Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
133~3
-- 1 --
BENZOTHIAZOLONES. AND THEIR PRODUCTION AND USE
This application is a division of our parent Canadian
application Serial No. 519,296 filed on September 29, 1986.
This invention relates to benzothiazolones, and
their production and use. More particularly, it relates to
novel benzothiazolones, a process for producing them, and
their use as herbicides.
Some benzothiazolone derivatives (e.g. 4-chloro-
2,3-dihydro-2-oxobenzothiazol-3-ylacetic acid (benazolin))
are known to be effective as herbicides [~erbicide ~andbook
of the Weed Science Society of America, 5th Ed., p. 40 --
(1983)]. However, their herbicidal activity is not neces-
sarily satisfactory.
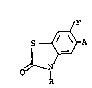
It has now been found that the benzothiazolones of
the formula:
~ , O
~ ( T )
. l O
~ .''''' , .
wherein R is a hydrogen atom, a Cl-C5 alkyl group, a C3-C4
alkenyl group, a C3-C4 alkynyl group, a halo(Cl-C4)alkyl
group, a halo(C3-C4)~1kenyligroup, a halo(C3-C4)alkynyl
group, a Cl-C2 alkoxy(C1-C2)alkyl group, a Cl-C2 alkoxy-
(Cl-C2)alXoxy(Cl-C2)alkyl group, a cinnamyl group, a cyano-
(C1~C3)alkyl group, a carboxy(Cl-C3)alkyl group, a Cl-C5
a}koxycarbonyl(Cl-C3)alkyl group, a halo(Cl-C5)alkoxy-
carbonyl(Cl-C3)alkyl group, a Cl-C2 alkoxy(Cl-C2)alkoxy-
carbonyl(Cl-C3)alkyl group, a Cl-C5 alkoxycarbonyl(Cl-C2)-
.~ .~ ~
13304~3
- alkoxycarbonyl(Cl-C3)alkyl ~ ~ Cyclo(c3-c6)
carbonyl(C1-C3)al~yl group, a Cl-C5 alXylaminocarbonyl-
(Cl-C3)alkyl group or a di(Cl-C5)alkylaminocarbonyl(C1-C3)-
alkyl group;show a high herbicidal activity against a wide
variety of weeds including broad-leaved weeds, Graminaceous
weeds, Commelinaceous weeds and Cyperaceous weeds in agri-
cultural plowed fields by fol~ar or soil treatment without
producing any material phytotoxicity on various agricultural
crops such as corn, sorghum, wheat, barley, soybean, pea~t
.,
and cotton. Examples of the broad-leaved weeds are wild
buckwheat (PolY~onum convolvulus), ladysthumb ( olYqonum
pessicaria), pale smartweed (PolY~onum lapathifolium),
common purslane (Portulaca oleracea), common chickweed
(Stellar~a media), common lambsquarters (Chenopodium album),
redroot pigweed (Amaranthus retroflexus), xadish (Raphanus
sativus), wild mustard (Sinapis arvensis), shepherdspurse
CaPsella bursa-Pastoris)~ hemp sesbania (Sesbania
exaltata), sicklepod (Cassia obtusifolia), velvetleaf
(Abutilon theoPhrasti), prickly sida (Sida spinosa), field
pansy ~Viola arvensis), catchweed bedstraw ~Galium aParine),
ivyleaf morningglory (IPomoea hederacea), tall morningglory
Ipomoea purPUrea)~ field bindweed ~Convolvulus arvensis),
jimsonweed ~Datura stramonium), black nightshade [Solanum
ni~rum), persian speedwell (Veronica persica), common
cocklebur (Xanthium p~nsYlvanicum), common sunflower
~ ~ (Helianthus annuus), scentless chamomile (Matricaria
,; perforata), corn marigold (ChrYsanthemum seqetum~, sun
.~., .
i 1~ spurge ~EuPhorbia helioscoPia)~ etc. Examples of Grami
,,. .
~, ':
--- -- 3 --
-: 133044~
naceous weeds are Japanese millet (Echinochloa frumentacea),
barnyardgrass (Echinochloa crus-qalli), sicklepod (Cassia
obtusifolia), large crabgrass (Diqitaria sanquinalis),
annual bluegrass (Poa annua), blackgrass (AloPecurus
mYosuroides), oats (Avena sativa), wild oats (Avena fatua),
johnsongrass (Sor~hum halePense), quackgrass (AqroPyron
repens), downy brome (Bromus tectorum), bermudagrass
(C~nodon dactylon), fall panicum (Panic~m dichotomiflorum),
etc. Examples of Commelinaceous weeds are asiatic dayflower
(Commelina communis), etc. Examples of the Cyperaceous
weeds are rice flatsedge (CYperus iria), etc.
- It is particularly notable that the-benzothiazolones
(I) exert a substantial herbicidal activity upon soil appli-
cation before ger~ination of undesired weeds with no material
chemical injury. For instance, they show a high herbicidal
potency on the broad-leaved weeds such as velvet-leaf, common
cocklebur, tall morningglory, sicklepod, prickly sida, jim-
sonweed, hemp sesbania, redroot pigweed, common lambsquarters
and black nighshade in fields of corn but afford no material
phytotoxicity to corn. In fields of sorghum, they exhibit a
good herbicidal activity on the broad-leaved weeds such as
redroot pigwed, velvetleaf, ivyleaf morningglory, tall morn-
ingglory and common cocklebur as well as Graminaceous weeds
such as johnsongrass, but exert no chemical injury on sorghum.
Undesired broad-leaved weeds such as catchweed bedstraw, com-
:~:
~ mon chickweed, field pansy, persion speedwell, scentless cham-
:
~ omile, pale smartweed, wild mustard, common lambsquarters,
:~
.';
,~
~ ,~
~; ~ ~
,: ~:
~ .
- 133~ 3
black nightshade and field bindweed and redroot pigweed in
fields of wheat or barley can likewise be sufficiently con-
trolled without exerting any material phytotoxicity on wheat
or barley. Problematic broad-leaved weeds in fields of soy-
beans or peanuts such as velvetleaf, common cocklebur, tall
morningglory, sicklepod, prickly sida, jimsonweed, hemp ses-
bania, redroot pigweed, common lambsquarters and black night-
shade, can also be controlled with no material phytotoxicity
to the soybeans or peanuts. Further, the compounds of the
invention are highly effective in controlling broad-leaved
weeds such as redroot pîgweed, prickly sida, ivyleaf morning-
glory, tall morningglory, black nightshade and common cockle-
bur in fields of cotton without causing any material chemical
injury to the cotton.
The benzothiazolones (I) of the invention are al-
so effective in exterminating paddy field weeds including
Graminaceous weeds such as barnyardgrass (Echinochloa ory-
zicola), broad-leaved weed such as common falsepimpernel
(Lindernia Procumbens)~ indian toothcup (Rotala indica) and
.,. ~ -~
waterwort (Elatine triandra), Ciperaceous weeds such as Sm.
fl. umbrella sedge (CYperUs difformis), hardstem bulrush,
(Scirpur i~_coides) and needle spikerush (Eleocharis acicu-
larLs) and others such as monochloria (Monochloria vaqinalis)
and arrowhead (Saqittaria ~vq~aea) without producing any
phytotoxicity to rice plants on flooding treatment.
