Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1328869
23189-6707
The present invention relates to dihydropyridlnamides, a
process for their preparation, and their use in medicaments, in
particular as circulation-influencing medicaments.
It is known that diethyl 1,4-dihydro-2,6-dimethyl-4-
phenylpyridine-3,5-dicarboxylate is obtained when ethyl
benzylideneacetoacetate is reacted with ethyl ~-aminocrotonate or
ethyl acetoacetate and ammonia [E. Knoevenagel, Ber. Dtsch. Chem.
Ges. 31, 743 (1898)].
It is furthermore known that certain l,~-dlhydro-
pyridines have interesting pharmacologlcal propertie~ IF. ~ossert,
W. Vater, Naturwissenschaften 58, 578 11971)1.
The present invention relates to new dihydropyridin-
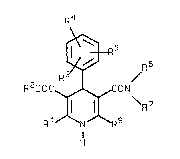
amlde~ of the general formula II)
R4
/~ ~-'-,
~ R 6
R3 1 / (I)
RZOOC ~ \R7
R1 N R8
H -
in which
l and R8 are identical or different and represent straight-
chaln or branched alkyl havlng up to 4 carbon atoms, each of which ~-
optionally ~ubstituted by phenyl,
R2 represent~i a stralght-chain or branched, saturated or
unsaturated hydrocarbon radical having up to 8 carbon atoms which
,,., ~ '
i~ - :~ .
~ ~i' ` ~ t
28869
231~9-6707
may be interrupted in the chain by an oxygen atom and which may be
substituted by fluorine, chlorine, cyano, hydroxyl or by a phenyl
group or by a-, ~- Gr ~-pyridyl,
R3 and ~4 are identical or different and in each case
represent hydrogen, fluorine, chlorine, alkyl having up to 4
carbon atoms, alkoxy having up to 4 carbon atoms, nitro or :.
trlfluoromethyl,
R represents a group of the formula -0-(C~2)n-X11, -
-S-(CH2)n-R11, -0-S02-R11 or -0-C0 (CH2)n-R11, in which n denotes
0 to 3, and R11 denotes phenyl which may be monosubstituted,
disubstituted or trisubstituted by identlcal or different
substituents selected from the group consisting of fluorine,
chlorine, nitro, trlfluoromethyl, alkyl havlng up to 4 carbon
atoms, alkoxy having up to 4 carbon atoms, amlno, alkylamlno
having up to 4 carbon atoms, dialkylamino having up ~o 4 carbon :
atoms in each alkyl group, acetylamino or denotes pyridyl, and -
R~ and R7 are ldentical or different and in each case ~-
represent hydrogen or cycloalkyl havlnq 3 to 7 carbon atoms or
represent straight-chain or branched alkyl or alkenyl which has up
to 14 carbon atoms and which may be substituted by fluorine, ~
chlorine, hydroxyl, alkoxy having up to 6 carbon atoms, alkylthio ~;; -
having up to 6 carbon atoms, carboxyl, alkoxycarbonyl having up to ~ :-
6 carbon atoms, by phenyl, or by a group of the formula -NR9R10, :
in which R9 and R10 are identical or different and in each case -:~
denote hydrogen, alkyl having up to 6 carbon atoms, benzyl, phenyl
or acetyl, or :
R6 and R7 ln each case represent phenyl which may be
.,"~ '"-'-'' ".
1~28869
23189-6707
monosubstituted, disubstituted or trisubstituted by identical or
different substituents selected from the group consisting of
nitro, fluorine, chlorine, alkyl having up to 4 carbon atom~,
alkoxy having up to 4 carbon atoms, trifluoromethyl,
trifluoromethoxy, amino, alkylamino having up to 6 carbon atoms,
dialkylamino having up to 6 carbon atoms in each alkyl group,
acetylamino and benzoylamino, or represent pyridyl,
and the physiologically acceptable salts thereof.
The compounds according to the invention exist in
stereoisomeric forms which behave either as image and mirror image
(enantiomers) or do not behave as image and mirror image
(diastereomers). The invention relates both to the antipodes and
to the racemic forms and to the diastereomeric mixtures. The
racemic forms and llkewise ~he diastereomers can be resolved in a
known fashion into the stereoisomerically unary components (cf.
E.L. Eliel, Stereochemlstry of Carbon Compounds, HcGraw Hill,
1962).
Physiologically acceptable salts can be salts of the
compounds according to the lnvention with inorganic or organic -
acids. Preferred salts are those with inorganic acids, such as,
for example, hydrochloric acid, hydrobromic acid, phosphorlc acid
or sulphuric acid, or salts with organic carboxylic or sulphonic
acids, such as, for example, acetic acid, maleic acid, fumaric
acid, malic acid, cltric acid, tartaric acid, lactic acid, benzoic ~-
acid, or methanesulphonic acid, ethanesulphonic acid, phenyl~
sulphonic acid, toluenesulphonic acld or naphthalenedisulphonic
acid.
R
.
1328869
23189-6707
Preferred compounds of the general formula (I) are those
in which
R1 and R8 are identical or different and in each case
represent straight-chain or branched alkyl having up to 4 carboD
atoms which is optionally substituted by phenyl,
R2 represents a straight-chain or branched, saturated or
unsaturated hydrocarbon radical which has up to 8 carbon atoms and
whicb may be interrupted in the chain by a oxygen atom and/or
which may be substituted by fluorlne, chlorine, cyano, hydroxyl or
by a phenyl, or by a-, ~- or ~-pyridyl,
R3 and R4 are identical or different and in each case
represent hydrogen, fluorine, chlorine, alkyl having up to 4
carbon atoms, alkoxy having up to 4 carbon atoms, nitro or
trifluoromethyl,
R5 represents a group of the formula -O~(CH2)n-R
-S-(CH2)n-R11, -0-S02-R11 or -0-C0-(CH2)n-R11, in which n denotes -~
0 to 3, and R11 denotes phenyl which may be monosubstituted,
disubstituted or trisubstituted by fluorine, chlorine, nitro,
trifluoromethyl, alkyl having up to 4 carbon atoms, alkoxy having ~-
up to 4 carbon atoms, methylthio, amino, alkylamino having up to 4 : -
carbon atoms, dialkylamino having up to 4 carbon atoms in each
alkyl group, acetylamino or denotes pyrldyl and :- ~
R6 and R7 are identical or different and in each case .
represent hydrogen or cycloalkyl having 3 to 7 carbon atoms or
represent straight-chain or branched alkyl or alkenyl which has up
to 14 carbon atoms and which may be substituted by fluorine,
chlorine, hydroxyl, alkoxy having up to 6 carbon atoms, carboxyl,
.~ ,':
.;.'
1328869
23189-6707
alkoxycarbonyl having up to 6 carbon atoms, by phenyl and/or by a
group of the formula -NR9R10, in which R9 and R10 are ldentical or
different and in each case denote hydrogen, alkyl having up to 6
carbon atoms, benzyl, phenyl or acetyl, or
R6 and R7 in each case represent phenyl which may be mono-
substituted, disubstituted or trisubstituted by nitro, fluorine,
chlorine, alkyl having up to 4 carbon atoms, alkoxy having up to 4
carbon atoms, trifluoromethyl, trifluoromethoxy, amino, alkylamlno
having up to 6 carbon atoms, dialkylamino having up to 6 carbon
atoms in each alkyl group, acetylamino or by benzoylamino, or
represent pyridyl,
and the physlologically acceptable salts thereof.
Particularly preferred compounds of the general formula
(I) are those in which
Rl and R8 are ldentical or different and in each case
represent methyl, ethyl or benzyl,
R2 represents a straight-chain or branched hydrocarbon ::
radical which has up to 6 carbon atoms, may be interrupted in the
chain by an oxygen atom and~or may be substituted by fluorine, ~: -
chlorine, cyano, hydroxyl, phenyl, a~ or ~-pyridyl, ~ -
R3 and R4 are identical or different and in each case
represents hydrogen, fluorlne, chlorlne, methyl, ethyl, methoxy,
ethoxy, nitro or trifluoromethyl, r ,"
R5 represents a group of the formula -0-CH2-Rll, -S-CH2-R
or -0-S02-Rll in which Rll denotes phenyl which may be monosubsti-
: tuted or dlsubstituted by fluorine, chlorine, nitro, trifluoro- :~.
methyl, methyl, methoxy, amino, methylamino, dime~hylamino,
f ~,
:'
1328869
23189-6707
ethylamino, diethylamino or acetylamino, the substituents belng
identical or different, or denotes an a-, ~- or a ~-pyridyl group,
R6 represents hydrogen or alkyl havlng up to 4 carbon atcms, :
and
R7 represents hydrogen, cyclopropyl, cyclopentyl or cyclo-
hexyl, or represents straight-chain or branched alkyl or alkenyl
which has up to 10 carbon atoms and which may be substltuted by
fluorine, chlorlne, hydroxyl, alkoxy having up to 4 carbon atoms,
alkylthio having up to 4 carbon atoms, carboxyl, alkoxycarbonyl
having up to 4 carbon atoms, phenyl and/or a group of the
formula -NR9R10, in which R9 and R1~ are ldentical or different ;~.
and denote hydrogen, alkyl having up to 4 carbon atoms, benzyl,
phenyl or acetyl, or - -
R7 represents phenyl which may be monosubstituted or
disubstituted by nitro, fluorine, chlorine, methyl, methoxy, ~-
trifluoromethyl, trifluoromethoxy, amino, alkylamino having up to . .
2 carbon atoms, dialkylamino having up to 2 carbon atoms in each
alkyl group, acetylamino or benzoylamino, the substituents
'.,,
'' ' ' ' '
132~8~9
-
being identical or different, or
represents a-, B- or y-pyridyl,
and the physiologically acceptable salts thereof.
The compounds of the general formula (I) accord-
ing to the invention
in which
R1 _ R8 have the abovementioned meaning,
are obtained in a process in which
CA] aldehydes of the general formula ~II)
R4
~ R5 (II)
R ¦
CHO
in which
R3, R4 and R5 have the abovementioned meaning,
and ~-ketocarboxylates of the general formuLa (III)
R2OOC~ (III)
R~
in which
R1 and R2 have the abovementioned meaning,
- are reacted ~ith B-ketocarboxamides of the general ~
formula (IV) . -
.
