Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
" 1328714
~' PEROXIDE BLEACHING OF MECHANICAL PULPS
. .
Field of the Invention
Present invention relates to the brightening of
5 mechanical pulps, more particularly the present invention
relates to hydrogen peroxide brightening of mechanical
pulps by in situ formation of the perhydroxyl ion to
provide a brightened pulp and a peroxide residual of at
least 35% of the peroxide added and to a process wherein
residual from treatment of a first pulp is used to treat a
i. second pulp.
;~ Background of the Present Invention
In conventional peroxide brightening of mechani-
.1 15 cal pulp a brightening liquor containing perhydroxyl ions
. is first formed by reacting hydrogen peroxide with sodium
hydroxide to form the perhydroxyl ion in water. This
liquor containing the brightening chemical (perhydroxyl
. ion) is then added to the pulp and functions to oxidize
color compounds in the pulp to colorless form thereby
increasing the pulp whiteness or brightness.
,~,
The ratio of peroxide to caustic (sodium
~, hydroxide) in the brightening solution must be optimized
for each pulp. Too little caustic would leave some of the
hydrogen peroxide in unreacted form while too much caustic
. .,
causes the perhydroxyl ions to decompose thus wasting some
.- of the perhydroxyl ion and further could result in alkali
. darkening of the pulp. ~ompounds are produced during the
i 30 pulp brightening that tend to utilize the sodium hydroxide
so that the optimum caustic to peroxide ratio varies
. depending on species and quality of the wood being
- brightened.
:,
,. ~
. .~
. - 1 -
'':
,,''
,
.
, ,
1328714
; Generally, magnesium sulfate and sodium silicate
are incorporated in the brightening liquor, i.e. liquor
containing the perhydroxyl ions to protect the perhydroxyl
ions from decomposition until it reacts with the pulp,
i.e. in storage and until the actual brightening reaction
takes place in the pulp.
In a typical composition, magnesium sulfate and
sodium silicate are first added in amounts related to the
~- 10 bone dry weight of the pulp being brightened. Peroxide
addition is keyed to obtain the desired degree of pulp
brightness thus is based both on desired degree pulp
brightness and on amount of pulp while the sodium
hydroxide usage is dependent on the peroxide used (pulp
^ 15 brightness desired) and obviously as above indicated on
the species being treated.
.
Because of the reactivity of the perhydroxyl ion
the brightening solution has to be made by specific
-~ 20 sequential additions of the individual chemicals. Normal-
~`,r, ly, a cascading system is used wherein magnesium sulfate
is first added to water and then sodium silicate is added
and intimately mixed followed by the addition of caustic
~^ and finally by the addition of hydrogen peroxide, the
resulting liquor then is stored in a bleach liquor storage
~; tank until used.
~ r
~;; This process obviously results in some decompos-
` ition of the reactive perhydroxyl ions in storage and
~` 30 requires a significant amount of process equipment such as
tanks, pumps, mixers, etc for each of the separate ingred-
ients. Also, the magnesium and silicate present in the
mixture form complexes which precipitate and sometimes
result in scaling and plugging problems.
. .
~`~ - 2 -
"
,
. "
-' 132871~
It will be apparent that if there is a rapid
change in wood quality it is not a simple matter of vary-
~ ing the brightening solution usage on wood, rather it is
; necessary to change the whole composition of the brighten-
.3 5ing solution. Clearly, when such changes are made they
.~ are not reflected in the system until the previously made
`~ brightening solution has been used thereby causing an
I inherent time delay when a change in the brightening
composition for the pulp is implemented.
.~ 10
Canadian Patent 686,115 issued May 12, 1964 to
Guard describes a system of bleaching wood pulp wherein
the wood is first treated with a chelating agent to remove
~,
- or deactivate catalytic ions followed by thickening to
15typicaliy about 14%-15%. Initially the resultant thickened
pulp is treated with hydrogen peroxide followed by the
addition of a buffered sodium hydroxide, i.e. sodium
hydroxide and silicate solution, thereby silicates are
incorporated into the system. This two stage bleaching
20technique is claimed to improved the brightness relative
to conventional process, however, it is believed in this
case the improvement in brightness was probably due to
~.,
preliminary treatment with a chelate followed by thicken-
, ing with possibly some improvement being attributed to the
25two stage process.