, Among the benzothiazolones (I), preferred are
those wherein R is Cl-C5 alkyl, C3-C4 alkenyl, C3-C4
.,~ ,
;~:
.~
:~:
~ ~ .
p" !~
- s - :
~3304~3
alkynyl, halo(Cl-C4)alkyl, halo(C3-C4)alkyl, halo(C3-C4)-
alkynyl, Cl-C2 al~oxy(C1-C2)alkyl, Cl-C2 alkoxy(C1-C2)-
alkoxy(C1-C2)alkyl, etc. More preperred are those wherein R
is C2-C4 alkyl, C3-C4 alkenyl, C3-C4 alkynyl group, halo-
propynyl, etc. The most preferred are those wherein R is
C2-C3 alkyl, C3-C4 alkenyl, C3-C4 alkynyl, etc. Typical
examples of the preferred compounds are 2-~6-fluoro-3-(1-
methylethyl~-2(3H)-benzothiazolon-5-yl]-4,5,6,7-tetrahydro-
2H-isoindole-1,3-dione, 2-[6-fluoro-3-(2-propenyl)-2(3H)-
benzothiazolon-5-yl]-4,5,6,7-tetrahydro-2H-isoindole-1,3-
dione, 2-t6-fluoro-3-~2-propynyl)-2(3H)-benzothiazolon 5-
yl]-4,5,6,7-tetrahydro-2H-isoindole-1,3-dione, etc. ; -
The benzothiazolones (I) of the invention are
obtainable by reacting an amine compound of the formula:
. /F
~ NH
~ ~ (II)
~ N
wher-in R is as definëd above with 3,4,5,6-tetrahydrophthalic
; ~ anhydride, usually in a solvent at a temperature of 80 to
200-C for a period of 1 to 24 hours. The compounds of formula
~ tII) above are the subject of the present divisional application.
...
Thus, according to the invention there is provided a
~ compound`of the formula
:~. /F
~ S ~ A (II)
';~
l ~
.
~ .
~ ! ~ ,. .~ ~ . .. ,. ' i - ` " ~" `
- 6 - ~ 33~
wherein R is a hydrogen atom, a C,-C5 alkyl group, a ~-C4 alkenyl
group, a C3-C4 alkynyl group, a halo(C1-C4)alkyl group, a halo-
(~-C4) alkenyl group, a halo(C3-C4)alkynyl group, a C1-C2 alkoxy-
(c1-c2)alkyl group, a c1-c2 alkoxy(C1-C2)alkoXy(C1-C2)alkYl group,
a cinnamyl group, a cyano(C1-~)alkyl group, a carboxy(C1-~)alkyl
group, a C1-C5-alkoxycarbonyl(C~-C3)alkyl group, a halo(C1-Cs)alkoxy-
carbonyl(C1-~)alkyl group, a cl-c2 alkoxy(C1-C2)alkoxycarbonyl
(C1-C3)alkyl group, a C1-C5 alkoxycarbonyl(C1-Cz)alkoxycarbonyl-
' (C1-C3)alkyl group, a cyclo(C3-C6)alkoxycarbonyl(C1C3)alkyl group,
: a (C~-Cs)-alkylaminocarbonyl(C~-~)alkyl group or a di(C1-C5)-
alkylaminocarbonyl (C~-C3) alkyl group and A is an amino group or a
~ nitro group. The compound is useful as an intermediate for the
3 preparation of herbicides when reacted with 3,4,S,6-tetrahydro
3 phthalic anhydride.
The 3,4,5,6-tetrahydrophthalic anhydride may be used in an
amount of about l to 3 equivalents to the amino compound (II).
Examples of the solvent are aliphatic hydrocarbons (e.g. hexane,
heptane, ligroin), aromatic hydrocarbons (e.g. benzene, toluene,
xylene), ethers (e.g. diisopropyl ether, dioxane, ethylene glyol
i ~ dimethyl ether), fatty acids (e.g. formic acid, acetic acid,
propionic acid), water, etc. Their mixtures are also usable.
After completion of the reaction, the reaction mixture is -
subjected to ordinary post-treatment such as addition of water and
collection of precipitated crystals by filtration. Also, it may be
,:,
subjected to extraction with an organic solvent and concentration.
If desired, any conventional purification procedure such as
chromatography or recrystallization may be adopted.
Typical examples of the benzothiazolones (I) which may be
obtainable through the above procedure are shown in Table l.
~. ~
~ ~:
~ - 7 -
.. . .
13304~3
~ ~able 1
O
S ~ ~ ~ (T)
1 ' 0~ 0 ' '
I . R
R R R
~! 5 H CH2=CHCH2- CF3CF3-
.. , ,,p~
i . CH3 CH3CH=CHCH~- Cl\ / CH2
., 2 S CH2=f-CH2- H ~ Cl
', . n-C3H7 ca3
!j i C3 7 C6H5CH=c~c~2 Cl / Cl
n-C4Hg CH-CCH2- CH C /CH2
i-C4Hg CH3C3CCH2- 2 ~ Cl
~: sec-C4H~ CH-C-fH- ClCH2CH=CHcH2- :
t-C4Hg CH3 C~ (Cl~C=CHCH -
. n C5H11 FCH2- ClCH=CHCH2-
i C5H11 ClCH2- . C=C
C H ~ BrCH - H~ ~ r
.~ : 2 5 CHCH - 2
CH3~ F2CH- . ~ ~C C/ CH2
. 2-CSHll C12CH- Br / ~Br
3-C5Hll Br2CH- CH C/CH2
(CH3)2CHfH- . CF3- \Br
. CH3 CC13- BrCH2CH=cH~2
~i .
neo-C5H11 cBr3- CH3(Br)c=cHcH
3 \ FCH2CH2_ BrCH=CHCH2-
; CH3-/c- ClCH2CH2 ClC-CCH2-
~ C2H5 BrCH2C 2 BrC3CCH2-
::~
:,~ ~
~:
- - 8 -
; 13~04'~3
. ~ .; .. ,
R R R
IC3CCH2- Ho-C-fH-n-C3H70-1CI-fH- :
