Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 132588~
The invention relates to a contlnuous production proces~ to
simultaneously obtaln elementary sulphur from H2S-gases and from
S02-gases, especlally from sour gas and from S02-rich gas derived
from the desulphurlzatlon of flue gas.
To obtain elementary sulphur by means of a catalytlc Claus reactlon 18
standard lndustrlal practice but requlres, however, a stolchlometrlc
H2S : S02 ratlo and greatly dlluted feed gases with a limited moisture
content.
In refining plants with ad~oinlng power station, H2S-gases as well a~ -
S02-gases are generally availabIe. The quantity of the sulphur compounds
depends on the capacity of the plants as well as on the processed raw
materials and, therefore, fluctuates widely without ever reaching the
stoichiometric H2S : S02 ratio over a prolonged period of
time. Furthermore, the concentrations, which reach over 50 molar % of
H2S in sour gas and more than 90 molar ~ of S02 in rich gas are by far
too high.
:, . ~ .
.
2 1325880
It was therefore suggested to first of all lower the concentration of
sulphuric compounds by way of tirect thermal oxidation of the ~2S
according eo
2 ~2S ~ 2 ~ 2 S + 2 ~2
or by the thermal or catalytic reduction of the S02 according to
2 + 2 ~2 f S + 2 ~2~
~owever, ehese eauations do not correspond to the actual course of
react$on. Depending on the temperature, the equilibrium in both cases
19 somewhere bet~een 60 - 70Z which always results in a greaeer oxidized
or reduced compound. Even lf it were possible to continue with the
reactions to éhe extent of attaining the low concentration of sulphuric
compounds required for the catalytic Claus reaction, these gases could
only conditionally be introduced to the Claus installation i~ view of
the quantities of water which will si~uleaneously form and on account of
ehe increased steam content which will result in considerable activity
losses of the catalyst. It has been established that the activity 1088
over a three-month period will result in a decrease of conversion of
approximately 20Z if customary Claus catalygtq are used. Since a water
separation by way of conden3ation i3 not feagible also for reason3
of heat economy, the only other alternative i5 the diluting with
sulphur-frQe, inert gases which la achieved, e.g. by combu~tlng the
~2S with air instead of oxygen. ~owever, thi3 procedure result3 in an
undesirable increase in the capacity of the apparatuses as well as in
the heat supply. Another very aggravating problem is the discolouration
of the sulphur that has been for~ed. This will occur i ehe 80ur gag,
which is generally pre3en~, contalns hydrocarbons. Furthermore~ the
lnvestment costs and, especially wlth respect to reduction~ the
, ;,
~ 3 ~ 1325880
operating costs are very high.
Specification DE-OS 2346247 attempts, therefore, to obtain
sulphur by way of a direct non-catalytic conversion of the H2S
with SO2, in which the SO2 is made available partly through H2S
oxidation and partly through foreign supply. Although it is
insinuated that the application should also be possible in gases
with an overstoichiometric SO2 content, the aforementioned
specification refers only to a procedure involving gases with a
H2S excess.
The specification states further that the retention time is
determined by the dimensions of the H2S combustion chamber.
However, it is not clear how the rate of reaction of the direct
sulphur formation which is lower in comparison to oxidation,
could be taken into consideration.
Therefore, the ob~ect of this invention was to find a possibly
economical method to simultaneously produce qualitatively
acceptable elementary sulphur from H2S-gases and from S02-gases
where the ratio to each other as well as the quantity would be
subject to great fluctuations and where it should be possible to
use waste gas directly as supply gas for a catalytic Claus
lnstallation.
This task has been accomplished by the invented method as
mentioned at the outset and according to which
a) all of the H2S-gas is being subjected to partial combustion,
in the presence of a 2 -gas, preferably air, at a H2S : 2
molar ratio of less than 2 : 1, and in the presence of a
portion of the
132~880
- 4 -
S02-gas at 8 ~Zs: S02 molar ratio of re than 4 : l. This
process takes place ln the frontal area of a 1st reaction zone
at temperatures ~n the 800-1400C range and thus results in
a reaction mi~ture of elementary sulphur with a ~2S and S02
quantity at a molar ratio of 2 : l up to l : lO;
b) the balance of the S02-ga~ 19 converted into elementary
sulphur in a 2nd reaction zone by way of reaction ~ith a
reducing gas at temperature~ in the 1000 - 1500C range
and into a reaction mixture containing ~2S and S02 at a molar
ratio of 2 : 1 to 10 : 1;
c) the ~i~ture of the reaction flow~ originating from stages a)
and b), as formed in the rear area of the 1st reaction zone,
is converted into elementary ~ulphur at a temperature in the
800 - 1300C range in a ~ub-~equene 3rd reaction zone without
requlri~g a further direct heat supply. The wagte gas at a
~2S : SO2 molar ratio of approximately 2 : l contains maximum
approximately 20 Z of ~2S and less than approximately 65
molar Z of ~2;
d) the waste gas from stage c) 1~ being con~erted into addi-
tional elementary sulphur in a mult~-~tage Claug lnstallatiOn
ln the customary manner ~ubsequent to partial cooling, heat recovery
and sulphur condensation.