, 1
When using hydrogen peroxide as a bleaching
agent it has normally been found that to obtain a given
. degree of brightness for a given pulp requires a specific
- 30percentage of hydrogen peroxide and that variations in the
,
~ process usually do not materially change the total appli-
-~cation of peroxide to pulp to obtain the given degree of
brightness.
.,
'
i; - 3 -
:
:
"
,'~ " '
~ ^ 13287~
, :
` It is also known that there is residual peroxide
in the bleaching liquor accompanying the bleached pulp
from the bleaching tower, however, this residual generally
does not exceed about 30% of the added peroxide thereby
5 rendering the recovery of this residual chemical unjusti-
S fiable-
. .
Brief Description of the Present Invention
- 10 It is an object of the present invention to
provide a more versatile and efficient system of bleaching
mechanical pulp using hydrogen peroxide by forming the
perhydroxyl ion in situ in the pulp slurry to brighten the
" ~
~ pulp, separating residual chemical from the brightened
:~ 15 pulp and using the residual to brighten another pulp.
r
~j, Broadly, the present invention relates to a
;, process for peroxide bleaching of mechanical pulps compri-
sing washing said pulp to reduce the reactive components
,''~f~ 20 including heavy metal ions in said pulp to a level so that
fl significant quantities of hydrogen peroxide is not consum-
.. ~ ed when peroxide is added to the pulp, mixing hydrogen
.i peroxide and an aqueous solution of peroxide compatable
: chelating agent, if required, with said washed pulp to
.. `~ 25 apply at least 1-1/2% hydrogen peroxide based on the oven
i dry weight of the pulp to uniformly disperse said hydrogen
.- peroxide throughout the pulp, mixing an aqueous solution
.< of caustic (sodium hydroxide) into said pulp containing
-~ the hydrogen peroxide to disperse same uniformly there-
~. 30 through and reacting over a period of at least 45 minutes
`~ said sodium hydroxide with said peroxide to produce
perhydroxyl ions in-situ in said pulp and reacting said
perhydroxyl ions so formed with the pulp to brighten the
pulp and provide a residual peroxide of at least 35% based
. 35 on the peroxide added (and in any event at least 20% more
:
-- 4 -
'.- ' ~ : ' '
.
, . ~ .
,' ' : ' .
~, . . . ,. :
1~28714
.1
~ than that available if the same pulp were treated in a
: conventional one stage chemical addition process wherein
the peroxide and caustic are added together in a solution)
separating said residual from the brightened pulp and
using the residual to treat a second pulp.
s As indicated the above process increases the
residual bleaching chemical relative to the amount that
~- would normally be found when a conventional bleaching
operation is used. In a preferred process specially
. 10 adapted to use this residual, the washed pulp is first
divided into two portions with the first portion being
treated as above described by reacting perhydroxyl ions
~ formed in situ in the pulp followed by separating a
~ residual liquor containing residual brightening chemical
from the brighten pulp, uniformly mixing the said residual
liquor with the second portion of the pulp to distribute
; said chemicals throughout said second portion, adjusting
the pH of the second portion of the pulp with said chemi-
`: cal distributed throughout thereby to brighten said second
portion.
; This increased residual may also be used for
-............ example, in deinking systems in mills that incorporate
deinking systems or in any other suitable process requir-
ing peroxide wherein the residual chemical is compatible,
. 25 for example, to upgrade stone groundwood.
f The residual featuring a second pulp may also be
` separated and used to treat a third pulp, for example,
when the brightness of the third pulp need not be raised
to a high value.
Brief Description of the Drawings
Further features, objects and advantages will be
evident from the following detailed description of the
` preferred embodiments of the present invention taken in
; 35 conjunction with the accompanying drawings in which:
-- 5
~'
".