ClC_C-CH- 2 5 0 C~3
CH3 CH30-1CI-CH2-i-C3H70-1C-fH-
BrC=C-CH- 0 0 CH3
C 3 C2H50-C-CH2-~-C4H90-1Cl FH
IC-C-fH- 0 0 CH3
l~: CH3 n-c3H7o-lcl CH2i-C4HgO-C-f~-
CH30CH2- 0 CH3
C2~50CH2- i-c3~7-fi CH2sec-C4HgO-~-CH-
CH30C 2 2 0 CH3
C2H50CH2CH2 n-C4HgO-~-CH2-t-C4HgO-ICI-fH-
3CH2CH2- 0 CH3
CH30CH2CH20CH2- i-c4Hg-fi C~2n-C5Hll-ll-fH
3 C2H50CH20CH2 : 0 CH3 .
.. . CH30CH20CH2CH2- seC-c4H9o-B CH2 i-C5HliO-C-CH-
C2~5CH2CH2CH2 0 CH3
C2H5CH2CH2CH2 t-C4HgO-ICI-CH2- 3-C5Hl10-1CI-fH-
:. 15 C2H50CH2CH20CH2CH2 .0 CH3
NCCH2- n-C5H110-C-CH2- CH30-1Cl-cH2c~2
.j i NCCH2CH2- 0 0
NC-fH- i-C5H1l0-lc, CH2 C2H50-9-cH2cH2
CH3 0
HO-C-CH2- 3-C5~11-~9 CH2 n-C3H70~1CI~cH2cH2
O ,
: 20 H0-C-CH2CH2- CH30-C-fH-i-C3H70-fi-CH2CH2
`~ 0 0 CH3 0
:~ HO--ICI-fH- C2H50--C-fH--n--CJ~HgO--ICl-cH2c~2 -
~ :- 0 CH3 0 CH3
.,
~ :
~ ~ .. $ -.... ;.. - ~ ...... ~
_ 9 _
t33~
R . R
i-C4HgO--CI-CH2CH2--FCH2cH20-lcl-fH ~.
O O C~3
n-C5HllO~ji-cH2cH2ClcH2cH2o-lcl-fH-
O O CH3 .
i-c5Hllo-lcl-cH2cH2BrCH2CH20-jCI-fH-
. 0 0 CH3
FCH20-8-CH2 CH30CH20-C-CH2-
~ . O
ClCH20-C-CH2-C2H50CH20-ll CH2
O O 'i~
BrCH20-1CI-CH2- CH30CH2C 2 11 2
~, O O
FCEI2CH2 fi; CH2 C2H50CH2CH20 ~1 CH2
O
2CH20-1Cl CH2 CH30CH20-1-CH-
O CH3
~ .BrCH2CH20-1CI CH2 C2H50CH20-1CI-fH- '~
.. ~. O CH3: ~ lo ~1 f CH30CH2CH20-lCI-fH-
i: ~ O CH3 0 CH3
.ClCH20-1C~ - ' C2H50CH2CH20-1CI-fH--
~i O CH3 O CH3 ' ~-
.~ ~ BrCH20-CI-fH- ~CH30-1Cl-cH20-lcj ~H2
O CH3 0 0
C2H50-1CI-CH20 lCI C~2
O O
:`..
-- 10 --
1 3 3 o ~ ~ 3
R R
CH30-1CI-fHO-ICl-CH2- C2H NH-C-CH2--
CH30-1CI-CH20-~ CH- n-c3H7NH-fi CH2
O O CH3 0
C2H50-lcl-fHo-lcl C~2 i-C3H7NX-ICI-CH2
o C~3
C2H50-CI-CH20-1j-fH- n-C4~19NH-fi-C~2-
O O C~3 0
Il S 11 f 11 f i-C4HgNH~fi~CH2~
0 ~3 0 CH3
n-C H NH-C-CH -
5 11 ll 2
O ~ .
i-c5Hl lNH-ICI CH2
D---ICI--CH2CH2-- ~'
O n-C3H7NX~ICl~lcH~
i-C3H7NH-C-fH-
. O CH3
-~-CH2cH2- . n-C4HgNH-CI-l~-
O O CH3 ¦
~ ICI ICH- i-C4HgNH~C~CH~
' ' n~C3H7NH~ICI~ ICH- ¦ .
O--ICI--ICH-- C2H5
~, ~ O C~3 i-C3H7NH-fi-lH-
;~:: O C2H5
,~ CH3NH-ICl-CH2_
:~ O
~ 1
x~ `
: . 1330~'~3
R R
n-c4H9~H-lcl-clH- 3 - N-~-CH-
C2H5 n-c4~9 I CH3
i-c4H9NH-lc-cH- C 3
C2~5 n-C5Hll ' 11 f
(CH3)2N-ll CH2 0 CH3
(C2H~)2N-lcl-clH-
CH3~ 0 CH3
C2H5/ ~j
. O
CH3~
n-c3H7 / fi 2
O
3~N-C--CH2--
n-C 4Hg/
.- CH3\
N-C-CH2- '`
n-CSHll/ 11
2 5 \ N-C-CH2- -`
2HS / 11
(CH ) N-C-CH-
O CH3
103 ~ N-C-CH-
,;;~ ~ . C2H5 o C}~
n-c3H ~ 11 f
~,
.
~o ~ . ,.
r ~ 12 -
1330~3
Practical and presently preferred embodiments for
production of the benzothiazolones (I) are illustratively
' shown in the following Examples.
I Example 1
3 5 S-Amino-6-fluoro-3-(2-propynyl)-2(3H)-benzothia-
zolone (0.43 g) and 3,4,5,6-tetrahydrophthalic anhydride
, (0.32 g) were suspended in acetic acid (5 ml), and the
resultant suspension was heated under reflux for S hours.
I After being allowed to cool, water was added thereto, and
;~ ~0 the resultant mixture was extracted with ethyl acetate. The
extract was washed with water and an aqueous sodium bi-
carbonate solution, dried and concentrated. The residue was
purified by silica gel thin layer chromatography with a
mixture of ethyl acetate and hexane (1 : 4) to give 2-t6-
fluoro-3-~2-propynyl)-2(3~)-benzothiazolon-5-yl]-4,5,6,7-t-
etrahydro-2H-isoindole-1,3-dione (0.13 g). m.p., 193 - ¦
; 195C. Recrystallization from 2-propanol gave the purified
pr~duct. m.p., 198 - 199C. ¦
IR ~XBr, CHC13) cm 1 3300, 3020, 1720, 1685,
~490, 1385, 1215, 760, 665.
,~ i
HNMR (CDC13, ~): l.S - 2.0 (4H, br.~, 2.1 - 2.6
I (SH, br.), 4.61 (2H, d, J = 2.6 Hz), 7.06 (lH, d, J = 6.0
,
Hz), 7.;27 (lH, d, J = 9.0 Hz).
Example 2
~; 25 5-Amino-3-ethoxycarbonylmethyl-6-fluoro-2~3H)-
benzothiazolone (140 mg) and 3,4,5,6-tetrahydrophthalic 1
anhydride (90 mg) were suspended in acetie acid (3 ml), and
~¦ the resultant suspension was heated under reflux for 5
~.. ~ :
. ~
.~i : ~:
: ~: ,"~: :.:: ,- ~,. ",". ,,.,,,,.,"",.,: "," .
_ l3 ~3
....
hours. After being allowed to cool, water wa~ added
thereto, and the resultant mixture was extracted with
' ethyl acetate. The extract was washed with water and
¦ sodium bicarbonate solution, dried and concentrated. The
, 5 residue was purified by silica gel thin layer chromato-
7, graphy with a mixture of ethyl acetate and hexane ~1 : 2)
i to give 2-[3-ethoxycarbonylmethyl-6-fluoro-2~3H)-benzothia-
zolon-5-yl]-4,5,6,7-tetrahydro-2H-isoindole-1,3(2H)-dione
(40 mg). M.p., 155.5 - 156.5C.