~CON
~R7 . ( I V )
0~ ~ 8
in which
R6, R7 and R8 have the abovementioned meaning,
and ammonia, if appropriate in the presence of inert
solvents, .
or in a process in ~hich
Le A 25 087
A ~:
~ 1328~69
~8~ aldehydes of the general formula (II) are reacted
~;th B-ketocarboxYlates of the general formuLa (III)
and enam;nocarboxam;des of the generaL formula (V) -~
,~R6
~ CON 7 (V)
H2N 8
in which
R6, R7 and R8 have the abovementioned mean;ng,
if appropriate in the presence of inert solvents,
or in a process in ~h;ch
CC] aldehydes of the general formula ~II) are reacted
~ith ~-ketocarboxamides of the general formula ~IV) and
enaminocarboxylates of the general tormula (VI)
R200C ~ (VI)
R1 H2
in ~hich
R1 and R2 have the abovementioned mean;ng,
if appropriate in the presence of inert solvents,
or in a process in ~hich
-~ CD~ B-ketocarboxYlates of the general formuLa (III) are
~h rea~cted ~ith ammonia and y~idene-B-ketocarboxamides of the --
general formula (YII)
~R4
R5 ~VII)
~- R3 ~ ,~R6
R7
~R8
in ~hich
R3 - R8 have the abovementioned meaning,
if appropriate in the presence of inert solvents,
~; or in a process in ~hich -
Le A 25 087
'
1328869 :-
tE~ ~-ketocarboxamides of the general formula SIV) are
reacted with ammonia and ylidene-B-ketocarboxylates of the
general formula (VIII)
R5
~ ~VIII)
R200C~H
Rl~O
in wh;ch
R1 _ R5 have the abovementioned meaning,
if appropriate in the presence of inert solvents,
or in a process in ~hich
tF~ ylidene-B-ketocarboxamides of the general formuLa (VII)
are reacted with enaminocarboxylates of the general formula
(VI), if appropr;ate in the presence of inert solvents,
or in a process in ~hich
CG~ y~idene-~-ketocarboxylates of the general formula
tVIII) are reacted w;th enaminocarboxamides of the general
formula (V), if appropriate in the presence of inert solvents,
or in a process in which
~H~ dihydropyridinemonocarboxylic acids of the general
formula tIX)
R4
R ~ (IX)
R2OOC~ ~ ~COOH
1~ ~N~ ~RB
H ~ -
in which
R1 _ R5 and R8 have the abovementioned meaning,
if appropriate via a reactive acyl derivative, are reacted
uith amines of the general formula tX)
Le A 25 087 q
d`~'. . :.
~ .
....
1328869 ~
,~R6 ::
~R7 (X)
, ,' :, . . ':
in which
R6 and R7 have the abovementioned meaning, .. ~1
if appropriate ;n the presence of an inert organic solvent. ~
Reactive acyl derivatives which may be mentioned -
as examples are: activated esters, hydroxysuccinimide
esters, acyl imidazolides, acyl halides, mixed anhydrides,
or the reaction in the presence of cyclohexylcarbodiimide.
Depending on the type of starting materials used,
the variants for the synthesis of the compounds according
to the invention can be represented by the following
equations: :-
;
.
~ ~ :
:. :
: ~ :
,,
Le A 25 G87 /0 - : :
:
1328869
~A~ ~
~- C1~2 - C 6H5
CHO
H3COOC ~ ~ O-NSCH3)2
H3C H3
N~3
-:-
H3COO ~ ON(CH3~2
H3C H3
'.-
tB~ ~
~S-CH2-C6H5
CHO
HSC200 ~ X O-NH-CH3
H3 l H2 H3 -- ~:
~ : ,
~S-CH2-C6H5 ~ '
5c2oocc~o-NH-cH3
;
Le A 25 087 A~
r,.. ~, '. ... ; ., , " , , .
` 132~69
..........
tc~
-502-~6H5
CHO
H3COOC ~~ O-NH-C6H5
H3C H2H3 .
' .
502-C6H5
H3COOC ~ CO-NH-C6H5 .
H3c~N~cH3
lD~ . ;
-CH~-C6H5
H3COOC~ H ~ CO-NH-C2H5 ~ :-
H3C~l~O ~ CH3
3 - -
- ' '
"'~ '
H3COOC~O-NH-C2H5
~: H3C H3 :.
': ' ',.
~'` :''
Le A 25 087 1~ .
1328~69
tE~
~o-cH2-c6Hs
H7C300C ~ H ~ CO-NH-C3~7
H3C~l~O CH3
, ~:
¢~0 CH2-c6Hs
H7C300C ~ O-NH-C3H7
~3 H H3 ~:
tF3
,
H3COOC ~ H ~ CO-NH-CH3
H3C ~ NH2 H3 ~:~
O CH -C H
H3Co ~ O-NH-CH3 :`
H `;
Le A 25 087 1~ ~
~ ;';
1328869
~G] ~ ~-CH2-C6H5
H3COoC ~ H H ~ CO-N(CH3)2
H3C O H2 CH3
f~O-CH2-C6H5 :,
~ , '' ~'.
H3COOC ~ O-NtCH3)2
H3 H H3
tH~ :
¢~0- CH2 - C6H5 '~ '
H3COOC ~ OOH ~
H3 H H3 - :.
N=~ ~=N
. ~ N-C-N J
O :.~ :'-.. :
: ~ -CH ~ H5
H3COOC~CO-NJ
3C~NV^~CH3 :
H2~1
~' .'".
~O-t H2-c6H5
H3COO ~ O-N ~ 1
~3 H H3
' ` :'':
~ Le A 25 087 ~ /~
' .
132~869
- Process variants A - G
_ .
Suitable solvents are water or all ;nert organic
solvents which do not react under the reaction conditions.
These preferably include alcohoLs, such as methanol, eth-
anol, propanol or isopropanol, ethers, such as diethylether, dioxane, tetrahydrofuran, glycoL monomethyL ether
or glycoL dimethyl ether, or amides, such as dimethylform-
am;de, d;methylacetam;de or hexamethyLphosphor;c tr;am;de,
or glac;aL acet;c ac;d, d;methyl sulphox;de, aceton;tr;Le
or pyr;d;ne.
The react;on temperatures may be var;ed w;th;n a
relat;vely w;de range. In general, the process ;s carried
out between +10C and ~150C, preferably between +20C
and +100C. In particular, at the boiling point~of the
particular solvent.
The reaction can be carr;ed out at atmospheric
pressure, but also at increased or reduced pressure. In
general, the process is carr;ed out at atmospheric pressure.
When carrying out the process variants A - G
ZO accord;ng to the invention, the ratio of the substances -
participating in the reaction is immaterial. In general,
however, ~olar amounts of the reactants are used. The ~ -
substances accord;ng to ;nvent;on are preferably ;solated --
and pur;f;ed by remov;ng the solvent by d;st;llation ;n
vacuo and recrystalliz;ng the residue obtained in
crystalline form, if desired, only after ice cooling, from a
suitab~e solvent. In some cases, it may be necessary to
purify the compounds according to the invention by
chromatography.
The aldehydes of the general formuLa tII) employed
as starting materials are known or can be prepared by
known methods ~German Offenlegungssehr;ften 2,165,260;
2,401~665; 7.D. Harr;s, G.P. Roth, J. Org. Chem. 44, 20Q4
~1979); ~.J. Dale, H.E. Henn;s, J. Am. Chem. Soc. 78,
35 2543 ~1956); Chem. Abstr. 59, 13929 ~1963)~. ; -
The B-ketocarboxYlates of the genera~ formula
Le A 25 087 ~i
:
~' ;
, . . . .~ . . , ... , , .. , .... . ~,. . . . .
. ~ 132~869
- (III) employed as starting materials are known or can be
prepared by known methods ~D. 60rrmann in Houben ~eyl's
"Methoden der organischen Chemie" ~Methods of Organic -
Chemistry~ Vol. VII/4, 230 (1968); Y. Oikawa, K. Sugano,
O. Yonemitsu, J. Org. Chem. 43, 2087 (1978)~.
The B-ketocarboxamides of the general formula
~IV) employed as starting materials are known or can be
prepared by kno~n methods CGerman Offenlegungsschrift
1,142,859].
- 10 The enaminocarboxam;des of the general formuLa (V)
e~ployed as start;ng mater;als are kno~n or can be pre-
pared by kno~n methods tGerman Offenlegungsschr;ft 2,ZZ8,377].
The enaminocarboxylates of the general formula
tVI) employed as start;ng materials are known or can be
prepared by known methods CF.A. Glickman, A.C. Cope,
J. Am. Chem. Soc. 67, 1017 (1945)].
The ylidene-B-ketocarboxamides of the general
formula tVII) employed as starting materials and the yl-
idene-B-ketocarboxylates of the general formula (VIII)
employed as starting materials are kno~n or can be pre-
pared by known methods C6; Jones "The Knoevenagel Conden-
sation" in Organic Reactions Vol. XV, 204 t1967)].
Process variant H according to the invention is
carried out based on the literature-kno~n method of con-
verting carboxylic acids into carboxamides. In thismethod, the carboxylic acid is initially converted into
an activated form, such as, for example, the acyl chloride
or the imidazolide, ~hich are either isolated as such and
reacted in a second reaction step, or which are amidated
directly in situ to form the compounds according to the
invention. Lesides inorganic halides, such as thionyl
chloride, phosphorus trichloride or phosphorus pentachloride,
or carbonyldiimidazole, activating reagents which may be
mentioned as examples are carbodiimides, such as cyclohex-
ylcarbodiimide or 1-cyclohexyl-3-C2-(N-methyl-morpholino)-
ethyl~carbodiimide p-toluenesulphonate, or N-hydroxyphthalimide
Le A 25 08? 16
~ - .
'
.. .: . . .. .. . . . : . .