1328714
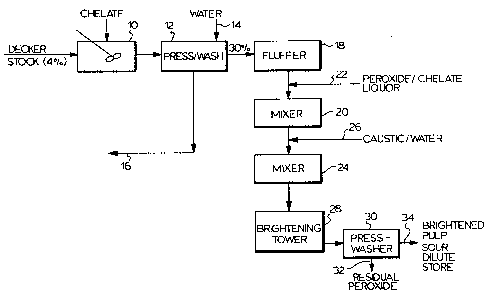
Figure 1 is a schematic representation of a
brightening process incorporating the present invention.
Figure 2 is a schematic illustration of a pro-
cess using recycle residual perhydroxyl ions for brighten^
ing a portion of the pulp.
i
Description of the Preferred Embodiments
In describing the invention the peroxide has
been described as hydrogen peroxide and the percentages
given are based on hydrogen peroxide as this is the
preferred source of peroxide. It will be apparent that
other suitable peroxides are also contemplated and that if
other peroxides are used the amounts used will require
J appropriate adjustment to provide the requirement amount
of active chemical.
As shown schematically in Figure 1 a pulp slurry
at low consistency passes from a decker or the like (not
shown) into a mixer 10 wherein a suitable chemical such as
a chelate is added to the pulp stock. Generally, the pulp
stock at this stage will be at a consistency of less than
5% preferably 1% to 3%. The chelating agent preferably
-~ will be a relatively powerful agent effective to complex
-- and solubilize reactive heavy metals in the pulp, for
example, diethylene triamine penta acetic acid (DTPA) or
the chelate discussed in EPC patent application publica-
i tion No. 0186990 published 10.12.85 or other similar or
~. .
suitable compounds or mixtures thereof. The pulp is then
transported into a washer 12 wherein the pulp is washed to
remove the solubilized heavy metals and heavy metal
complexes as well as soluble organic extractives. Prefer-
ably, washing will be through a double or twin wire press
washer in which a final washing stage is a displacement
- washing stage. Generally, the pulp leaves the washer 12
at a consistency of at least 20% and preferably over 30%
and contains a significantly reduced metal ion content.
: ' `' - ' .: ~
~.
132~7~4
.~ .
Fresh water enters the press washer 12 as indi-
cated by the arrow 14 and displaces effluent (liquor and
contaminants from the pulps) as indicated by the arrow 16.
~l The washed pulp at high consistency then passes
to a fluffer 18 (optional) and into a mixer 20 where the
washed pulp is mixed with a hydrogen peroxide li~uor
preferrably, in fact essentially, except in exceptional
circumstances containing a protective chelate entering as
indicated by the arrow 22. In the illustrated arrangement,
~3 10 the hydrogen peroxide is shown as entering between the
^Y fluffer and mixer 20. It can be introduced directly into
the mixer 20 if desired. The pH of the pulp containing
added per~oxide and chelate solution will be below about 7
to minimize reactions of the peroxide with the pulp.
It is important that the mixer 20 mix the pulp
and hydrogen peroxide (plus chelate) liquor to uniformly
distribute the hydrogen peroxide throughout the pulp.
After the hydrogen peroxide and pulp have been
uniformly mixed in the mixer 20, the so formed mixture is
j 20 then fed to a second mixer 24 where caustic is added as
; indicated by the line 26. The caustic is shown as added
before the mixer 24, but it could also be added directly
l to the mixer it being important that the mixer 24 uniform-
x ly distribute the caustic throughout the pulp, so that,
;, 25 the caustic can react with the peroxide in situ in the
pulp and form the perhydroxyl ions in situ. Generally the
-~ pH after caustic addition will be above about 8. The pulp
containing caustic and peroxide uniformly distributed
throughout is passed to a brightening tower 28 wherein
reaction of caustic (sodium hydroxide) with the peroxide
? (hydrogen peroxide) continues to form perhydroxyl ions and
- the perhydroxyl ions so formed in situ in the pulp react
with the pulp to brighten same.
,, ~
',:
-- 7 --
. .
:- .
.
.