In the same manner as above, the benzothiazolones
(I) as shown in Table 2 were obtained.
~ .
li~ .
::~
:~;
~ .
.. -- 1~ --
~3304~3
.-.
- Table 2
o
O ~
O
. _ _ _
: Compound R Physical property
No. .-
l H m.p., 247.5C
1, 2 CH3 m.p., 189.5-190.5C
I . 3C2H5 - m.p., 117-119C
:. 4n~C3H7 m.p., 158-159C
5i-C3H7 n24.4 1 5633
6~ n-C4Hg m.p., 183-184C :~
. . 7i-C4Hg m.p., 182.5-184.0C
. 8sec-C4~9 m.p., 180.5-181.0C~
. 9 n-C5H11 . nD 1.5691
. . 10 i-C5H11 nD1 7 1.5640
~ 15 11 3-C5Hll m.p., 168.5-170.0C
,: . 12 CH2=CHCH2- m.p., 136.5-138.5C
s . 13 CH3CH-CHCH2- m.p., 152.5-154.0C
14 f m.p., 85-86C -.
. ; ' CH3 . .
C6H5CH=CHCH2- m.p., 62.5-64.0C
16 CHaCCH2- m.p./ 193-195C
~ . 17 CH=C-fH- m.p., 110-111
:~ . CH3 _ _ I _
~'
.~
::
~ '
-- 15 --
1 3 3 ~
(Continued)
. . .
No. R Physical property
18 FCH2CH2 n25 2 1.5763
19 ClCH2CH2- m.p., 180.5C
BrCH2C 2 m.p., 195C
21 Cl \ / CH2 m.p., 59-60C
j H Cl
,: 22 H \ / CH2- m.p., 191-192C
'~.'' /C=C\ .
~ 23 ~ CH2- m.p., 151-153C
. CH2=C ~ .
24 ClCH2CH=CHCH2- n2i.0 1 5824
CH3(Cl)C=CHCH2- nl8-5 1.5870
26 ClCH=CHCH2- n20.0 1 5844
27 BrC--CCH2- m.p., 192.5-194.0C
28 CH3OC~2- m.p., 155-156C
29 C2H5OCH2- m.p., 194-195C
CH3ocH2cH2ocH2 m.p., 123-125C
31 ~CCH2- m.p., 195-196C ;
, 32 NCCH2CH2 m.p., 192C
33 HO-C-C~2- m.p., 289.4C
, O .
34 fi m.p., 222.0-223.0C
,~,. '~ O .
1 35 I C2l5O-C-C52 1 ~ p., 155.5-156.5C I ~
'
..
- 16 -
:: - 13 3 0 4 ~ 3 (Continued)
. _ . _ _ .
Compound R Physical property
. . _
36 n-C5H11 ICl CH2 n27- 1.5520
37 CH30-C'-CH- m.p., 164.0-165.0C
38 CH3O-ICl-CH2CH2 m.p., 174.9C
¦ 39 ¦ n~C4HgO-C-CH2C52~ ¦ D 1.5616
¦ 40 ¦ C CH2CB2O-1C-CH2- 1 O9~0 1.5800
4i CH3OcH2cH2O 1l CH2 m.p., 200.5-202.0C
, 42 C2H5O-C-CH-O-fi-CH2- nD9' 1.5531
O CH3 O
. 43 ~ O-fi-CH2- n27-7 1.5508
~ . ' ,
. 44 ~ O-fi-CH2CH2- m.p., 80-82C
G O-ll-CH2CH2- n29 o 1.5752
; ¦ 46 ¦ (C53 2CHCH2N~-CI-I - ¦ 2 5 1.5383
47 3 N-C-CH- nD 1.5464
n-C H ~ 11 1
- 17 -
~3304~3
......
The amino compound (II) as the starting material
in the process of this invention may be produced according
to the following scheme:
~ .
R
reduc- ~ \ \ reduc-
tion / \ ~ ion
I / \ reduc-
F / \ tion - F
(III ~ ~
tion ~ ~ (VI)
: S ~ 2
o~ N H
R3 1 R4 (VII) ~ (VIII)
ni~r-
. ~ ~ ation
H2N ~ ~ F
- 18 -
.. '.,: ,, 1~304~3
wherein R is as defined above and R has the same meaning as
R except car~oxyethyl, 2-(Cl-C5)alkoxycarbonylethyl, 2-halo-
(Cl-C5)alkoxycarbonylethyl, 2-(Cl-C2)alkoxy(Cl-C2)alkoxy-
carbonylethyl, 2-(Cl-C5)alkoxycarbonyl(Cl-C2)alkoxycarbonyl-
ethyl, 2-cyclo~C3-C6)alkoxycarbonylethyl, 2-(Cl-C5)alkyl-
aminocarbonylethyl and 2-di(Cl-C5)alkylaminocarbonylethyl,
R
R2 has the same meaning as Rl except -CH-R4, R3 and R4 are
each Cl-C4 alkyl but the total numbers of carbon atoms in R3
and R does not exceed 4, X is chlorine, bromine, iodine,
methanesulfonyloxy or p-toluenesul~onyloxy and Y is
hydroxyl, Cl-C5 alkoxy, halo(Cl-C5)alkoxy, Cl-C2 alkoxy-
(Cl-C2)alkoxy, Cl-C5 alkoxycarbonyl(Cl-C2)alkoxy, cyclo-
(C3-C6)alkoxy, Cl-C5 alkylamino or di(Cl-C5~alkylamino.
Each reaction as set forth above will be herein-
after explained in detail.
(1) Production of the compound ~II) from the
compound (III), ~IV) or ~VIII) ~Procedure 1):-
The compound (II) is obtainable by reacting the
compound (III), (IV) or (VIII) with a reducing agent (e.g.
s 20 iron powder) at a temperature of 60 to 120C for a period of
10 minutes to 12 hours. Preferably, the reaction is
effected in a solvent such as acetic acid and ethyl acetate.
In the reaction, the reducing agent is used in an amount of
~;~ 3 to 30 equivalents, preferably of S to 20 equivalents, to
~` ; 25 one equivalent of the compound ~III), (IV) or lVIII).
After completion of the reaction, the residue is
collected by filtration, and the filtrate is washed with an
},~
-- 19 --
1~30~3
organic solvent. The extract is washed with water and a
sodium bicarbonate solution and subjected to concentration
to obtain the compound (II). If necessary, any purification
method such as recrystallization or chromatography may b~
applied to the product.