1325880
The invented method is applied mainly in refining plants with an
ad~oining power station for the purpose of reprocesslng simultaneously
the sour gas from the refining plant and the S02-rich gaR derived from
the desulphuri2ation of the flue gas. However, the process is equally
suitable for gas flows of other provenances.
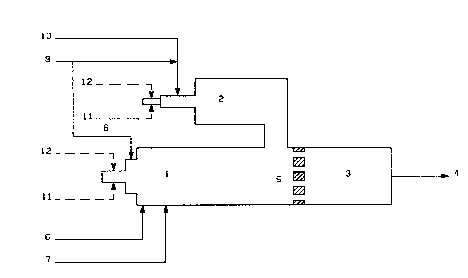
All of the H2S gas is fed into the oxidation zone whereas the S02-gas is
being dlvided, i.e. preferably quantitatively controlled, between the
oxidation and the reduction zones. The quantity of H2S to be oxidized
to S02 will only be as much as is required for flame 6tabillty. This
quantity increases only when proces6ing 8ases with a great surplus of
H2S. Depending on the S02 quantity to be processed, a larger or lesser
portion is being reduced to sulphur and H2S. The only limitation is the
quantity of water (generally less than approximately 65 molar ~) that i8
being tolerated by the subsequent catalytic Claus ln6tallatlon. It would
be po66ible to exceed said water content if H2 were used as reducing
gas. It could be counterscted by using a methane and/or C0-reducing gas.
In addition, a 02-gas, e6pecially air, is being fed into the oxidation
zone. The quantity of 2 correspond~ to the quantity of H2S which i6 to
be oxidized to S02. H2, C0 and/or low hydrocarbons such a6 methane,
ethane, etc. may be u6ed a6 reducing gas. Gas mixtures which contain
methane, H2 and C0 and which are frequently available at low cost in
refining plants can be used without detrimental effects.
With respect to heat economy, it has proven especially successful to
combine to a certain degree the production of the reducing gas and
13258~0
the heat requlred for the S02 reduction. In thls case, the heating gas,
whlch consists mostly of methane, is being dlvided into 3 partial
streams at a ratio of about (20 to 50) : (79 to 30) : (1 to 20) with the
total equalling 100, i.e. preferably 35 : 50 : 15. The 1st stream i6
being combusted understoichiometrically with air, preferably with ~
equalling approximately 0.6 - 0.95 or most preferably if ~ equals about
0.7. The hot combustion gases ranging between 1700 - 1900C, depending
on the heat losses, are being mixed with the 2nd stream and ~ubsequently
fed into the actual reduction zone. The 3rd stream, after possibly being
indirectly preheated, e.g. to 150C, will be fed into the reduction zone
together with the S02-rich gas. The reducing gas made available in the
above-described manner contains methane, hydrogen and carbon monoxide as
reducing components and ranges in temperature from 1350 - 1550 C,
depending on heat 1088. For example it can be composed as follows:
CH4 20.0 molar %
N2 50-9 molar %
C2 6.8 molar %
2 8.7 molar %
C0 2.9 molar
H20 10.7 molar ~
In order to effect further sa~ings ln energy, the S02-gas, which is to
be fed into the 2nd zone, can be divlded into two partial streams at a
ratio of sllghtly more than 2 : 1, i.e. preferably after belng preheated
by indirect heat exchange , e.g. to 450C. The larger stream is being
llreduced in the actual reduction zone by means of the reducing gas
obtalned in the above-descrlbed manner at a resulting mixing temperature
132~880
of about 1200C. ThQ remainder of the S02 part~al stream is fed into
ehe react~on ~ixture obtained fro~ the reduction zone prior to combining
the reaction mixture ~ith the ~;Yture from the oxidation zone which, in
view of a m~ing temperature in excess of 900C a~d an already adequate
retention time, leads to the formation of elementary sulphur. A checker
wall advantageously separates the actual reduction zone from the
preceding c bustion zone and, provlded one e~ists, from the subsequent ..
sulphur forming zone.