1328869
or N-hydroxy-benzotriazole in the presence of dicyclohexyl-
carbodiimide. Naturally, the dihydropyridinemonocarboxy-
~ic acids can also be employed in the form of their salts.
CThe amidation method is described, for example: Fieser
~ Fieser, Reagents for Organic Synthesis, John Wiley
Sons Inc. (1967), page 231 - 236; J.C. Shihan and G.P.
Hess, J. Am. Chem. Soc. 77, 1067 ~1955); U. Goodman, G.W.
Kenner, Adv. in Protein Chem. 12, 488 (1957); ~.A. Bonner,
P.I. McNamee, J. Org. Chem. 26, 254 (1961); H.A. Staab,
Angew. Chemie Int. Ed. 1, 351 (1962); Fieser ~ Fieser,
Reagents for Organic Synthes;s, John ~iley ~ Sons Inc. 1967,
116, 114; H.C. 8eyerman, U.O. van der ~rink, Re. Trav. 80,
1372 ~1961); C.A. ~uehler, D.E. Pearson, John Wiley ~ Sons,
Volume I (1970), page 895 ff, Volume II, t1977)~.
9esides ~ater, suitable solvents for process var-
iant H are all inert organic solvents ~h;ch do not react
under the reaction cond;tions. These preferably include
ethers, such as diethyl ether, dioxane, tetrahydrofuran,
glycol monomethyl ether or glycol dimethyL ether, or hal-
ogenated hydrocarbons, such as d;chloromethane, trichloro-
methane or tetrachloromethane, or amides, such as d;methyl-
tor~am;de, d;methylacetamide or hexamethylphosphoric tr;-
am;de, or hydrocarbons such as benzene, toluene or xylene,
or acetonitr;le, n;tromethane, pyrid;ne, d;methyl sulphox;de ~ -
or ethyl acetate. M;xtures of the solvents ment;oned may
l;kew;se be used. If the act;vated ;ntermed;ates of the
d;hydropyr;d;nemonocarboxyl;c acids are isolated, the
amines of the formula ~X) can also be used alone as d;luents.
The react;on temperatures may be varied ~ithin a -
30 relat;vely w;de range. In general, the process is carried ~;~
out ;n a range from -70C to +140C, preferably from -20C
to ~100C.
The react;on can be carried out at atmospher;c
pressure, but also at ;ncreased or reduced pressure. In
general, the process ;s carr;ed out at atmospher;c pressure.
When carry;ng out process variant H according to ~ -
Le A 25 087 ~7
A~ - ~
. ~
1328869
23189-6707
the invention, the ratio of the substances participating in the
reaction is immaterial. In general, however, molar amounts of the
reactants are used. However, it has proven favourable to employ
the amine in a 5- to 10-fold molar excess. The amine is
particularly expediently employed directly as solvent in large
excess.
The dihydropyridinemonocarboxylic aclds of the general
formula (IX) employed as starting materials are known or can be
prepared by known methods [German Offenlegungs~chriften 2,847,236;
3,206,671 and 2,962,241].
The amines of the general formula (X) employed as
starting materials are known or can be prepared by known methods
[Houben Weyl's "Methoden der organischen Chemie" [Methods of
Organic Chemistry] Vol. XI~l; Paulsen, Angewandte Chemie 78, 501 - -
566 (1966)].
The compounds according to the inventlon exhibit an
unforeseeable, valuable pharmacological spectrum of action. They
influence the contraction power of the heart, the tone of the
smooth muscles and the electrolytlc and liquid balance.
They can therefore be employed in medicaments for
treatment of pathologically changed blood pre~sure and heart
insufficiency, and also as coronary therapeutic agents. They can
be employed to combat clrculation and cardiocirculation disorders.
In addition, the can be employed for treatment of heart
rhythm disturbances, kldney insufficiency, cirrhosis of the liver,
.
ascites, lung oedema, cerebral oedema, pregnancy oedema, glaucoma
or diabeteq mellitus.
. ~
18 -
:- 132~869
23189-6707
The cardioactive effect of the compounds accordlng to
the invention was found on isolated, stimulated papillary muscle
of the guinea pig heart. To this purpose, the experimental
animals (guinea pigs of both sexes weighing 200 g) were killed,
the thorax was opened, and the heart was removed. For the
experiments, the smallest possible papillary muscles were subse-
quently in each case removed from the right ventricle and fixed
horizontally in an organ bath. During this procedure, one end of
the muscle was held by two metallic electrodes, which simultan-
eously served to stimulate the preparation, whereas the other end
of the muscle ~as connected via a thread to a force transducer.
The papillary muscle was stimulated above the threshold at a
frequency of 1 Hz. Here Krebs-Henseleit solution (concentration
in mmol. NaCl 118; NaC03 25; KCl 10; KH2P04 1.2; MgS04 1.2; CaC1
1.8; glucose 10, pH 7.4) was passed continuously through the organ
bath, which had a volume of about 2 ml, at a rate of 4 ml/min at a
temperature of 32C. The contractions of the papillary muscle
were measured lsometrically via the connected force transducer and
recorded on a recorder.
The substances according to the invention were dissolved
in the Krebs-Henseleit solution at a concentration of 10 ~g/ml, if
approprlate using a solubilizer ~DMS0 to a concentration of 0.5%). --
The dihydropyridine-carboxamides according to the invention ~
exhibited inhibition of the contractile force of the papillary ~ ;
muscle of more than 10% in this test, relative to the control
values.
The new active compounds can be converted in a known
,,,~, ', -" ' ' ,
- 1328869
23189-6707
fashion into conventional formulations, such aæ tablets, coated
tablets, pills, granules, aerosols, syrups emulsions, suspensions
and solutions, using inert, non-toxic, pharmaceutically suitable
excipients or solvents. In the abovementioned case, the
therapeu~ically active compound should in each case be present in
a concentration of about 0.5 to 90% by weight of the total
mixture, i.e. in amounts which are sufflcient to achieve the
abovementioned dosage range. The invention also extends to
commercial packages containing a compound of the invention a~
active pharmaceutical ingredient, together with instructions for
its use in combating circulation disorders and disorders of the
electrolyte and liquid balance.
The formulations are prepared, for example, by extending
the active compounds with solvents andtor excipients, if
appropriate using emulsifiers and/or dispersantæ, and, for example ~ -
when using water as a diluent, organic solvents can optionally be
uæed as auxiliary solvents.
:~: : " '
':: ' . - '
132~869
Examples of auxil;ary substances which may be
mentioned are: water, nontoxic organic solvents, such
as paraffins (for example mineral oil fractions), veget-
able oils (for example groundnut/sesame oil), alcohols
(for example: ethyl alcohol, glyceroL), excipients, such
as, for example, ground natural minerals (for example
kaolins, clays, talc and chalk), ground synthetic minerals
(for example highly disperse silica and silicates), sugars
(for example sucrose, lactose and glucose), emulsifiers
(for example polyoxyethylene fatty acid esters, polyoxy-
ethylene fatty alcohol ethers, alkylsulphonates and aryl-
sulphonates)~ detergents (for example lignin, sulphite
~aste liquors, methylcellulose, starch and polyvinyl
pyrrolidone) and lubricants (for example magnesium stearate, ;
talc, stearic acid and sodium lauryl sulphate).
Administration takes place in a conventional
fashion, preferably orally or parenterally, in particul-
arly perlinguaLly or intravenously. In the case of oral
adm;nistration, tablets can of course also contain addit-
ives, such as sodium citrate, calcium carbonate and dical-
cium phosphate, together with various additional substances,
such as starch, preferably potato starch, gelatin and the
like in addition to the excipients mentioned. Furthermore,
lubr;cants, such as magnesium stearate, sodium lauryl sul-
phate and talc can be co-used for tabletting. In the case
of aqueous suspensions, various flavour improvers or col-
orants can be added to the active compounds in addition
to the abovementioned auxiliaries.
In the case of parenteral administration, solut-
ions o~ the active compounds can be employed using suit-
able liquid excipient materials.
In the case of intravenous administration it has
generally proven expedient to administer amounts from
0.001 to 1 mg/kg, preferably about 0.01 to û.5 mg/kg of
body ~eight in order to achieve effective results, and in
the case of oral administration, the dosage is about 0.01 ~ -
e A 25 087 -~
- 21 -
".
, ' : ' :, . , . : . : ' ' . .: : , . , : , - , -. - , .: . -
~328869
to 20 mg/kg, preferably 0.1 to 10 mg/kg of body we;ght.
Nevertheless, it may at t;mes be necessary to
deviate from the amounts ment;oned, and in particular to
do so as a funct;on of the body weight and the nature of
S the administration method, the indiv;dual behaviour to-
wards the medicament, the nature of the formulation of
the medicament and the time or interval over which admin-
istration takes place. Thus, it may in some cases be
sufficient to manage with less than the abovementioned
minimum amount, whereas in other cases it is necessary to
exceed the upper limit ~entioned. ~hen relatively large
amounts are administered, it may be advisable to divide
these into several individual administrations over the
course of the day.
Preparation examples
Rf values: Merck TLC aLuminium foil, coating thickness
0.2 mm, s;lica gel 60 F 254; mobile phase toluene/ethyl
acetate in the volumerratio 1:2. -
Example 1
Methyl 5-(dimethylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
~-C~2~
COO ~ 0-N(CH3)2
H3~ H
Process variant A
2.12 9 (10 mmol) of 2-benzyloxy-benzaldehyde are
boiled for 18 hours w;th 1.16 9 (10 mmol) of methyl aceto- -
acetate, 1.29 9 of N,N-dimethylacetoacetamide and 1 ml of
ammonia. The mixture is cooled and evaPorated. The evap-
oration residue is taken up in ethyl acetate, washed tw;ce
with water, dried and evaporated. It is purified over a
Le A 25 087
- 22 -
' ' ' i ' ' '. '.' :,` ' -~ ' : 1 . . . .
1328869
s;Lica gel column using toluene/ethyl acstate mixtures.
The pure fractions are colLected and evaporated. The
product crystallizes on trituration using ether/ethyl
acetate 10:1. 0.8 9 ~19% of theory) of colourless crys-
tals of melting point 162 - 163C is obtained.