(2) Pro~uction of the compound lIII) from the
compound (VIII) (Procedure 2):-
The compound tIII) is obtainable by reacting the
compound (VIII) with the compound (V) in the presence o a
base (e.g. sodium hydride, potassium carbonate, sodium
hydroxide, potassium hydroxide) at a temperature of 0 to
120C for a period of 30 minutes to 24 hours in a solvent
such as an aromatic hydrocarbon (e.~. toluene, benzene),
an amide (e.g. N,N-dimethylformamide), a sulfur compound
15. (e.g. dimethylsulfoxide), a nitrile (e.g. acetonitrile) or
water, or their mixture. -In the reaction, the compound (V~
. and the base are respectively used in 1 to 1.5 equivalents
to one equivalent of the compound (VIII). The recovery of
the compound (III) can be accomplished by adding water to
the reaction mixture, extracting the resultant mixture with
an organic solvent and concentrating the extract. When
desired, any purification procedure such as recrystalli-
za~ion or chromatography may be adopted.
(3) Production of the compound (IV) from the
compound IVIII) (Procedure 3):-
The compound (IV) can be obtained by reacting the
compound (~III) with the compound tVI) in the presence of a
base (e.g. benzyl trimethylammonium hydroxide) at a temper-
^ 20
133~3
ature of 50 to 100C for a period of 30 minutes to 24 hours,
ordinarily in a solvent such as an aromatic hydrocarbon
(e.g. toluene, benzene), an amide ~e.g. N,N-dimethylform-
~mide), a sulfur compound (e.gO dimethylsulfoxide), a
nitrile (e.g. acetonitrie) or water, or thier mixture. In
the reaction, the compound (VI) and the base are respec-
tively used in 1.0 equivalent to excess and l.0 to 3 equi-
valents to one equivalent of the compound (VIII). The
- recovery of the compound ~IV) can be accomplished by adding
water to the reaction mixture, extracting the resultant
mixture with an organic solvent and concentrating the
extract. When desired, any purification procedure such as
recxystallization or chromatography may be adopted.
(4) Production of the compound (VIII) (Procedure
4):-
- The compound (VIII) is obtained by reacting the
compound (X) with a nitrating agent (e.g~ a mixture of
sulfuric acid and nitric acid) at a temperature of -lO to
; 10C instantaneously or within 5 hours. In the reaction,
sulfuric acid and nitric acid are used respectively in 1
equivalent to excess and 1 to 1.2 equivalents to one
equivalent of the compound (X). After completion of the
reaction, the rèsultant mixture is subjected to conventional
post-treatment. For instance, it is poured into ice-water, -
and precipitated crystals are collected by filtration and
washed with water. When desired, the resulting product may
be further purified by recrystallization or chromatography.
Still, the starting compound (X) may be prepared
~ '
I
~ - 21 -
~330~3
according to the method as described in G. Mazzone and G.
Pappalaro: Farmaco, Ed., Sc., 32 (5~, 348 ~1977).
(5) Production of the compound (III) (Procedure
5):-
The compound (III) may be obtainable by reacting
the compound (VII) with a nitrating agent (e.g. a mixture of
sulfuric acid and nitric acid) at a temperature of -10 to
10C instantaneously or within 5 hours. In the reaction,
; sulfuric acid and nitric acid are used respectively in 1
equivalent to amount and 1 to 1.2 equivalents to one e~ui-
valent of the compound (VII). After completion of the
reaction, the resultant mixture is subjected to conventional
post-treatment. For instance, it is poured into ice-water,
and precipitated crystals are collected bv filtration and
washed with water. When desired, the resulting product may
be further purified by recrystallization or chromato~raphy
(6) Production of the compound (VII):-
,
. The compound ~VII) can be prepared from the
compound (IX) by the method as described in Japanese Patent
Publication (unexamined) Nos. 123480/1985 and 166673/1985.
Said intermediates, i.e. the co~pounds tII),
;.:.
~III), (IV) and ~VIII), are novel and can be represented by
the general formula: ~
" ' '`,
~F
S ~ A
I \ =J (XI)
~ ~ N/
¦~ R
~':
: ..
~ - 22 -
1330~3
.; .
wherein R is as defined above and A is amino or nitro.
Typical examples for production of said inter-
mediates are illustratively shown in the following examples.
Example 3 (Procedure 1)
Electrolytic iron powder (0.77 g) was suspended in
5 ~ acetic acid (l.S ml), and the suspension was heated to
80C. A solution of 3-allyl-6-fluoro-5-nitro-2(3H)-benzo-
thiazolone (0.70 g) in acetic acid (2.8 ml) and ethyl
acetate (2.8 ml) was added thereto. The resultant mixture
was heated under reflux at a temperature of 60 to 80C for 3
hours. After being allowed to cool, water and ethyl acetate
were added thereto, the precipitate was collected by filtr-
ation, and the filtrate was extracted with ethyl acetate.
The extract was washed with water and sodium bicarbonate
solution, dried and concentrated to give 0.60 g of 5-amino- ;~
~- 3-allyl-6-fluoro-2(3H)-benzothiazolone. nD 1.6236
Example 4 (Procedure 1)
Electrolytic iron powder (0.2g g) was suspended in
S % acetic acid ~2 ml), and a solution of 3-ethoxycarbonyl-
methyl-6-fluoro-5-nitro-2~3H)-benzothiazolone (0.31 g) in
.. .~
acetic acid (1 ml) and ethyl acetate (1 ml) was dropwise
added thereto while heating. The resultant mixture was
heated under reflu'x for 3!hours. The reaction mixture was
subjected to filtration with celite, and the filtrate was
extracted with ethyl acetate. The extract was washed with
~, ~
water and sodium bicarbonate solution, dried and concen-
trated to give 0.20 g of 5-amino-3-ethoxycarbonylmethyl-6-
fluoro-2(3H)-benzothiazolone. m.p., 172.5 - 173.5C
~:
'~
~
.~
;~
- 23 ~-3 3 ~ ~ ~ 3
In the same manner as above, the compounds (II) as
shown in Table 3 were obtained.
Table 3 ~ ~ -
S ~NH2
(II~
O~ I " - ,
. .
.,. _ ~ ::
R Physical property
H _ _
CH3 m.p., 188-189C
C2H5 - m.p., 117-119C
n C3 7 n25-3 1.6000
i-C3H7 n24'4 1 6056
~ ~ n-C4Hg nD 1.5910 ~ -
; i-C4Hg m p., 113-114C
; sec-C4Hg nD 1.5933
n C5Hll n22 0 1.5691
i-C5H11 n21 7 1.5824
i- 3 C5Hll m.p., 102C
CH2=CHCH2- nD 1.6236 ~ -
CH2=C-CH2- m.p., 96-97~C
~, 3 ~ j , , ~
; C6H5CH=C~CH2 m.p., 104.5-105.0C '
CH_CCH2- m.p., 124-126C
~; CH--C-C~- m.p., 128.5-129.5C
,- 3
"~ . . _ . .
. . ~ .
,,~
, ~ .
- 24 -
4 ~ 3
. (Continued)
_ _
R Physical property .
