
Sulfur-free crosslinking with N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfide in natural rubber
filled with carbon black and silica suppresses reversion
and at the sa~e time produces a concentration-dependent
increase in modulus.
,
.
- 42 -
132~80~
EXAMPLE 29
Crosslinking of silica-filled NR with N, N'-substituted
bis-(2, 4-diamino-s-triazin-6-yl)-tetrasulfide
RSS 1 100100
Ultrasil VN 3 Gran. 50 50
ZnO RS 5 5
Stearic acid 2 2
Naftolen ZD 3 3
Vulkanox 4010NA 2.52.5
Vulkanox ~S 1.51.5
Protector G35
Vulcacit MOZ 1.43
D - 7
Sulfur 1.5
:,.
max (max+60 )(%) 33.07.8
max min
at 170C vulcanization
temperature
Vulcanizate data
(t95~) at 170C
Modulus 300% 2.74.3
Sulfur-free crosslinking with N,N'-substituted bis-(2,4-
diamino-s-triazin-6-yl)-tetrasulfide in silica-filled
natural rubber leads to a substantially reduction in
reversion and at the same time to an increase in the
- modulus value at 300~ elongation.
- 43 -
. .
13258~5
EXAMPLE 30
Acceleration of N220/silica-filled NR with N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide
RSS 1 100100100 100100 100 100
CORAX N220 25 25 25 25 25 25 25
Ultrasil VN3 Gran. 25 25 25 25 25 25 25
ZnO RS 5 5 5 5 5 5 5
Stearic acid 2 2 2 2 2 2 2
Naftolen ZD 3 3 3 3 3 3 3
Vulkanox 4010NA 2.52.52.5 2.52.5 2.5 2.5
Vulkanox HS 1.51.51.5 1.51.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43 - - - - - -
B - 2 3 4 5 6 7
Sulfur 1.5 0.51 0.76 1.02 1.27 1.53 1.79
Reversion:
max (max+60')(%) 44.73.21.7 0 0 0.8 1.7
; max mln
at 170C vulcanization
temperature
Vulcanizate data
(t95%) at 170VC
Modulus at 300% 3.63r55 5 7.08.3 9.2 9.8
The sulfur vulcanization of N220/silica-filled NR with
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
sulfide reduces reversion to 0% and produces a marked
increase in the modulus value.
- 44 -
132~80~
EXAMPLE 31
Acceleration of silica-filled NR with N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide
RSS 1 100 100
Ultrasil VN3 Gran.50 50
ZnO RS 5 5
Stearic acid 2 2
Naftolen ZD 3 3
Vulkanox 4010NA 2.5 2.5
Vulkanox HS 1.5 1.5
Proteetor G 35
Vulkacit MOZ 1.43
B - 7
Sulfur 1.5 1.79
Reversion:
max (max+60')(%)33 0 4 5
max min
at 170C vulcanization
temperature
Vuleanizate data (t95%)
at 170C
Modulus 300% 2.7 ,5.3
The sulfur vuleanization of siliea-filled NR with
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-tetra-
sulfide is possible with virtually no reversion and
produees an increase in the modulus value at 300~.
.
:
\
132~80~
EXAMPLE 32
Acceleration of N220/silica-filled NR with N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide in the
presence of Si 69
RSS 100 100 100
CORAX N220 25 25 25
Ultrasil VN3 Gran.25 25 25
Si 69 3.75 3.75 3.75
ZnO RS 5 5 5
Stearic ac,id 2 2 2
Naftolen ZD 3 3 3
Vulkanox 4010NA 2.5 2.5 2.5
Vulkanox HS 1.5 1.5 1.5
Protector G35
Vulkacit MOZ 1.43 - -
B - 2 3
Sulfur 1.50.49 0.7q
:
Reversion:
max (max+60 )(%)18.1 0 0
max min
at 170C vulcanization
temperature
Vulcanizate data (t95~)
at 170C
Modulus 100~ 1.4 1.6 2.4
Modulus 200~ 3.5 4.6 7.3
Modulus 300% 7.1 9.5 13.9
... .