132871~
It is important that the peroxide solution be
added to and be dispersed uniformly through the pulp
before the caustic is added, if the full benefit of the
inventions are to be obtained. If the caustic is added
too soon after or at the same time as the peroxide, the
- caustic may well react with the pulp or with the per-
hydroxyl ions being formed in quantities sufficient to
lose some of the benefit of the invention. If the caustic
is added before the peroxide it is likely to react with
and darken the pulp. Thus while separate mixers are used
i in the various embodiments described herein it will be
apparent that both peroxide and caustic may be added in an
appropriate single mixer provided the above described
sequences are effected to ensure the benefits of the
invention are attained.
After a suitable time in the brightening tower
28 the brighten pulp is withdrawn and passed to a suitable
washer such as the press washer indicated at 30 to separ-
ate residual liquor containing residual peroxide from the
pulp as indicated at 32. The brighten and washed pulp
s~ exits the washer 30 in line 34 and any remaining bleaching
^ chemical is soured and the brightened pulp delivered to
pulp storage.
i The washing of the fresh pulp in the press
J 25 washer 12 is to produce a pulp that is relatively free of
peroxide destroying chemicals. The peroxide solution, i.e.
hydrogen peroxide in water is, as above indicated, prefer-
ably and normally protected by a suitable protective agent
such as a chelating agent that is compatible with the
peroxide and does not combine therewith to reduce the
effectiveness of the peroxide in the process. This pro-
tecting agent is added to the peroxide before it is added
to and uniformly distributed throughout the pulp slurry.
Obviously, if this pretreatment stage (addition of per-
oxide) is to be efficiently carried out there must be few
.,
-- 8 -
:
.
.
:.
' ~ ' -,
-
~32871~
,
'
if any peroxide destroying components available in the
pulp to react with the peroxide and render same useless
for brightening. The protecting agent (chelate) in the
~ peroxide protects same and increases the amount of these
- 5 impurities that may be tolerated. If the pulp is substan-
tially free of such peroxide destroying chemicals and
mixing is carried out in nonreactive atmosphere, i.e. in
, equipment made of suitable materials that do not contri-
bute to the deterioration of the hydrogen peroxide, the
use of a protecting chelating agent with the hydrogen
peroxide may not be necessary. It will be apparent that
the later circumstances are seldom if ever available and
`' in any event as a safety measure normal practice will be
` to include a suitable protecting chelating agent in the
hydrogen peroxide solution.
.,
It is believed that the efficiency of the
- process of the present invention is dependent on maximum
removal of contaminants in the press washing stage, proper
~ distribution of the hydrogen peroxide in the pulp slurry
-i 20 and rapid and proper dispersion of the sodium hydroxide
~ ions through the peroxide treated pulp slurry.
:,j The quantities of peroxide and caustic used for
brightening when following the teaching of the present
` invention will normally be essentially the same as with
prior art peroxide brightening process to obtain a speci-
fic degree of brightness and thus will depend on the
~ brightening required (peroxide) and on the specific pulp
-~ being treated (caustic). In any event to obtain the
required increase in and/or an amount of active chemical
in the residual liquor after bleaching for use in accord-
ance with the present invention the minimum amount of
peroxide will be about 1-1/2% peroxide based on hydrogen
-~ peroxide and oven dry weight of the pulp. Obviously there
; must be sufficient caustic to cause the peroxide to be
converted to perhydroxyl ions with an excess to neutralize
,
,, g
.
, .
- : :
`~
1328714
. .
acids formed in the brightening operation. Too much
caustic is detrimental to the process as is too little
caustic, the precise amount being determined for the pulp
-, being treated. Generally when sodium hydroxide is the
caustic this will be at least 50% by weight of the
, hydrogen peroxide added.
,
With such a system of in situ perhydroxal ion
formation a residual containing at least 35% and normally
at least about 50% of the hydrogen peroxide applied is
available after the normal brightening reaction. The
- residual chemical from the in situ perhydroxal formation
-~ method normally contains at least in an amount of hydrogen
peroxide 20% higher than the amount of hydrogen peroxide
'~ 15 available from a residual liquor obtained by the conven-
tional one stage (or single addition) peroxide brightening
process wherein both peroxide and caustic are added simul-
taneously. Furthermore, the peroxide in this residual
formed in situ is relatively stable at temperatures up to
` 20 about 80 to 90C and can be stored and used to treat other material (pulp).