The gas mixture formed in the reduction zone, either with or without a
subsequent sulphur- forming zone, ig being combined with the gas mixture
from the oxidation zone in the rear area of the latter zone a~d is then
subsequently fed into a 3rt reaction zone. In order to ensure a thorough
~lxing of the gases, the 3rd zone i9 separated from the preceding zones
preferably by a vertical laetice--shaped or honeycombed checker wall with
a dlfferential pressure of approximately 3 - lO mbar.
Whereas in the oxidation zone, under conditiong which deviate from
stolchlometrlc rat~os, a conversion of ~zS and SO2 to sulphur worth
mentioning is not accomplished and, whereas only small qua~tities of
sulphur are being formed in the reduction zone, the production of
sulphur, therefore, concentrates on zone 3. Due to the physical
separation of the sulphur formation, the following advantages are
realized:
1. indl~idual retention time and con~equently an optimum approximation
to the thermodynamic equilibrium;
2. Production of a 3ulphur of great purity due to the fact that the
hydrocarbons, which are contain~d in the heatlng gag ag well as ln the
., 1
- 1325880
sour gas, are combusted almost completely and/or that the resulting
carbon reacts with the reaction components to COS and CS2
owinq to the
indi~idual retention time, thus preventing soot from forming.
The temperature in the zones should preferably be as follows:
zone 1 900 - 1250C; zone 2 1150 to 1300C; zone 3 900 - 1200 C.
In order to reach these temperatures, it ~ay be necessary to burn a
hydrocarbon heating gas geparately in Zone 2 andlor to add same to the
sour gas in Zone 1. In additlon, the S02-gag, the reduction gas, the
sour gas andtor the combuqtion air can be heated by way of indirect heat
e~change prior to being fed into the respective zones. In view of the
puriey of the sulphur which formg already ln zone 2, it is preferable to
. . ,
conduct at least a-portion of the heat indirectly to zone 2. This
measure is a posslbility especially If methane is-available as reducing
gas. Any economical low hydrocarbon mi~ture 3ay be used as heating
gas. Depending on the construction of the burner, lt is possible in
princlple to use a liquid fuel. Since none of the three reaction zones
has a catalyst, it is not necessary to make any special demands on the
heatlng gas with respect to caealyst poi~on. ~n principle, a catalytic
reduction would be e~pediene only if such catalygt would tolerate
temperaeures of 800 - 1500C.
For practical reasons, the cemperature of the 1st and 2nd zone is
appropriately in the upper ran8e of the gpecified temperature interval.
Thus the formation of COS and CS2 is advantageouglr reduced and,
secondly, the temperature in zone 3 can be retained within the in~erval
wiehout burning additlonal heaeing gag. In view of the fact that the
sulphur forming reaction 19 ~lightly endothermic, ehe temperature of the
3rd zone is re~pectively gomewhat lower than that of ehe precediny
zones.
1325880
_ 9 _
The tivisios of the S0 -gas flow, as descr~bed above, and the e~tent ofthe oxidat~on and reduction reactions are decisive for the composition
of the waste gas in the 3rd zone. In order to render further processing
in a catalytic Claus installation possible without the recovery of a
possible surplus component, the H2S : S02 ratio should be approximately
Z or, to be more preclse,
H25 + ( COS . a ~ + ( 2 C52 b )
=2
SO2
a and b can ln this case be either the same or may differ; however, they
represent the degree of conversion of COS and/or CS2 in the 3ubsequent
Claus installatian. I~ addition, the ~2S concentration i9 to be l~;ted
to about 20 molar ~ ma~i~um. S~nce the equilibrium of the
sulphur-forming react~on ig somewhere between 60 - 70Z, depending on the
tenperature, a continuous, partlal and premature removal of the sulphur
ls a possibility. ~owever, this measure i~ llmdted to special
ind~vidual cases for rea30ns of heat economy.
Normally, the entlre sulphur, as for~ed in zoneg 1 to 3, ls ~eparaeed
after the 3rd reacti.on zone subsequent to cooling
the g?S m~xture below the condensat~on temperature o~ gulphur while at the same
time recovering the heat, e.g. in the form of steam. By subjecting
the gas from which the s~ulphur has ~een removed in the above
described-manner to a catalytic Claus installation and, if necessary,
by slightly reheating the ~as, further 5ul~hur i5. beino o~ta~ned.