~','
Acceleration with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfide in the presence of Si 69
enables reversion to be returned to 0~ for a considerable
- increase in the modulus values.
:,
`:
- 46 -
132~5
EXAMPLE 33
Acceleration of silica-filled NR with N,N'-substituted
bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide in the
presence of Si 69
RSS 1 100 100 100100
Ultrasil VN3 Gran. 50 50 50 50
Si 69 7.5 7.5 7.57.5
ZnO RS 5 5 5 5
Stearic acid 2 2 2 2
Naftolen ~D 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.52.5
Vulkanox HS 1.5 1.5 1.51.5
Protector G35
Vulkacit MOZ 1.43
B - 2 3 4
Sulfur 1.50.49 0.74 0.99
Reversion:
max (max+60 )(%)16.6 0 0 0
max min
at 170C vulcanization
temperature
Vulcanizate data (t95~)
at 170C
Modulus value 100%1.5 1.7 2.53.2
Modulus value 200%3.4 4.3 6.68.3
Modulus value 300~6.3 8.2 12.3 15.0
Even with pure silica filling, NR is vulcanized without
any reversion with N,N'-substituted bis-(2,4-diamino-s-
triazin-6-yl)-tetrasulfide and sulfur in the presence of
Si 69, the modulus values also being very considerably
increased.
- 47 -
132~8~5
EXAMPLE 34
Sulfur-free crosslinking of N220/silica-filled NR
with N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide in the presence of Si 69
RSS 1 100100100 100 100
CORAX N220 252525 25 25
Ultrasil VN3 Gran. 252525 25 25
Si 69 3 75 3.75 3.75 3 75 3 75
ZnO RS 55 5 5 5
Stearic acid 22 2 2 2
Naftolen ZD 33 3 3 3
Vulkanox 4010NA 2.52.52.52.5 2.5
Vulkanox HS 1.51.51.51.5 1.5
Protector G35
Vulkacit MOZ 1.43
D - 1 2 3 4
Sulfur 1.5
Reversion:
Dmax D(max~60 )(%) 18.1 0 0 0
max Dmin
at 170C vulcanization
temperature
Vulcanizate data (t95%)
at 170C
Modulus 200% 3.52.64.96.5 8.6
Modulus 300% 7.15.810.2 12.9 16.0
In carbon black/silica-filled natural rubber, sulfur-free
crosslinking with N,N'-substituted bis-(2,4-diamino-s-triazin-
6-yl)-tetrasulfide, even in the presence of Si 69, prevents
reversion and produces a considerable concentration-
dependent increase in the modulus values.
'' .
~,
- 48 -
, ~
132~80~
EXAMPLE 35
Sulfur-free crosslinking of silica-filled NR with
N,N'-substituted bis-(2,4-diamino-s-triazin-6-yl)-
tetrasulfide in the presence of Si 69
RSS 1 100 100 100100 100
Ultrasil VN3 Gran. 50 50 50 50 50
Si 69 7.5 7.5 7.57.5 7.5
ZnO RS 5 5 5 5 5
Stearic acid 2 2 2 2 2
Naftolen Z,D 3 3 3 3 3
Vulkanox 4010NA 2.5 2.5 2.52.5 2.5
Vulkanox HS 1.5 1.5 1.51.5 1.5
Protector G35
Vulkacit MOZ 1.43
D - 1 2 2 4
Sulfur 1.5
Reversion:
max (max+60') (%)16.6 0 0 0 0
max min
at 170C vulcanization
temperature
Vulcanizate data
(t95~) at 170C
Modulus value 200~3.4 2.8 3.95.0 7.6
Modulus value 300%6.3 5.0 7.6 11.3 13.9
The crosslinking of silica-filled natural-rubber with N,N'-
substituted bis-(2,4-diamino-s-triazin-6-yl)-tetrasulfide
in the presence of Si 69 again prevents reversion and
also produce a considerable increase in the modulus values.
~ - 49 -