Process variant E
2 9 (6.45 mmol) of methyl 2-benzyloxy-benzylidene-
acetoacetate are refluxed for 18 hours ~ith 0.83 9 (6.45
mmol) of N,N-dimethylacetoacetamide and 0.53 ml of ammonia.
The mixture is cooled and evaporated. The evaporation
residue is taken up in ethyl acetate, shaken twice with
water, dried and evaporated. The product mixture is pur-
ified over a silica gel column using a toluene/ethyl
acetate mixture. The pure fractions are collected and
evaporated. The product crystallizes on trituration with
ether and a l;ttle ethyl acetate. The crystals are f;l-
tered off under suction. 0.6 9 (22.9~ of theory) of a
colourless substance of melting point 163 - 165C,
Rf value = 0.118, is obtained.
ExampLe 2
Methyl 5-(phenylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
dihydro-2,6-d;methyl-pyr;d;ne-3-carboxylate
¢~0 CH2~
H3COOC~0-N!~O
H3C H H3 . ~:
' :.
Process var;ant G
. . .
3.1 9 (10 mmol) of methyl 2-benzyloxy-benzylidene-
acetoacetate are boiled for 3 hours in 20 ml of ethanol
with 1.76 9 (10 mmol) of N-benzyl-3-aminocrotonamide. The
mixture is cooled and evaporated. The solid evaporation
Le A 25 087 . `
- 23 -
132~869
- residue is stirred with ether, filtered off under suction
and recrystallized from acetonitrile. 2.4 9 ~51.3% of
theory) of coiourless crystals of melting point 194C
are obtained.
Example 3
Methyl 5-(cyclopropylcarbamoyl)-4-(2-benzyloxy-phenylj-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~0^ CH2--O :
H3COOC~O-NH-
H3C H H3
Process variant H
.
20 9 ~45.14 mmol) of methyl 5-~imidazolylcarbamoyl~-
4-(2-benzyloxy-phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate are dissolved in 130 ml of absoLute dimethyl-
formamide, 5.63 ml (80.1 mmol) of cyclopropylamine are
added, and the mixture is stirred for 20 hours at 90-1~0C
under argon. The mixture is cooled and evaporated. The
evaporation residue is taken up in ethyl acetate, ~ashed
with water, 1N hydroch~oric acid, ~ater, sodium bicarbon- ~
ate solution and again with water, dried and evaporated.
The evaporation residue is stirred with toluene, filtered
off under suction and ~ashed with toluene. After recrys-
tallization from about 60 ml of toluene, 14.95 9 (76~7X
of theory) of colourless crystals are obtained. Melting
point: 191 - 193C.
Example 4
Z5 Methyl 5-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2- -
(3-trifluoromethyl-benzyloxy)phenyl]pyridine-3-carboxylate
Le A 25 087
- 24 -
' ,
1328869
CF
~-CH2~ :
H3COOC~O-NH-CH3
H3C H H3
3.5 9 (6~8 mmol) of methyl S-(imidazolylcarbamoyl)-
1,4-dihydro-Z,6-dimethyl-4-C2-(3-trifLuoromethyl-benzyloxy)-
phenyl~pyridine-3-carboxylate (obtained from monomethyl
5 1,4-dihydro-Z,6-dimethyl-4-C2-(3-trifluoromethylbenzyloxy)- ;-~
phenyl~pyridine-3,5-dicarboxylate were reacted with carb- ~ ~
onylbisimidazoLe in tetrahydrofuran) are st;rred overnight ~ -
without further purification with 40 ml of methyla~ine
solution (40X strength). The preeipitaeed product is
10 filtered off under suction and washed well with water. It ~ -
.
;~ is dried and recrystallized from a little toluene. 1.3 9 ~-
~; (40.3Z of theory~ of a colourless product are obtained. ~ ~ -
Melting point: 156 - 157C. ~-~
The~following ~ere prepared analogously to Examples ~
~;~ 15 1-~to 4: ~ `
~-~ Example 5 -~ -
Methyl 5-(~cyclopropyLc~arbamoyl)-1,4-dihydro-2,6-dimethyl- ~ -- - -
4-t2-(3-methyl-benzyloxy~phenyl~-pyridine-3-carboxylate
CH2~ .
~ ~,, , , , . ~ : ~ ,,.
- H3COOC ~ ~ CO-NH-
; H3C ~ ~ 5
Melting point: 176C
L~ A 25~087--
- 25 - ~ -
- 1328869 ~:
Example 6
Methyl 5-(cyclopropylcarbamoyl)-4-t2-(4-chloro-benzyloxy)-
phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
CH2~1
H3COOC~o-NH--a
H3C ~3
,' ' ,' '
5 Melting point: 178C :
Example 7
Methyl 5-((2-pyridyl)carbamoyl)-4-t2-(4-chloro-benzyloxy)-
phenyl~-1,4-dihydro-2,6-d;methyl-pyr;d;ne-3-carboxylate
CH2~3c1
H3COOC~CO-NH~
H?C N CH3 - .
~ .
~`: 10 Melt;ng point: 201 - 204C
: Example 8
Me:thyl 5-((2-pyr;dyl)carbamoyl)-4-(2-benzyloxy-phenyl)-
1,4-dihydro-2,6-d;methyl-pyridine-3-carboxyLate
~-C~2~ .' ':: -
H3COOC~O-NH~;~
H3C H H3
15 Melting point: 164 - 165C ~ -
Le A 25 087 :
- 26 -
`~
Exa~ple 9 1328869
Methyl 5-((2-diethyla~ino-ethyl)carbamoyl)-4-tZ-(4-chloro-
benzyloxy)phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate
~ ' :
~ O-CH2 ~ ~C2H5
H3COOC ~ 2 2 ~C2H5
H3C N CH3
H
Melting point: from 80C
ExampLe 10
,
Methyl 5-t(2-d;ethylamino-ethyl)carbamoyl)-4-(2-benzyloxy- : :
phenyl)-1,4-dihydro-2,6-dimethyL-pyridine-3-carboxylate
,- . - '-- . ' '
~ -CH2 ~ ~ ~C2H5 ~-
H3COOC ~ ~C2H5
H3C H H3
,:
Melting point: ~rom 95C
: Example 11 ;
.
MethyL 5-~(4-carbamoyl-phenyl)carbamoyl)-4-(2-benzyloxy~
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~
~0 CH2--0 ,
: , f~
H~COOC ~ ~ CO-NH ~ ONH2
: H,C N~CH3 - --: H
Melting point: > 300C .:
~: Le A 25 087 ~
- 27 - - . -
~ ,;'''
' ' ' '
1328869
Example 1Z
MethyL 5-((4-acetylaminophenyl)carbamoyl)-4-(2-benzyloxy-
phenyl)-1,4-d1hydro-2,6-dimethyl-pyridine-3-carboxylate
¢;~ CH2--0
H3COOC ~ O-NH ~ NH-CO-CH3
H3C H3
H
Melting point: 298C decomposition
ExampLe 13 - -
Methyl 5-((4-benzoylaminophenyl)carbamoyl)-4-(2-benzyloxy- : .
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
O-CH ~
_ ~--\ /~\ ' '
H3COOC ~ ~ CO-NH ~ NH-CO
3 H 3
..
: 10 Melting point: 197C : ~
~: Example 14
Methyl 5~-~(1-phenylethyl)carbamoyl)-(R,S)-4-~2-benzyloxy-
phenyl)-1,4-dihydro-2:,6-dimethyl-pyridine-3-carboxylate :
~R, S mixture)
2 ~ ~ -
¦ (R,S)
~;~ H3COOC ~ ~ CO-NH-IH
H3C ~ NH~^~CH3 CH3
-~ 15~ Melting point: from 162C -~
L- A a5 087 ~- :
- - 28 -
~' . ' .
1328869
Example 15
. .
Methyl 5-((1-phenyLethyl)carbamoyl)-(R)-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyr;dine-3-carboxylate
(R form) :
O-CH2 ~
(R) /-='\ . :
H3COOC ~ ~ CO-NH-CIH ~ --
H3C CH3 CH3
Melt;ng point: 82C
Example 16
Methyl 5-((3-dimethylaminopropyl)carbamoyL)-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate -. --
.',~-
-CH2 ~
~3COO ~ O N 2 3 ~CH3
Melting point: 89 - 90C
Example 17 .
Methyl 5-~cyclohexylcarbamoyL)-4-(2-benzyloxy-phenyl)-1,4- . .
dihydro-2,6-dimethyl-pyridine-3-carboxylate - :
~ -.:~
~o- CH2--O .
H3COOC ~ C~-N
H3C N CH3
Melting point: 108 - 111C :
~: Le A 25 087 :-
. .
' '~
' ''
1328869
Example 18
,
Methyl S-(tert.butylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
dihydro-2,6-d;methyl-pyridine-3-carboxylate
~ - CH2~
ICH3
H3COOC ~ CO-NH - C - CH3
H3C~N~CH3 CH3
Melting point: 148C
Example 19
Methyl 5-~propylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
'
'~0-CH
2 ~
H3COOC ~ CO-NH-CH2-CH2-CH3
H3C N H3
H
10~Foam
Rf ~ 0.37
Examp:le:20 : ~
-~ Methyl 5~ 2-methyl-propyl)carb:amoyl)-4-(2-benzyloxy-phenyl)-
1,4-d;hy~dro-2,6-dimeth~y~l-py~ridin~e-3-carboxylate
: H3COOC ~ CO-NH-CN2-CH~CH3)2
H3 ~ N'~CH3
H
Me~lt;ng point: 134C
Le A 25 :087 - ~ :
.
- 30 -
:,~ ' '--:,:
-: ~- ~ :
132~8~9 -, :-
Example 21
.
Methyl 5-(N-benzyl-N-tert.butylcarbamoyl)-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
..~.....
CH2--0 -
H3COOC ~ ~ CO~ CH
H3C N CH3 C(CH3)3
Melt;ng point: 128C
Example 22
Methyl 5-(methylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4- :-
dihydro-2,6-dimethyL-pyridine-3-carboxylate
~ ,, .