_ _ _
2C 2 m.p., 133-134C
ClC 2C 2 m.p., 126.5-128.0C
BrCH2CH2 m.p. ! 113-114C
C1 C C/ C 2 m.p., 140-142C
Cl/ ~ Cl m.p., 142.5-l43.5C
/ CH2- m.p., 136-137C
ClCH2CH=CHCH2- m.p., 237-239C
0 ~3C ~C C/ H m.p., 103-104C .
C1 / \ CH2- :-
C=C/ m.p., 90.5-91.0C-
H3C / \ CH2 .
C=C/ m.p., 139-140C
. Cl / ¢ CH2- n21 0 1.6323
.. BrC_CCH2- m.p., 174.5-175.0C
CH3OCH2- . m.p., 112-113C . ~-:
2H5C 2 m.p., 77-78C .
:: CH3CH2CH2CH2 m.p., 99.5-100.5C ;~
NCCH2- . m.p., 153.5-154.5C
~ NCCH2CH2 m.p., 175C
1:
1:
l ~'
I ~;~
.
- 25 -
- ~ 1330~3
.. ~ ..................... .
(Continued)
_ . _ _ _ _
R Physical property
H0-fi-CH2- resinous
CH30-1CI-CH2- m.p., 155.5-156.5C
O .
5 C2H50-C, CH2 m.p., 172.S-173.5C
:. ll
,' ' O .
. n-C5H1l0-c CH2 ~7-7 1.5651
11
.. O
CH30-C-CH- 'm.p., 120.0-125.0C.
11 1
0 CH3
CH30-fi-CH2CH2 m.p., 124.1C
O
~ n-C4HgO~~ H2cH2 m.p., 79.1C
lo CH30CH2CH20 fi CH2 m p.~ 155-157C
~ ' . O ~ .
.,.................. C2H50-1CI-fHO fi CH2 visous solid
. 0 CH3 0
,,.,$
~ 0-C-CH2- ' n27'7 1.5642
~ O i . . , ~
~: ~ 0-1CI-CH2CH2- n28.0 1 5744
.~ ,. O
.~
,,
~:
~:
~:
~ .
~ i.", ~
~; ' '.". . .~ ~ ` ~ . i. ' ',' . ' ', ` ': ~ r
l r , . ~
- 26 -
~ 330~3
~Continued)
. _ .
R Physical property
. ,
O-fi-CH2CH2- nD90 1.5655
O . .
(CH3)2CHCH2NH-Il-fH- resinous
C2H5
3~ N-C-C~- nD8 1.5556
n C4 9 1l IH I
Example S (Procedure 2)
60 ~ Oily sodium hydride 10.21 g) was suspended in
N,N-dimethylformamide (7 ml~, and the resultant suspension
was cooled to 0C. 6-Fluoro-5-nitro-2(3~)-benzothiazolone
(1.0 g) was added thereto at 0 to 5C, and the mixture was
.. 10 stirred for 30 minutes. Allyl bromide (0.62 g) was added to
the reaction mixture, and the temperature was gradually
elevated to a temperature of 50 to 60C. The mixture was .
allowed to re~ct at that temperature for 3 hours. Water was
added to the mixture, which was extracted with ethyl
acetate. The extract was washed with water, dried and
concentrated. The residue was purified by silica gel thin
. layer ahromatography with a mixture of ethyl acetate and
; toluene (1 : 9) to give 0.70 g of 3-allyl-7-fluoro-6-nitro-
2(3H)-benzothiazolone. m.p., 112.5 - 113.5C.
Example 6 (Procedure 2)
50 % Oily sodium hydride (0.21 g) was suspended in
::
~; N,N-dimethylformamide (5 ml), and the resultant suspension
':
x,.
r, ~
- 2~ -
~33~L3
was cooled to 0C. 6-Fluoro-5-nitro-2(3H)-benzothiazolone
(1.0 g) was portionwise added thereto at 0C, and the
mixture was stirred at the sam,e temp~rature for 30 minutes.
Ethyl bromoacetate (0.86 g) was added to the reaction
mixture at 0C, and the temperature was gradually elevated
to a temperature of 50 to 60C, followed by stirring at that
temperature for 3 hours. Water was added to the mixture,
which was extracted with ethyl acetate. The extract was
washed with water, dried and concentrated. The residue was
purified by silica gel thin layer chromatography with a
mixture of ethyl acetate and toluene (1 : 9) to give 0.35 g
of 3-ethoxycarbonylmethyl-6-fluoro-5-nitro-2(3H)-benzothia-
zolone. m.p., 139.0 - 140.0C.
Example 7 (Procedure 3)
A mixture of 6-fluoro-S-nitro-2(3H)-benzothia-
. zolone (1.0 g), methyl acrylate (5 ml) and a 40 % methanolic
solution of benzyltrimethylammonium hydroxide (1.95 g) was
heated under reflux for 2 hours, followed by cooling; Water
was added to the reaction mixture, which was extracted with
; 20 ethyl acetate. The extract was washed with water, dried and
; concentrated to give 1.12 g of 6-fluoro-3-(2-methoxy-
carbonyl)ethyl-5-nitro-2(3H)-benzothiazolone. m.p.,
224.3C.
Example 8 (Procedure 5)
` 25 6-Fluoro-3-(1-methylethyl)-2(3H)-benzothiazolone
(299 g~ was dissolved in conc. sulfuric acid (8750 g), and
the resultant mixture was cooled to a temperature of -5 to
0C. 98 % fuming nitric acid (d = 1.52) (94.68 g) was
- 28 -
~330~
.......
dropwise added thereto while keeping tAe temperature at 0C,
followed by stirring for 1 hour. The reaction mixture was
poured into ice-water (16 kg), and the precipitated crystals
were collected by filtration. Crystals were washed with
water and dried to give 325 g of 6-fluoro-3~ methyle~hyl)-
5-nitro-2(3Hj-benzothiazolone. m.p., 153-153.5C.
In the same manner as above, the compounds ~III)
or (IV) as shown in Table 4 were obtained.
Table 4
. . .
S ~N02
~ ~
O ~.
_ _ .
R Physical property
CH3 m.p., 148-149 C
C2H5 m.p., 128-128.5C :
n-C3H7 m.p., 71-73C
i-C3H7 m.p., 153-153.~C
n ~4H9 m.p., 83-84C
i C4~9 m.p., 101.5-103.0C
sec-C4Hg m.p., 87-88C
n-C5Hll m.p., 68.5-69.5C
i C5 11 n22 0 1.5900 .3 C5H11 m.p., 110-111C
CH2=CHCH2- m.p., 112.5-113.5C
CH3CH=CHCH2- m.p., 84-85C
.
~' . .
.;~
:~
:
- 29 -
1 3 3 0 4 4 3
(Continued)
_ _ .
R Physical property
_ _
f m.p., 83-84C
CH3
C6H5CH=CHCH2 m.p., 106-107C
CH-CCH2- m.p., 123.5-124.5C
CH-C-CIH- m.p., 183.5-184.5C
CH3
FCH2CH2- m.p., 136-137C
ClCH2CH2- m.p., 125-126C
BrCH2CH2 m.p., 128.5-129.5C
Cl ~ /C~2 n22 5 1.6224
C-- ~
H \ / CH2- m.p., 133C
, .' /C=C\ .