, ~,
In a preliminary trial using 3% hydrogen
peroxide brightening on chemithermomechanical pulp from
MacMillan Bloedel's Powell River pulp mill, samples were
washed with a chelating agent to remove metals and were
treated in the conventional single addition manner and by
the formation of the perhydroxyl ion in situ in the pulp
in accordance with the present invention. ThP final
brightnesses of the two treated pulps were essentially the
same (in one test 73.2 and 73.1 respectively points
`~ Elrepho brightness), however, the residual reactive
bleaching chemical (peroxide) after the brightening step
was 50% hlgher using the in situ method as compared to the
conventional method, i.e. following the conventional
`:
- 10 -
..
` ~
: , : ;, .
.
~328714
method reactive chemical equivalent to about 40% of the
starting peroxide was in the residual and following the in
situ method reactive chemical equivalent to about 60% of
the starting peroxide was available in the residual.
Example
Wash Stage: 25 b.d. 9 of hembal CTMP (0.8%
sulfonation) was washed to remove metals and soluble
contaminants by diluting the pulp to 1% consistency with
`~ 10 deionized (DI) water and treating this slurry with 0.14%
chelate diethylene triamine penta acetic acid (DTPA).
Chelate percentage based on weight of bone dry pulp. The
dilute slurry was kept at 60C for 10 minutes to allow
complete chelation to take p1ace and then 97% of the
soluble contaminants were removed by thickening to approx-
. imately 30% consistency.
Hydrogen Peroxide Addition: The thickened pulpwas diluted to 20% consistency with deionized water, heat-
ed to 60C and then reacted with an aqueous solution
0 containing 3% hydrogen peroxide and 0.6% Dow~chelate
XU-11082.00L (see European patent application publication
No. 0186990 published 10.12.85) to give a pulp slurry
having a consistency of 17% w/w. Peroxide and chelate
percentages are based on the amount of pulp fiber (bone
dry basis) present. Adequate mixing was carried out to
ensure excellent dispersion of the peroxide molecules
through the pulp slurry.
Sodium Hydroxide: Sodium hydroxide (2.1% based
on b.d. pulp) was dissolved in sufficient deionized water
at 60C so that when added to the pulp containing the
~ peroxide the resultant consistency is 8X w/w. The alkaline
;~ solution is mixed to ensure efficient contact between the
peroxide and the hydroxide molecules in the slurry.
The resultant mixture was allowed to stand at
60C for 90 minutes for pulp brightening.
~', - 11 -
. ' . " ' '
. .
. :
,. .
;; -` 1328~14
~:;
.; .
Pulp samples were then taken, the residual per-
oxide content, and the pulp brightness after souring to pH
5.0 with sulfurous acid, were measured.
.,
Brightness Residual H22
.
-' Results points % of Starting
~ . ,,
Optimized Conventional 73.7 39.7
~s Process Using 3% H22
, In Situ Process Using 73.2 59.0
103% H22
,
It will be apparent that the quantity of resid-
o ual reactive bleaching chemical (hydrogen peroxide) in the
residual liquor separate from the brightened pulp is now
16 very significant, it is relatively stable and economically
recoverable for brightening further pulp or for any other
' useful purpose.
The consistency of the pulp containing peroxide
and hydroxide was deliberately lowered to the relatively
~ 20 low value of 8% to test the invention under adverse condi-
- tions (and it operated effectively), but normally the
consistency at this point, i.e. in the tower 28, will be
in the range of about 22% to 28%.
The process schematically illustrated in Figure
25 2 illustrates a preferred way to recover and use the
residual reactive bleaching chemical in the residual
separate from the pulp. In this system washed pulp from
` said pulp washer 12 of Figure 1 is conveyed by line 36
into the system shown in Figure 2.
The pulp from line 36 is divided into two
portions, one in line 3~ and the other in line 40. As
above indicated about 50% of the peroxide is left in the
residual liquor from the bleaching operation and therefore
it is preferred to divide the flow into one-third in say
35 line 38 and two-th~rds o~ t~e pulp flow in line 40.