~- CH2--O
H3COOC~O-NH-CH3 :
H3C H3
H
10 MeLting point: 105C ~:
Example 23
~;~ Methyl 5-(ethylcarbamoyl)-4-(2-benzyLoxy-phenyL)-1,4- -~
dihydro-2,6-dimethyl-pyrid;ne-3-carboxyLate
~ CH 2{~ '.
~1~ H3COOC~O-NH-CH2-CH3 ~""'
H3C N H3 ~ 1
15 MeLting point: 172C :
,
Le.A 25 087
. - 31 - :
~-'
,, , ,'.
132~869
Example Z4
Butyl 5-(cyc~opropylcarbamoyl)-1,4-d;hydro-2,6-dimethyl-
4-C2-(2-pyridyl)methoxy-phenyl~-pyrid;ne-3-carboxylate
~ 1 2-
~ -CH
H9C400C~0-NH~
H3C H H3
Foam
Rf = 0.1
Example 25 .
8utyl S-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2- :
(2-pyr;dyl)methoxy-phenyl]-pyridine-3-carboxylate
~ 'H2~ ~ ~ -
H9~400C~C0-NH-CH3 ~ -
H3C ~ CH3
--
Foam
Rf = 0.07 :~
Example 26
~utyl 5~ methylpropyl~carbamoyl)-1.4-dihydro-2,6-dimethyl-
4-C2-(2-pyridyl)methoxy-phenyl~-pyridine-3-carboxylate
~ ' ' ,'. '
--r -CH2 3
H9C400~0-NH-CI H-CH2-CH3 . .
H3 H3 CH3
~ Le A 25 087
: - 32 -
:
Foam 1 3 2 8 8 6 9
Rf = 0.25
Example 27
.
MethyL 5-(cyclopropylcarbamoyl)-4-C2-(3,4-dichloro-benzyloxy)-
S phenyl]-1,4-d;hydro-2,6-dimethyl-pyridine-3-carboxylate
~0 - CH2~ 1
!i3COOC~O-NH~
~ 3 C N H 3 . .
', . .. .
Melting point: 195C
Example 28
Methyl 5-(cyclopropylcarbamoyl)-4-C2-(2,6-dichloro-benzyL-
10~ oxy)phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~-C~2~ ::
H3COO~O-NH~ .
:~ ~3 H3 -- :
'' '
Melting point: 178C
Example 29
MethyL 5-t(1-methyl-propyl)carbamoyl)-4-C2-(2,6-dichloro-
benzyloxy)phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-
; carboxylate
Le A 25 087
- 33 -
; ~ ? ~
1328869
Cl
~ - CH
Cl
H3COOC ~ ~ CO-NH-IH CH2_CH~
H3C N CH3 CH3
Rf = 0.36
Example 30
Methyl 5-tmethylcarbamoyl)-4-C2-(2,6-dichloro-benzyloxy)-
phenyl]-1,4-dihydro-2,6-dimethyL-pyr;dine-3-carboxyLate
~ Cl
~-C~2~
H3COO ~ ~ O-NH-CH3 ~ -
H3C H . ; ~ :.
Melting point: 133C -
Example 31
Methy~ 5-(me~thyltarbamoyl)-4-C2-(3,4-dichloro-benzyloxy~-
phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~; : ~ O-CH2 ~ I -
~: ~: : :
H3COOC ~ ~ CO-NH-CH3
H3C CH3
.
Melting point: 120C
Le A 25 087
_ 34 _
~ " ,, ,,,,,, .,,~"" ,, , , ",, ,, j,", , ,,~, , ~,, , ~;~ ", ~," ~ ".,, ~ ".,; - , , "
132~8~9
Example 32
Methyl S-(cyclopropylcarbamoyl)-4-C2-(3-fluoro-benzyloxy)-
3-methoxy-phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate
. ~ OCH3 F
~0 CH2~ ~ ;
H3COOC~CO-NH~
H3C~N~--CH3
Melting point: Z02C -
Examp(e 33
Methyl'S-~cyclopropylcarbamoyl)-4-C2-(2-chloro-benzyloxy)- -
phenyl]-1',4-dihydro-2,6-d;methyl-pyridine-3-carboxylate :
"
~ c i ,
[I ,~o ~ .: :~ - CH2~ , . .
H3C OOC~O - NH <¦
H3C H H3
Melting point: 1R5C
Example 34
Methyl 5-~methylcarbamoyl)-4-C2-(2-chloro-benzyloxy)phenyl]-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~ .
:
~ 15
;~
Le A 25 087
,,
.'
13288~9
CH
H3COOC ~ O-NH-CH3 .
~3C H H3
Melting po;nt: 170C
Example 35
Methyl 5-tt2-methyl-propyl)carbamoyl)-4-CZ-(3-fluoro- -~
5 benzyloxy)-3-me~hoxy-phenyl~-1,4-d;hydro-2,6-dimethyl- ~ -
pyridine-3-carboxylate :~
: H3COOC ~ Co-NH-cH2-cH(cH3)2 .
3 H 3 :
Melting paint: 98C
Example 36 :-
~: 10 Methyl 5-tcyclopropyl~carbamoyl)-1,4-d;hydro-2,6-dimethyl-
4-CZ-(3-trifluoromethyl-benzyloxyjphenyl~-pyridine-3- :
carboxylate
H3COO ~ O NH ~ ;
;~ H3 M3 ~:-
Melting point: 148C :
Le A 25 087
- 36 -
~ .
: : .
.~ 132886~ -
Example 37
Methyl 5-t~1-methyl-propyl)carbamoyL)-1,4-dihydro-2,6-
dimethyl-4-t2-(3-trifl~oromethyl-benzyloxy)phenyl~-pyridine-
3-carboxylate
CF3
~ -C~2 ~
: H3COOC ~ O-~H-CH-CH3
H~C N H3 CH2-CH3
Foam
Rf = 0.44
Example 38
Methyl S-~isopropylcarbamoyl)-4-C2-(3-fluoro-benzyloxy)-
3-methoxy-phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate
OCH3 F
CH2~
H3COOC ~ O-NH-CH(CH3)2
H3C H3
, ~. '
~elting point: 106C
Example 39
1-methyl-propyl 5-(cyclopropylcarbamoyl)-1,4-dihydro-2,6-
di~ethyl-4-~2-(4-methyl-benzyLoxy)phenyl]-pyridine-3-
carboxylate
; Le A 25 087 ~ -
:::
: ~ 37 ~
. ' ' ' .
r. ~ . ? ~
~328869
~ O-CH2 ~ CH3
H3C-HC-OOC ~ O-NH ~ ~
H~C-H2C H3C H H3 -: .
Foam -
Rf = 0.31
Example 40
Methyl 5-tcyclopropylcarbamoyl)-4-(4-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyr;d;ne-3-carboxylate
/--\ '
I - CH2~ ~
¢~
H3tOOC ~ O-NH
H3C H H3
Melting point: 197C
-~ ~E~xample 41 ~ -
1-methyl-propyl 5-((1-methyl-propyl)carbamoyl)-1,4-d;hydro-
2,6-dimethyl~-4-C2-(4-met~hyl-benz~yloxy~phenyl]-pyrid;ne-3-
carboxylate
.
O-CH2 ~ ~3
;: H3C-CH-O~OC ~ O-NH-CH-CH3
3C-H2C -H3C N H3 CHz-CH3
Melting po~nt: 126C -
Le A 25 087
.
~ - 38 -
1328869
Example 42
. .
1-methyl-propyl 5-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-
4-CZ-(4-methyl-benzyloxy~phenyl]-pyridine-3-carboxyLate
~ ''~
"
~O- CH2~CH3
H3C-CIH-OOC ~ 0-NH-C~3
~3C-H2c H3C H~
Foam
Rf = 0.48
Example 43
Ethyl 5-~cyclopropylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-
C2-~3-nitro-benzyloxy)pheny~]-pyridine-3-carboxylate
~ 1~2
~ 0-CH
H5C200C~CO-NH~
~:~ H
~ , ''' -
Foam
Rf = 0.24
Example 44
Methyl 5-(prapylcarbamoyl)-4-(4-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
'
~ Le A 25 087
.
- 39 -
, :
~ .
1328~69
O - CH2--O
H3COOC ~ 0-NH-CH2-CH2-C~3
H3C H3 - .
H
Melting point: 210C
Example 45
Ethyl 5~ phenyl-ethyl)carbamoyl)-(R)-4-C2-(4-fluoro-
S benzyloxy~phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate (R form)
0-CH2 ~ F
tR~
5C200C ~ C0-NH-CIH ~
H3~A`N CH3 CH3 : :
H ~:
Foam ~ ~
~; Rf - 0~56 ---
Example 46 ~ --
Methy~ 5-t~2-methyl-propyl)carbamoyl)-4-~4-benzyloxy-phenyl)-
1,4-dihydro-2,6-dinethyl-pyridine-3-carboxylate
~; 0-CH2 ~ ~
H3C00 ~ 0-NH-CH2-CH(CH3)2 . - .
3 ~ H H3
Me~ting point: 202C ~-
Le A 25 087
- 40 -
:~
.
' '`~ ''" ''i~ ~ ~ ; A ;
- 1328869
Example 47
EthyL 5-(cyclopropylcarbamoyl)-4-~2-(4-fluoro-benzyloxy~-
phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~ -.
~0- CH2~3F
H5C200C ~ O-NH
H3C N H3
H
Melting point: 159C
Example 48
Methyl 5-(cyclopropylcarbamoyl)-4-C2-(4-fluoro-benzyloxy)-
phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~ -CHz ~ F :~
H3COO ~ O-NH
~` H3C H3
H
:,`~ ~ , ,
MeLting point: 154C
~-- E~-a~ 9
Methyl 5-(~1-phenylethyl)carbamoyl)-~S)-4-(4-benzyloxy-
phenyl~-1,4-dihydro-2,6-di~ethyl-pyridine-3-carboxylate
0-CH2 ~
"
( S )
H3COOC ~ O-NH-CH-CH3
H3C CH3
Le A 25 087 ~
- 41 - ~ -
132~869
Melting point: 141C
Example S0
Methyl 5-(isopropylcarbamoyl)-4-(4-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
O- CH2--
S ~ , "
H3COOC ~ O-NH-CH(CH3)2
H3C H3
H
Melting point: 162C
Example 51
Methyl 5-(t1-phenylethyl)carbamoyl)-(R)-4-t4-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
- 10 (R form, diastereomer A)
_~ ~h.::
~ ~1 .;.