; ~CH2- n22-2 1.6340
CH2=C\
ClCH2CH=CHCH2- ~1.7 1.6217
CH3(~l~c=cHcH2- n18 5 1.6153
ClCH-CHCH2- n18 5 1.6287
BrC3CCH2- m.p., 134.5-136.0C
CH30CH2- m.p., 125-126C
C2H5OCH2- m.p., 1'14-115C
3C~2CH2CH2 m.p., 78-80C
. NCCH2- m.p., 146.5-147.5C
NC 2 2 m.p., 174-176C
HO-C-C~2- m.p., 264.2C
o
.:~
~ .
- 30 -
~L330~3
, (Continued)
. Physical property
O m.p., 163.5-165.0C
C2H5O-~-CH2- m.p., 139.0-140.0C .
.. n-cSHllo-c~ CH2 m.p., 75.0-76.0C
¦ O CH3 ¦ m.p., 124.5-125.5C
CH30- IJ -CH2CH2 m.p., 224.3OC
n-C4HgO-Il-CH2CH2- m.p., 79.1C
. ClCH2CH2O 1l C~2 m.p., 118.0-120.0C
. CH3OCH2CH2O jl CH2 m.p., 90.5-91.5C .
C2H5O-CI-ClHO-Cl-CH2- m.p., 110.0-112.0C
: . O CH3 O
-lj-C32 1 ~.p., 1-1.5-123.0C
O-CI-C~2 32- I nD9'0 1.5914
O-ICl-CH2CH2- . nD 1.5743
.. ''~ ~ O _ ~
:
;;'~
- 31 -
13304L43
tContinued)
~ _ _
R Physical property
28 5~
3~ N-C-C~- nD 1.5445
n-C H ~ ¦¦ ¦
Example 9 (Procedure 4)
6-Fluoro-2(3H~-benzothiazolone (47.58 g) was
S dissolved in 100 % sulfuric acid (760 ml), and the resultant
; mixture was cooled to 0 to 5C. 98 % fuming nitric acid (d
- 1.52) (18.79 g) was gradually added thereto at a temper-
ature of 0 to 5C, followed by stirring at the same temper-
ature for 60 minutes. The reaction mixture was poured into
ice-water. The precipitated crystals were collected by
filtration, washed with water and air-dried to give 48.48 g
of 6-fluoro-S-nitro-2(3H)-benzothiazolone as pale brown
.
crystals. m.p., 180 - 182C. -
On the practical usage of the benzothiazolo~es
(I), they may be applied in conventional preparation forms
such as emulsifiable concentrates, wettable powders, sus-
pensions and granules in combination with conventional solid
or liquid carriers or diluents as well as surface active
agents or auxiliary agents. The content of the benzothia- -
~; zolones (Ij as ~he active ingredient in such preparation `
forms is usually within a range of 0.05 to 90 % by weight,
preferably of 0.1 to 80 % by weight. Examples of the solid
...
`~ carrier or diluent are fine powders or granules of kaolin
,~ .
clay, attapulgite clay, bentonite, terra alba, pyrophyllite,
talc, diatomaceous earth, calcite, walnut powders, urea,
.
!
- 32 -
i3~0~3
ammonium sulfate and synthetic hydrous silicate, etc. As
the liquid carrier or diluent, there may be exemplified
aromatic hydrocarbons (e.g. xylene, methylnaphthalene),
alcohols (e.g. isopropanol, ethylene glycol, cellosolve),
ketones (e.g. acetone, cyclohexanone, isophorone), soybean
oil, cotton seed oil, dimethylsulfoxide, N,N-dimethylform-
amide, acetonitrile, water, etc.
The surface active agent used for emulsification,
dispersion or spreading may be any of the anionic and
non-ionic type of agents. Examples of the surface active
agent include alXylsulfates, alkylarylsulfonates, dialkyl-
sulfosuccinates, phosphates of polyoxyethylenealkylaryl
ethers, polyoxyethylene alkyl ethers, polyoxyethylene
alkylaryl ethers, polyoxyethylene polyoxypropylene block
~ 15 copolymer, sorbitan fatty acid esters, polyoxyethylene
- sorbitan fatty acid esters, etc. Examples of the auxiliary
agents include ligninsulfonates, sodium alginate, polyvinyl
alcohol, gum arabic, CMC (carboxymethyl cellulose), PAP
(isopropyl acid phosphate), etc.
Practical embodiments of the herbicidal composi- ;
tion according to the invention are illustratively shown in
the following examples wherein parts are by weight. The
compound number oflthe active ingredient corresponds to the
one in Table 1. 1 -~
:
Formulation Example 1
Fifty parts of Compound No. 2, 4, 34 or 35, 3
~` parts of calcium ligninsulfonate, 2 parts of sodium lauryl-
sulfate and 45 parts of synthetic hydrous silicate are well
. ~ ~
~: :
- 33 -
~3304~3
mixed while being powdered to obtain a wettable powder.
Formulation Example 2
Five parts of Compound No. 5, 14 parts of poly-
oxyethylenestyrylphenyl ether, 6 parts of calcium dodecyl-
S benzenesulfonate, 30 parts of xylene and 45 parts of cyclo-
hexanone are well mixed to obtain an emulsifiable concen-
trate.
Formulation Example 3
Ten parts of Compound No. 37 or 43, 14 parts of
polyoxyethylenestyrylphenyl ether, 6 parts of calcium
dodecylbenzenesulfonate, 25 parts of xylene and 45 parts of
cyclohexanone are well mixed to obtain an emulsifiable
concentrate.
Formulation Example 4
Two parts of Compound No. 3, 12, 16, 34 or 44, 1
part of synthetic hydrous silicate, 2 parts of calcium
ligninsulfonate, 30 parts of bentonite and 65 parts of
;~ ka~lin clay are well mixed while being powdered. The
".~ .
mixture is then kneaded with water, granulated and dried to
obtain granules.
_r~ L~ e~
Twenty-five parts of Compound No. 4, 16, 29, 37 or
41 is mixed with 3 parts of polyoxyethylene sorbitan~mono- -
oleate, 3 parts of carboxymethyl cellulose and 69 parts of
water and pulverized until the particle size of the mixture
- becomes less than 5 microns to obtain a suspension.
.,~:
Formulation Example 6
~ Five parts of Compound No. 2, 3, 4, 5, 12, 16, 17,
'~
,'~ :
:
~ - , . .. .
- 34 -
13304~3
28, 29, 31, 36 or 38, 14 parts of polyoxyethylenestyryl-
phenyl ether, 6 parts o calcium dodecylbenzenesulfonate, 30
parts of xylene and 45 parts of N,N-dimethylformamide are
well mixed to obtain an emulsifiable concentrate.
~he benzothiazolones (I) thus formulated in any
suitable formulation form are useful for the pre-emergence
or post-emergence control of undesired weeds by soil or
- foliar treatment as well as flood fallowing treatment.