'
, ' ' '
: ,
: ~ .
1328714
In the illustrated arrangement, the pulp in line
40 (two-thirds of the pulp) is directed to a mixer general-
ly indicated at 42 wherein hydrogen peroxide is mixed with
the pulp to uniformly distribute the hydrogen peroxide
throughout the pulp. Arrow 44 indicates the introduction
of the hydrogen peroxide. In some cases, depending on the
condition of the pulp and the particular mixing equipment
and bleaching equipment used, it normally will, as above
indicated, be necessary to protect the hydrogen peroxide
from deterioration by the addition of certain protectors
particularly chelates to tie up any heavy metal that may
i be contacted by the hydrogen peroxide.
' The mixture of pulp and peroxide is directed
` from mixer 42 to a second mixer 46 wherein caustic is
introduced as indicated by line 48 and the caustic uni-
formly mixed with the mixture of pulp and hydrogen
peroxide so that there is a uniform distribution of
caustic throughout the pulp peroxide mixture.
~` The caustic (NaOH) reacts with the peroxide to
form as above described the perhydroxyl ion which reacts
with the pulp to brighten same. The mixture formed in
mixer 46 is carried into the bleaching vessel 50 wherein
the brightening of the pulp is completed.
Preferably, the brightened pulp from the bleach-
ing vessel 50 is first diluted as indicated by the addi-
tion of dilution water via arrow 52 into the mixer 54 and
, the diluted brightened pulp is then carried to a press
washer 56 wherein the residual brightening solution con-
taining residual reactive bleaching chemical (peroxide) is
washed from the pulp and is collected in a suitable tank
as indicated at 58. The brightened pulp is then diluted
and soured in the conventional manner and passed on to the
next stage and storage as indicated by the arrow 60.
- 13 -
,
1~287~4
,~
.,
The one-third of the pulp carried in line 38 is
moved into a mixer 62 wherein it is mixed with the resid-
ual liquor containing the residual bleaching chemical
(peroxide) carried via line 64 from the residual peroxide
tank 58. The mixer 62 thoroughly mixes the residual liquor
from line 64 with the pulp from line 38 to uniformly
distribute the bleaching chemical containing peroxide
uniformly throughout the pulp.
The homogeneous mixture of pulp and residual
:~ 10 liquor from mixer 62 is, in the illustrated arrangement,
carried to a mixer 66 wherein caustic is added as indi-
cated by line 68 and mixed with the mixture of residual
liquor and pulp to adjust the pH to the required level for
conversion of peroxide in the residual bleaching chemical
;~ 15 to perhydroxyl ions and the perhydroxyl ions to react with
the pulp (pH will normally be in the range of 9-11).
The pH adjusted mixture of pulp and residual
~ liquor passes from mixer 62 into a second bleaching vessel
i~ 70 wherein the brightening of the pulp from line 38 is
completed. The brightened pulp leaves the system (vessel
70) via line 72 and is diluted and soured in the conven-
~ tional manner.
',`,!'~ The flows in line 60 and 72 have slightly
different brightnesses and may be mixed in suitable pro-
' 25 portions to obtain a desired degree of brightness of the
resultant pulp, or all of the flow from line 60 may be
blended with the flow from line 72 as required.
:: As is well known, the consistency during bleach-
ing has an effect on the final brightness on the pulp
being produced. In a specific example the pulp enters the
system in line 36 at a consistency of about 30% and is fed
to the mixers 42 and 62 at 30%. However, the addition of
the aqueous solutions of peroxide and caustic in the
mixers 42 and 46 respectively will dilute the stock from
line 40 down to about 26% consistency in the tank 50. This
. . .
, - 14 -
- ~ .
1328714
~:.
brightened pulp is then further diluted in the mixer 54
and the residual liquor is washed from the pulp in the
washer 56 by the addition of fresh water as indicated by -
the arrow 57 so that the residual peroxide liquor collect-
ed in the tank 58 is significantly less concentrated thanthe hydrogen peroxide added in line 44.