H 3 C OOC~O - NH - C H~
H ~C N H3 CH 3
Melting point: 196C
Example 52
Methyl 5-(cyclopropylcarbamoyl)-4-l2-~3-chloro-benzyloxy)-
phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-carbsxylate .
."''',
' ''~:',''.. ''
Le A 25 087 -
- 42 -
1328869
Cl
~0- CH
~3~00 ~ 0-NH-~
H3C H H3
Melting point: 174C
Example 53
Methyl 5-(cyclopropylcarbamoyl)-4-t2-t4-fluoro-benzylthio)-
phPnyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~5 - CH2{~F
H3COOC ~ CO-NH
H3C H H3
Melting point: 202C
ExampLe 54
Methyl 5-((1-phenylethyL)carbamoyl)-(R3-4-(4-benzyLoxy-
~` 10 phenyL)-1,4-d;hydro-2,6-dimethyL-pyr;dine-3-carboxylate
(R form) (diastereomer 3)
~, , .
CH f~
~: f 2 ~
~I3COOt~O-NH-C~ . "
H3C H3 CH3
,~
MeLting point: ZOZC
Le A 25 087 -
- - 43 - ~
~' ~' , '': ' '
1328869
Example 55
Methyl 5-((1-phenyLethyl)carbamoyl)-(R)-4-(4-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyrid;ne-3-carboxylate
(R form, diastereomeric mixture)
O CH2--
~'.
(R) ~
H3COOC~O-NH- IH~>
H3C H3 CH3 :- :
Melting point: 110 - 166C
Example 56 - -
Methyl S-((1-phenylethyl)carbamoyl)-(S)-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
(S form, diastereomer A)
: ~ ~O-CH2{~ ;
H3COO~O-NH- I ~ S ~CH3 , " ,
~': ~ . ~ ' ' "'
Melting point: 172C
Example 57 -
-5 Methyl 5-(~1-phenylethyl)carbamoyl)-~5)-4-~2-benzyloxy-
15phenyL)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~S form, diastereomer 8) --
~ " `,.': "
:-
Le A 25 087
~ 44 ~
13288~
~ - CH2--
H3COOC ~ CO-NH-fH-CH3
H3C H `3 0
Foam
Rf = 0.53
Example 58
.
Methyl S-((1-phenyl-ethyl)carbamoyl)-(R)-4-t2-~4-fluoro-
benzyloxy)phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3- -
carboxylate tR form)
,.' ',
O-CH2 ~ F
H3COC ~ O-NH-IH ~
H3C H~ CH3 . ~-
Foam
~ 10Rf = 0.52
-j Example 59 ~ ~-
`~ Butyl 5-~cyclopropylcarbamoyl)-1,4-dihydro-2,6-dimethyl-
4-C2-(methyl-benzyloxy)phenyl~-pyridine-3-carboxylate
CH2 ~ CH3
C400C ~ CO~NH ~
:~ ~3C~A~N~A~CH3 :
1~ 15 Foam
~:: R~ = 0.31
~ : .
~ Le A ZS 087
~ 45 ~
1~ ' .-
Example 60 13288~ .
ButyL 5-((1-phenyL-ethyL)carbamoyL)-(R)-1,4-d;hydro-
Z,6-dimethyL-4-CZ-(3-methyL-benzyLoxy)-phenyl~-pyr;dine-
3-carboxyLate (R form)
CH
tR) ~=~
HgC400C~; 3~0-NH-CI H~
H3C H H3 (:H3
Oi~ -
R~ = 0.88
Example 61
Methyl 5-((1-phenyl-ethyl)carbamoyl)-(R)-4-r2-(3-chloro-
10 benzyloxy)phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3- ;
carboxylate (R form)
,~, C 1 : :
~0 CH2~
H3COOC~O-NH-CH~ . -
H3C H3 CH3
~: '" `~ . '.
,~ . , .
Foam
~ Rf s 0.73
-~ 15 ExampLe 62 -
Methyl 5-((1-phenyl-ethyl)carbamoyl)-(R)-4-(3-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyL-pyridine-3-carboxylate
(R form)
' ,
Le A 25 087
- 46 -
. .
' - ' .
1328869 :
/=\
¢~0 - CH2~
1 (R) /~=~\
H3COOC ~ O-NH-~
H3C ~ H3 CH3
Melting point: from 143C
Example 63
Metkyl 5-(cyclopropylcarbamoyl)-4-(3-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
¢;~0 - CH2--O
COOC ~ O-NH
~3C H3
Melting point: 144C
Example 64
Methyl 5-(~1-phenyl-ethyl)carbamoyl)-(R)-4-C2-t4-fluoro- ~ -
benzyLthio)phenyl~-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate (R for~)
8-CH
~R) ~5=~
H3COO ~ ~ O-NH-
H3 H3 CH3
H
Foa~ -
Rf = 0.47
~:: 15 - -'
Le A 25 087
- 47 -
~ ;,,.
~: .
132~869
. Example 65
:
Methyl 5~ phenyl-ethyl)carbamoyl)-(S)-4-C2-(4-fluoro-
benzylthio)phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-
carboxylate (S form)
~
~ S-CH2 ~ F
(S) .
H3COOC ~ ~ O-NH-CH-CH3
H3C H3 ~
Foam - .
Rf = 0.48 `:~.
Example 66
.
Methyl 5-(allylcarbamoyl)-4-~2-(4-fluoro-benzylthio)phenyl]-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~S - CH2~F ; .
H3COOC ~ O-NH-CH2-CH=CH2
H3 H3
~;~ Melting point: 177C
Example~67 ~
Methyl 5-(allylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-dihydro- -.
2,6-dimethyl-pyridine-3-carboxylate
3COOC ~ Co-NH-cH2-cH=cH2 ~
H3C CH3 ~ .
Melting point: 148C -
Le A 25 087
"~
1328~69
Example 68
Methyl 5-~(1-phenyl-ethyl)carbamoyl)-(R~-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
(R form)
¢;~0 - CH2--0
~R) f_~
H 3COOC~Co-NH- I H~;)
H3C H3 CH3
Melting point: 175C
Example 69
Methyl 5-((1-phenyl-ethyl)carbamoyl)-(R)-4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~R form, diastereomer ~)
~ ,
--0 CH
~R)
H3COOC~; ;3~0-NH-CH--O :
~3C H3 CH3 :
Melting point: 169C
Example 70
Methyl 5-((2-phenyl-ethyl)carbamoyl)-4-(2-benzyloxy-phenyl)-
151,4-dihydro-2,6-di~ethyL-pyridine-3-carboxyLate ~ ~-
¢;~ CH2~
;~ H3coo~o-NH- CH2~ CH2~0
H3 H3
Le A 25 087
- 49 -
1328869
Example 71
Methyl 5-(benzylcarbamoyl)-4-(2-ben2yloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
~0- H2--0 "
H3cooc~o-NH-cH2--O :
H3C H3
Melting point: 148 -149C .
Example 72
Methyl 5-(ethylcarbamoyl)-4-t2-~4-fluoro-benzylt-hio)phenyl~-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate ~ :
CH2 ~ F
~ : - .
H3COOC ~ ~ O-NH-CH2-CH3
H3C H3
10 Melt;ng point: 202 - 204C -~ -
~ Exa~mple 73
i~: Methyl 5-~ethylcarbamoyl)-4-(3-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate .
-CH2
H3Coo ~ CO-NH-CH2-cH3
H3C H CH3
15 Melting point: 152 - 154C ~: -
Le Z5 087 : :
5 ~ ~:
' : ~.. ,'-'
1~2886~
Example 74
Methyl S-(ethylcarbamoyl)-~-(4-benzyloxy-phenyl)-1,4-
d;hydro-2,6-dimethyl-pyridine-3-carboxylate
O CH2~
H3COOC~CO-NH-CH2 -CH3
~{3C H CH3
Melting point: 183 - 185C
Example 75
Methyl 5-carbamoyl-4-(2-benzyloxy-phenyl)-1,4-dihydro-2,6-
dimethyl-pyridine-3-carboxylate
-CH
H3COOC~O-NH2
Melting po;nt: 188C
`-~ Example 76 ~
Methyl 5-(diethylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
~; dihydro-2,6-dimethyl-pyridine-3-carboxylate
C~2{~
H3COO~O-N ~ C2H5 ) 2
H3 H3
: ':' .
Melting point: 135C
Le A 25 087
- 51 -
, :
13288~9
~ Example 77
Methyl 5-(cyclopropylcarbamoyl)-1~4-dihydro-2,6-dimethyl-
4-CZ-t4-methyl-phenylsulphonyloxy)phenyl~-pyrid;ne-3-
carboxylate
s
~ 0-502 ~ } CH~
H3COOC ~ O-NH
H3C H3
Xelting point: 238 - 242C
Example 78
Methyl 5-(ethylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
~4-methyl-phenylsulphonyloxy)phenyl~-pyridine-3-carboxylate ~-
~ '; .' ~ ''
~ -SO2 ~ H3 ~:
%3COOC ~ ~ O-NH-C2H5 -.
3 H 3
Melting point: 228C .
Ex-mple 79 ~ -
Methyl S-(ethylcarbamoyl)-4-C2-(2,6-dichloro-benzyloxy)phenyl~
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
: I Cl -
H3COOC ~ O-NH-C~2-c~3
~3C H3
H
Melting point: 174 - 176C
Le A 25 087 :
''~
Example 80 1328869
Methyl 5-(ethylcarbamoyl)-4-C2-(3,4-dichloro-benzyloxy)-Phenyl]
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
Cl
~O-CH2~: 1
H3t:00C~O-NH-C~2-C~3
H3C H H~
Melting point: 178 - 180C
Example 81
Methyl 5-(ethylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
(2-pyridylmethyloxy)phenyl~-pyridine-3-carboxylate
~ ~' ,.