Surprisingly, lt was d~scovered that contrar7 to prevlou~ knowledge, the
partial oxidatio~ of the ~2S, when physically geparated from the
j~ ~ `J `
j,~7
132~880
- 10 -
sulphur-forming zone, can be csrried out without any difficultles even
in the presence of large quantities of S02 which may be many time~ thst
of the H2S. The invented method is not limited to a minimum rstio of
H2S : S02. It is especially suited for the reprocessing of gases with an
S2 surplu~. The sulphur thus produced is of great purity due to the
fact that impurities caused by hydrocarbons which have not been
completely combusted are to a large extent prevented by the featur2s of
novelty of the invention.
132~
The in~en~ion will be explained below by way of e~amples and by
referring to the atrached sche~atic drawing but, however, without
limiting it to the conditions given in the e~ample.
Example l:(use of natural gas as reducing gas)
23.9 I~molth of S02 rich gas (8) with a S02 content of 95.27 molar ~, preheated
to 450C, is fea into the oxidation zone (1), as well as 137.9
kmol/h of sour gas (7) with a H2S content of 89.20 molar ~ and 2~3.2
kmal/h of air ~61 via t~e ~_5 hurner which may consist of a system
comprised of several indi~idual burners. At the same time, the
reduction zone (2) 1~ being fed with the remaining Z1.4 kmollh of
preheated S02 rich gas (9) and with 10.79Dlol/h of a reducing gas (10)
with 97 5 molar Z of C~4,0.9% of C2~6, 0.3% of C3~8, 0~9~ of 2
f C2 as well 85 with 4.38 kmol/h of heati~g gas (12) ant 29.64 kmol/h
of combustion air (11) via a heating gaq burner. rhe heating ga~,
having the same compo-~ition as the reducing gas,is burned
understoichiometr~c311y.
A partlal retuc~ion of the SO2 take3 place in zone 2 at a temperature of
approximately 1250 C, thus resulting in a nLLxture with a ~2S : S02 ratio
of 1.9 : 1 and a vaporous sulphur (S2) content of 9.1Z. At the same time a hot
sulphur-containing mixture of approximately 1000C and with a ~2S
: 52 ratio of 1.96 : 1 is formed hy way of partial ~25 oxidation in
zone 1 in tne vicinity-of tn~-burn~r. BQth liiixturas are t~n
mixed in the rear area of zone 1 and fed into zone (3) via the
checker ~all (5). During a retention time of a~proximately 1
second, a further conversiOn to sulphur takes
1325880
-lZ -
place in zone 3. The temperature sets itself to a~out 1040 C. The
m~xture(4) leaving reaction zone (3) is then be~ng cooled to 240C by
way of 5 bar-steam production, and the condensing sulphur is
separated in the usual manner. The yleld ~8 2800 kg/h of sulphur with a
99.9% purity. After being indirectly heated to 260C, the rema;~ing gas
mixture containing 7.4Z of ~2S, 3.8% of S02, 0.2% of COS and CS2, as
~ell as 31% of a2o i9 converted into further elementary sulphur in the
customary manner in a catalyt~c Claus installation.
Example Z: (use of hydrogen as reducing gas)
The procedure is si3ilar to example 1 except for the preheating of the
S2 rich gas. Zone (1) is fed with 137.9 kmol/h of sour gas (89.2Z of
S~, 233~2 kmol/h of the oxidation air as well a3 23.9 kmol/h of S02
rich gas, thus produc~ng at a temperature of 1000C a m~Yture with a
H2S : 52 ratio of 1.9 : 1. At the same time zone (2) i9 fed with 21.4
kmol/h of S02 rich gas and 42.1 kmol/h of hydrogeu and converted by
means of partial reduction at 13Z0 C into a m~xtur~ ~ith a a2s : S02
ratio of 2 : 1 and a sulphur content (S2) of 11.6Z.
Subsequent to mixing, a further conversion to sulphur takes place in
zone 3 ae 1150 C and a retention tlme or 1 second. By cooling to 240 C,
2800 kg/h of sulphur of 99.9Z i~ separ3ted. The remaining gaq mixture
containing 7.6Z of ~2S, 3.~5% of S02, O.lZ of COS and CS2 and 32-72 of
a2o i5 further processed as de~cribed in Example 1.