,~
Nevertheless, this more dilute residual liquor
containing the perhydroxyl ions is added to pulp in line
38 and the mixer 62 thereby to dilute the pulp from line
' 38 which is further diluted by the addition of caustic in
mixer 66 so that the concentration in bleaching vessel 70
is significantly lower than the concentration in the
bleaching vessel 50 (generally, less than half say between
about 6% and 12% consistency). This lower consistency and
~ the fact that the residual bleaching chemical is not quite
i; as effective as fresh chemical may result in a slightly
- lower degree of bleaching, i.e. the brightness may be
slightly less in the pulp leaving vessel 70 than the pulp
;~ 20 leaving vessel 50 (assuming there is same percent peroxide
.~/ utilized on each pulp flow) partially because bleaching is
^ at a lower consistency and partially because the per-
hydroxyl ions in the residual liquor are not quite as
` effective as those formed in situ in the pulp. Obviously,
this can be remedied at least to a degree by increasing
the percent peroxide (residual liquor) added to flow 38 to
` compensate for the lower consistency, for example, by
- adjusting the division of pulp in line 38 and 40 so that
slightly more than two-thirds of the flow is in line 40
and less than one-third of the flow is in line 38 and
again assuming about a 50~ residual, the percent peroxide
to pulp will be increased in the material flowing along
line 38.
- 15 -
. :
, ~ .
.
'' ~32871~
It will be apparent that the in situ method of
brightening by forming the perhydroxyl ions in situ in the
pulp permits reducing the consistency of the pulp during
the bleaching or brightening stage without reducing
bleaching efficiently nearly to the extent it would be
reduced if a conventional bleaching operation were being
carried out at such low consistency.
~, Table I shows the results obtained by following
the method illustrated in Figure 2 and compares the stan-
dard one stage addition process with the brightening for
different time periods and pulp temperatures of the pulp
using fresh peroxide (pulp I) and that obtained using
residual peroxide (pulp II).
, j
TABLE I.
,
Pulp Chemithermomechanical Pulp
.~ Method Brightening Brightness H22 in Resid
Time, min points % H22 Added
. Temperature 60C 80C 60C 80C
..,
. Standard Process 90 74.2 32.67
. 25
Fresh H22 30 70.4 72.8 56 48
Pulp I 60 72.0 75.0 55 36
` Two Stage Process 90 72.9 74.0 54 44
~ 120 73.9 75.0 54
.; 30 _
Use of Residual
-. H22 30 67.3 71.5 57 34
Pulp II 60 70.0 73.1 49
' Two Stage Process 90 70.9 73.1 39 33
120 72.2 72.9 40 28
''
~ - 16 -
.~
,
,
!
. - ' ' " .
-` 132871~
It w;ll be apparent that the residual chemical
remaining after treatment of pulp II is still substantial
when following the present invention, e.g. assuming 5%
peroxide addition to pulp I with a .55% residual and a 50%
residual from pulp II the residual chemical available from
pulp II is equal to 5 x .55 x .5 = 1.375% of pulp I. This
- residual is a significant amount that may justify a
~ further separation of residual from pulp II by the incor-
- poration of a press or press wash similar to that employed
for pulp I and indicated at 56 in the line 72 to separate
the residual from pulp II before it is soured.
The residual chemical separated from pulp II is
dilute and may be used for bleaching other pulps but
normally will only be recovered and used to bleach pulps
where the increase in brightness (final brightness) is
relatively low but in many mills this will be significant
as it may, for example, be used in place of hydrosulfite
bleaching or brightening.
It will be apparent that it is preferred to use
the residual in the in situ method, i.e. to treat pulp by
first adding the residual and then the caustic as above
described, but in some instances the residual may be
treated in the conventional manner with the addition of
caustic and suitable stabilizers and then applied to a
pulp for brightening the pulp.
i The process lends itself well to bleaching of
wood pulp and had been described accordingly, but it may
well find use with other cellulosic materials.
Having described the invention, modifications
will be evident to those skilled in the art without
departing from the spirit of the invention as defined in
the appended claims.
.
- 17 -
-. -
. ~ - , .1
~,
. . .
.,' : ~ , , ;