H COOC3~0-NH-C2H5
H3C H H3
., ,
~
Melting point: 159 - 161C
Example 82 ~ ~ -
Meth~yl 5-(cyclopropylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-
t2-(2-pyridylmethyloxy)phenyl~-pyridine-3-carboxylate
~o-CH~?
;: H ~COOC~O-NH
~: H3C H H3
,: . ~'.,'
15 Melting point: 168 - 170C . ::
~ Le A 25 087 .~-
i:~ - 53 ~ ;:
132886~
Example 83
Methyl 5-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-(2-
phenylsulphonyloxy-phenyl)-pyridine-3-carboxylate
~ '~
~ -52
H~COOC ~ O-NH-CH3
H3C H H3
Melt;ng point: 171 - 173C :
Example 84 :-. .
Methyl 5-(ethylcarbamoyl)-1,4-dihydro-Z,6-dimethyl-4-(2-
phenylsulphonyloxy-phenyl)-pyridine-3-carboxylate H
: ~ -52
H3COOC ~ O-NH-C2H5
: Hj H H3
Melting point: 188 - 190C
-~ Example 85 :
Ethyl 5-(ethylcarbamoyl)-1,4-dihydro-4-~2-~4-fluoro-
benzyloxy)phenyl]-2,6-dimethyl-pyridine-3-carboxylate
~ . ~ .
1: ~- CH2~}F
1: H5C200C ~ O-NH-C2H5
¦- H3C H H3
.
¦ 15 Melting point: 147 - 149C
¦:~ Le A 25 087 : .
- 54 -
1 :-
1: .
1328869 - -
Example 86
-
Methyl 5-(ethylcarbamoyl)-1,4-dihydro-4-~2-(4-fluoro-
benzyloxy)phenyl]-2,6-dimethyl-pyridine-3-carboxylate
[~o-cH2~F " .
H3COOC~O-NH - C 2H5
H3C H H3
Melting point~ 113C
Example 87
Methyl 5-(allylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-~Z-
phenylsuLphonyloxy-phenyl)-pyridine-3-carboxylate
,
` ~ ~-52-0
H3COOC~o-NH-cH2-cH=cH;2
~: H
MeLting point: 154 - 156C
Example~ 88
Methyl 5-tt4-pyridyl)carbamoyl)-1,4-dihydro-2,6-dimethyl-4-
(2-phenylsulphonyloxy-phenyl)-prridine-3-carboxylate
S02~
H3COQC~CO-NH-CHz~N
H :
, ~ :
Melting point: from 240C (decompos;tion)
Le A 25 087 -
- 55 -
1328~69 : ~ -
Example 89
Methyl 5-(allylcarbamoyl)-4-C2-(4-chloro-benzyloxy)phenyl~- :
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~ ,
~ O-CH2--<} Cl ~,
H3COOC~O-NH-CH2-CH=CH2 - '.
H3C H 3 .
Melting point: 145C
S Example 90
Methyl 5-(allylcarbamoyl)-4-(3-benzyloxy-phenyl)-1,4-dihydro- ~ :
2,6-dimethyl-pyridine-3-carboxylate
- -
~O-CH2--V ~
~ . ,
H2C=CH-HzC-NH-OC ~ OOCH3
C H H3 :~-
~' . ..
;~ Melting point: 95 - 98C -
Example 91
Methyl 5-~allylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-t2- : :
- (4-methyl-phenylsulphonyloxy)phenyl]pyridine-3-carboxylate -~
-52 ~ H3 -
H3COOC ~ O-NH-CH2-CH-CH2
: ~ H ~ - ~
~ . . .:
Melting point: 168 - 17:0C : ~:
"' .
~ Le A 25 087 - 56 -
,: ~ . .
,', . ' ' :
132~8~9
Example 92
-
Methyl S-(allylcarbamoyl)-4-C2-~2,6-dichloro-benzyloxy)phenyl~- -
1,4-d;hydro-2,6-dimethyl-pyridine-3-carboxylate
~O-CH
Cl
H2C=HC -H2C-HN-OC~ ~COOCH3
H3C~N~CH3
Melting point: 132 - 134C
Example 93
Methyl 5-((2-hydroxyethyl)carbamoyl)-4-~2 benzyloxy-phenyl)-
1,4-d;hydro-2,6-d;methyL-pyridine-3-carboxylate
¢~0 CH2--
H3cooc~co-NH-cH2-cH2-~)H
11 11 .,.
H3~:~N~CH3
,
10 Melting point: 148 - 1SDC ~-~
Example 94
Methyl S-(methylcarb~moyl)-4-t2-(3-fluoro-benzyloxy)-3-
methoxy-phenyl~-1,4-d;hydro-2,6-dimethyl-pyridine-3- -
carboxylate
~OCH3 F -
CH2~ ~ :
H3COOC~O-NH-CH3
~' H3C H3
-.
~ ~ .,:.
Le A 25 087 - -
~ 57 ~ ~
.
" " ~ - " , ,~ ;' ,~ ,,-, ~ , : ~ , ,. ., .,.
~328869
Melting point: 171 - 173C
, .... .
Example 95
Methyl 5-(2-(4-pyridyl)ethylcarbamoyl)-4-(2-benzy~oxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
S
~ -CH~ ~
H3COOC ~ ~ CO-NH-CH2-cH2 ~ N
1 11. .
H3C~ ~N ~ CH3
H
Melting po;nt:
Example 96
Methyl 5-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
(3-pyridyl)methoxy-phenyl]-pyridine-3-carboxylate
, . . .
~ ~ , '
: . ~ O-CH2 ~ ::
H3COOC ~ o-NH-CH3
H3C H 3
` : ''' ~",
~: 10 Melting po;nt: from 210C
Example 97 :
~ Methyl 5-(ethylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
;~:~ (3-pyridyl)methoxy-phenyl]-pyridine-3-carboxylate
-' . '
N
H3COOC~O-NH-C2H5 '~
H3C H3
~ . ` :,
Melting point: 122 - 125C
Le A 25 087
- 58 - -
,~
,
1328869
Example 98
Methyl 5-(ethylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
(4-pyridyl)methoxy-phenyl~-pyrid;ne-3-carboxylate
~0- CH2--CN
H3COOC~o-NH- C2H5
H3C H 3
Melting point: 95 - 100C
Example 99
Methyl 5-(methylcarbamoyl)-1,4-dihydro-2,6-dimethyl-4-C2-
(4-pyridyl)methoxy-phenyl]-pyridine-3-carboxylate
~ -
~O - CH 2~N
H3cooc~o-NH-cH3 , ~ "
H3C~N H3
:: '
10 Melting point: from 203C (decomposition)
Example 100
Methyl 5~((cyclopropylmethyl)-carbamoyl)-4-(2-benzyLoxy- --
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
¦~ ~ CH2--O
H3COOC ~ CO-NH-CH
H3Cf~N~CH3
H : ~:
!~ 15 Melting point: 186C
~ Le A 25 08?
3~ ~ 59 ~
~ , '
Example 101 1328869
MethyL 5-(ethylcarbamoy~ )-4-(Z-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethy~-pyridine-3-carboxylate
¢~ - CH2--0 '
H3COOC ~ O-N~-CH2^CH3 . .
~3C H H3 :
Melting point: 147C
C~D20 = ~ 29.68
c = 0~91 (DMF)
Example 102
10 Methyl 5-tethylcarbamoyl)-~-)-4-~2-benzyloxy-phenyl)-1,4-
~' dihydro-2,6-dimethyl-pyridine-3-carboxylate
CH2'~
~ . .
: H3COO ~ ~ O-NH-CHz-CH3
H3 H 3
Melting point: 148C
~D20 = _ 29.92
c s 0.805 tDMF~ -
Exa~ple 103
Ethyl 5-~methylcarbamoyl)-4-~2-benzyloxy-phenyl)-1,4-dihydro-
2,6-dimethyl-pyr;dine-3-carboxylate
Le A 25 087
- 60 -
~ .
1328~
~ -CH
H5C200C~o-NH-cH3
~3C H H3
Melt;ng point: 179C
Example 104
.
Ethyl 5-(ethy~carbamoy~)-4-(2-benzyloxy-phenyl)-1,4-d;hydro-
2,6-dimethyl-pyrid;ne-3-carboxylate
' ~
' ',
-CH2
5C2QOC~O-NH-C2H5
H3C N H3 ~ :
. ''-. ': .
Melting point: 161 - 164C
Example 105
Methyl 5-~2-ethoxycarbonylethyl)carbamoyl)-4-(2-benzyloxy-
;~ 10 phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
` ~ O-CH
H3COOC ~ O-NH-(cH2)2-cooc2H5
~ H3C H H3
,~
Rf ~ 0.35
Le A Z5 087
61 -
1328869 -
Example 106
MethyL 5-~(ethoxycarbonylmethyL)carbamoyl)-4-~2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyr;dine-3-carboxylate
CH2--
H3COOC~o-NH-cH2-cooc2Hs
H3C H H3
S Rf = 0.408
Example 107 .
Methyl 5-~octylcarbamoyl)-4-t2-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxyLate
' ,
~1 . '- ~ ~
~O - CH2~
H3COO~O~ CH2 ) 7 - CH3
,
Rf = 0.53 ~
Example 108
~:~ - Methyl 5-(nonylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4-
-~ dihydro-2,6-dimethyl-pyridine-3-carboxylate
~O-CH2--0
H3COOC~O-NH- ~ CH2 ~ 8-CH3
H
'~
Rf = 0.55
~: Le A 25 087
- 62 - ~ .
''
1328~69
Example 109
MethyL 5-(decylcarbamoyl)-4-(2-benzyloxy-phenyl)-1,4- .
dihydro-2,6-dimethyl-pyridine-3-carboxylate
~- CH2--O
H3COOC ~ O NH ~CH2)9 CH3
H3C N H3
MeLting point: 111C
Example 110
Methyl 5-((2-methoxy-ethyl)carbamoyl)-4-(Z-benzyloxy-Phenyl)
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
H3COOC ~ O-NH-CH2-CH2-0CH3
Melting point: 145C
~ ,
Example 111 .~:
Methyl 5-((3-methoxy-propyl)carbamoyl)-4-(2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyL-pyridine-3-carboxy~ate
H3COOC ~ O-NH-(CH2)~-OCH3
H3C H3
15 Rf = 0.19 :~
- Le A 25 087
3 -
, , .
~ ` ''' .,; .. .,-
1~28869
Example 112
Methyl 5-((2-hydroxy-1-methyl-2-phenyl~ethylcarbamoyl)-
(R,R)-4-~2-benzyloxy-phenyl)-1,4-dihydro-2,6-d;methyl-
pyridine-3-carboxylate
S .'
--O-CH2--
CH3 ~
H3COOC~ ~CO-NH-Cil CH-OH
( R ) ~ R )
H3C~N--
H
Process variant H (direct coupling with dicyclohexyl-
carbodiimide)
1 9 (2.54 mmoL) of monomethyl 4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3,5-dicarboxylate
10 are dissoLved in 5 ml of dimethylformamide, and 0.477 ~ ~
(2.54 mmol) of L-norpseudoephedrin hydrochloride, 0.35 ml
t2.54 mmol) of triethylamine and 0.629 9 (3.05 mmol) of
dicyclohexyl-carbodiimide ~solid) are added. The mixture
is stirred at room temperature for 4 hours, the urea is
filtered off under suction, and the filtrate is evaporated.
Column chromatography: Silica gel 60, grain size 0.040 -
0.063 mm using CHCl3/CH30H/NH3
20 : 1 : 0.05
Since urea is only sparingly soluble in ether and
~ethylene chloride, the product is taken up several times
~; in ether or methylene chloride, filtered off and evaporated.
Yield: 0.9 9 (67.3% of theory)
Rf = 0.35
C~ D20 _ 47.93 (CHCl3)
.~ .
~ Le A 25 û87 - 64 -
. -'.
. '.
` ' ' ' ' ' . . . ... ' ' . ' . . ' . " '.. ' . " ' " .:' ~: ': ,.:. ' .' ' ' .. ' '.' :
1~288~
Example 113
Methyl 5-((2-hydroxy-1-methyl-ethyL)carbamoyl)-(S)-4-(2-
benzyloxy-phenyl)-1,4-dihydro-2,6 dimethyl-pyr;dine-3-
carboxylate
[~0 CH2--
' (S) ''.
H3COOC ~ ~ CO-NH-CH-CH20H . :
H3C CH3 CH3 . .
Rf = 0.13
~~ D20 ~7.34 (CHCl3)
Example 114
MethyL 5-((1-hydroxy-methyl-propyl)carbamoyl)-(S~-4-~2-
benzyloxy-phenyl)-1,4-dihydro-2,6-d;methyl-pyridine-3-
carboxylate
CH
: _ I C12H5
H3COOC ~ CO-NH-CH-CH20H
: H~C~N~CH3
H
Rf = 0.15
_9.3? (CHCL3)
Example 115
Methyl 5-((1-hydroxy-methyl-2-methyl-propyl)carbamoyl)- ~ -
- (S~-4-(2-benzyloxy-phenyl)-1,4-dihydro-2,6-dimethyl- -
pyridine-3-carboxylate
- Le A 25 0~7 -~
. .
~ 65
'-`,..
~32886~ ~
': . '
~0 - CH2--O '
(s) ,
H3C9OC ~ ~ CO-NH-CH-CH2-OH
H3CCH3 CH
H H3C ~ ~ CH3
Rf = 0.19
C~ 20: -10.8 (CHCL3)
Example 116
S Methyl 5-t~1-hydroxy-methyl-2-methyl-butyl)carbamoyl)-tS)-
4-~2-benzyloxy-phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-
3-carboxylate
CH ~ ~
S )
H3COO ~ ~ O-NH-CIH-CH2-OH
H3C H3C i ~ ~2H5
~ ~ .
L~ Z3; -17.37 (CHCl3
Example i17
;~ Me~th~yL 5-tt3-hydroxy-propylScarbamoyL)-4-t2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
Le~A 25 087
--66 -
:
1328869
~'^~v-CH~-~ >
H3COOC CO-N~-(CHz~3-~H
H3C~N~CH3
Melting point: 205C
xample 118
Methyl 5-t3-cyclopropylcarba~oyl)-(-) 4-(2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~ CH2
H3COOC ~ O-NH ~ ~
H3C H3 . .
- ~:
Melting point: 178 - lB1C
C~209=-38.28 (c=0.569, chloroform) ~-;
Example 119 -~ -
;~ 10 Methyl 5-(3-cyclopropylcarba~oyl)-(~) 4-(2-benzyloxy-
phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
-CH
- ~COOC ~ O-NH
H3cfA~N H3 :~
Melting point: 178 - 181C
ta~2589= +36.56 (c=0.52, chloroform) ~-~
Le A 25 087 - 67
'' ` ' '
132886~
Exa~ple 120
-
Methyl 5-hexylcarbamoyl-4-(2-benzyloxy-phenyl)-1,4-dihydro-
2,6-dimethyl-pyridine-3-carboxylate
f ~ 2
-CH
H3COO ~ O-NH-(C~2? 5-CH3
H3C H H3
5 Melting point 133C
Example 121 - -~
Methyl 5-carbamoyl-4-C2-(4-methyl-benzenesulphonyloxy)-
phenyl]-1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
.~: f~
~ S2 ~ ~'H3
-~ H~COOC ~ CO-NH2
H 3
~; 10 Melting point: 205C ~
Example 1ZZ
~,
Methyl 5-sec.-butylcarbamoyl-4-~2-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-2-carboxylate
. ~5eC~
H3COO ~ CO-NH-CH-CH3
H3C ~ ~ H3 CHz C~3
Melting point: 135C
Le A 25 087
.
~ - 68 -
~ .~ .
1328869
Example 123
Isopropyl 5-carbamoyl-4-(2-benzyloxy-phenyl)-1,4-dihydro-2,6-
dimethyl-pyridine-3-carboxylate
~ O-CH~-~' `j :
H3C~ ~
CHOOC ~ ~CO-NH2 ..
H3C~ ll Il, :
~l3C~1,~CH3
,' ' . '
5 Melting point: 190C -
Example 124 -:
2-Methoxy-ethyl 5-carbamoyl-4-(2-benzyLoxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-5-carboxylate
' "' '
3C-O-H2C-H2C-00 ~ -NH2 ~ ~
3 ~ 3 - ~ :
10 Melting point: 165C . ~:~
Example 125
,~ Methyl 5-butylcarbamoyl-4-~2-benzyloxy-phenyl)-1,4-dihydro- :~
2,6-dimethyl-pyridine-3-carboxylate ~- -
~ -CHz ~ ..
¦~: H3COO ~ O-NH-(CH2)3-CH3
;~: H3C H3 ~
f . H ~.:~ .-
~:~ Le A 25 087 .. -
-,~ 69 : .
3 -
Melt;ng po;nt: 144 - 148C 132886~
Example 126
Isopropyl 5-ethylcarbamoyl-4-(2-benzyloxy-phenyl)-1,4-
d;hydro-2,6-dimethyl-pyridine-3-carboxylate
f~
H3C~ `r -CH2--O
CH-OOC~O-NH-C2H5
H3C H H3
Melting point: 135C
Example t27
Methyl 5-(3-ethoxypropyl)carbamoyl-4-t2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
f~o CH2~
H3COOC--C`O-NH- ( Clt2 ~ 3--C2~5
H:3C~N H3
Melting point: 115C
Example 128 ~: .
Methyl 5-(5-hydroxypentyl)carbamoyl-4-(2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
H3COOC ~ O-NH- ( CH2 ) 5 OH
3 H 3
Le A 25 087
- 70 -
' . .
1328869
Melt;ng po;nt: 178C
Example 129
2-Methoxy-ethyl 5-methylcarbamoyl-4-(2-benzyloxy-phenyl)-
1,4-d;hydro-2,6-dimethyl-pyridine-3-carboxylate
I CHZ~
H3C-O-H2C-HzC-OOC~CO-NH-CH3 ' '
H3C~N~--CH3
Melting point: 133 - 135C
Example 130
2-Methoxy-ethyl 5-ethylcarbamoyl-4-(2-benzyloxy-phenyl)-
1,4-dihydro-Z,6-dimethyl-pyridine-3-carboxy~ate
~_C~2~ ,'~
H3C O H2C HzC-OO ~ CO-NH-C2H5
H3 H H3 M
, ~ - " '.'' , .
~` ' ' ':
Melting point: 111C `-
Example 131
Isopropyl 5-methylcarbamoyl-4-t2-benzyloxy-phenyl~-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
H3C~ I CH2-~
I CH-OOC ~ CO-NH-CH3
H3C~
H3C~A~N ~ CH3
Le A 25 087
. .
- 71 -
1 328~9
Melt;ng point: 140 - 143C
Example 132
Isopropyl 5-cyclopropylcarbamoyl-4-(2-benzyloxy-phenyl)-
1,4-dihydro-2,6-dimethyl-pyridine-3-carboxylate
~-CH2 7
113C~ --~_
C~l-UOC~ CO-NH~
~3C~
H3C~ ~N ~ CH3
~' ' -' ''
Melting point: 13Z - 135C
E~ample 133
Isopropyl 5-isopropylcarbamoyl-4-(2-benzyloxy-phenyl)-1,4-
dihydro-2,6-dimethyl-pyridine-3-carboxylate
` f~
~) - CH; ~
H3C~ ¦ ~CH3
H C~ 1 ,~CO NH C~CH
~: H3 H : -
, ' `
Melting point: from 110C
1 ~
',
'''.
¦ L2 A 25 087 -: :
I;: - 72 -
1 ..
I, .