These treatments include the application to the soil surface
prior to or after the transplanting or the incorporation
into the soil. The foliar treatment may be effected by
spraying the herbicidal composition containing the benzo-
thiazolones (I) over the top of the plants. It may also be
applied directly to the weeds if care is taken to keep the
chemical off the crop foliage.
The benzothiazolones (I) of the invention may be
used together with other herbicides to improve their
activity as herbicides, and in some cases, a synergistic
effect can be expected. Further, they may be applied in
combination with insecticides, acaricides, nematocides,
fungicides, plant growth regulators, fertilizers, soil
improvers, etc.
Furthermore, the benzothiazolones (I) can be used
as herbicides applicable to agricultural plowed field as
`-~ 25 well as paddy field. They are also useful as herbicides to
be employed for orchard, pasture land, lawn, forest, non-
agricultural field, etc.
The dosage rate of the benzothiazolones (I) may
.` ~ ~ .
~::
~ - 35 -
13304~3
vary on prevailing weather conditions, formulation used,
prevailing season, mode of application, soil involved, crop
and weed species, etc. Generally, however, the dosage rate
is from 0.02 to 100 grams, preferably from 0.05 to S0 grams,
of the active ingredient per are. The herbicidal composi-
tion of the invention formulated in the form of an emulsi- -
fiable concentrate, a wettable powder or a suspension may
ordinarily be employed by diluting it with water at a volume
of 1 to 10 liters per are, if necessary, with addition of an
auxiliary agent such as a spreading agent. Examples of the
spreading agent include, in addition to the surface active
agents as noted above, polyoxyethylene resin acid (ester),
ligninsulfonate, abietylenic acid salt, dinaphthylmethane-
disulfonate, paraffin, etc. The composition formulated in
the form of granules may be normally applied as such without
dilution.
The biological data of the benzothiazolones (I? as
herbicides will be illustratively shown in the following
Examples wherein the phytotoxicity to crop plants and the
herbicidal activity on weeds were observed visually as to
the degree of germination as well as the growth inhibition
and rated with an index 0, 1, 2, 3, 4 or 5, in which the
numeral "0" indicates no material difference is seen iin
comparison with the untreated plant and the numeral "5"
indicates the complete inhibition or death of the test
plants.
The compounds shown in Table 5 below were used for
comparison.
'~
::~ .
- 36
1330,~4~
- Table 5
_ _ _
Compound Chemical structure Remarks
No. _ _ ~ _ _
ACl CH COO~ Commercially
I ¦ 2 available herbi-
N~ cide "benazolin"
~S~O
_ _ , _ _
B Cl Commercially
available herbi-
. ~C~ cide "cyanazin"
. I ~ N .
. .N_C /C-N- ~ N~ C ~ C2 5 .
. . _
. Test Example 1
Cylindrical plastic pots (diameter, 10 cm; height,
. .-.
10 cm) were filled with upland field soil, and the seeds of ~ .
Japanese millet, oats, tall morningglory and velvetleaf were
.- sowed therein and covered with soil. A designed amount of.
: 10 the test compound formulated in an emulsifiable concentrate
. according to Formulation Example 2, 3 or 6 was diluted with
~ . water, and the dilution was sprayed onto the soil surface by
; means of a small hand sprayer at a spray volume of 10 liters :
per are. The test plants were further grown in a greenhouse
lS for 20 days, and the herbicidal activity was examined. The :
results are shown in Table 6. .
,~ ' .
.~
~' I
,~ I .
: ~(
1330~
Table 6
-
Compound Dosage Herbicidal activity
No. (g/are) .
Japanese Oats Tall Velvet-
millet morning- leaf
. glory
_ _ _
2 20 5 5 5 5
3 20 5 5 5 5
: 4 20 5 5 5 ~
S 20 5 5 5 S
6 20 5 5 . 5 5
7 20 g _ 5 5
8 22oo 5 5 55 5S
~ 4 4 5
12 20 5 5 5 5
13 20 4 4 4 5
14 20 4 4 5 5
16 20 5 5 . 5 5
17 20 5 5 5 5
18 20 5 5 5 5
19 20 5 5 5 5
4 4 5
22 20 5 _ 4 5
_ _ 5 5
. 26 20 . 5 4 5 5
. 27 20 5 5 5 5
. 28 20 5 5 5 5
29 20 5 5 5 5
. 5 5
31 20 5 5 5 5
32 20 5 5 5 5
.35 20 _ _ 5 5
. 37 20 5. _ 5 S
: 35 38 20 _ _ 4 5: 39 20 _ _ 5 5. 41 20 _ _ 5 5
. 44 20 _ , _ 5 5
.~ 45 20 _ _ 5 5
47 20 5 _ 5 5
: . ~ .
I , ''` '
l Test Example 2
l ~.
-~ Cylindrical plastic pots (diameter, 10 cm; height,
, ~
10 cm) were filled with upland field soil, and the seeds of
~ : Japanese millet, radish and velvetleaf were sowed therein
.,~
:~;45 and cultivated in a greenhouse for 10 days. A designed
,~
'~
. ;~!' .. '.", . '~ . , :, ~
- 38 -
133044~
amount of the test compound formulated in an emulsifiable
concentrate according to Formulation Example 2, 3 or 6 was
- diluted with water containing a spreading agent, and the
dilution was sprayed over the foliage of the test plants by
means of a small hand sprayer at a spray volume of 10 liters
per are. The test plants were further grown in the green-
house for 20 days, and the herbicidal activity was examined.
The results are shown in Table 7.
Table 7
Compound Dosage Herbicidal activity
No. (g/are) Japan~ise millet ¦ Radish ¦ Velvetleaf .
22o _ s5 ~ S
222oo 554 . 555 55
: 6 22o 5 55 5
8 20 5 . 5 S
:~ . i9o 22oo 55 55 55
11 20 5 5 S
12 20 5 5 5
~ 25 l5 22o . 5 5 55
.: 16 20 5 5 5. 19 20 5 5 5
.. 20 20 5 5 5
22 22oo 55 55 55
: 245 28 ' 5 55 5
22278 22 5 555 55
~ 29 20. 5 5 5
;~ 30 20 5 5 S
3312 220 5 5 55
~ 33 20 5 S 5 ~:.
..
., ':~
.
: "l,. i. ' '~
- 39 -
: 133~c~
- (Continued~
_ _ _ _
Compound Dosage Herbicidal activity
i No. (g/are) _
Japanese millet Radish Velvetleaf
34 20 5 _
38 20 4 5 5
39 20 4 5 5
401 20 5 55 5
42 20 5 5 5
43 20 5 5 5
~ 20 5 S 5 _
Test Exam~e 3
Cylindrical plastic pots (diameter, 8 cm,; height,
12 cm) were filled with paddy field soil, and the seeds of
barnyardgrass (Echinochloa orYzicola)~ broad-leaved weeds
(i.e. common falsepimpernel, indian toothcup, waterwort)
were sowed in 1 to 2 cm depth. Water was poured therein to
make a flooded condition. Buds of arrowhead were sowed in 1
to 2 cm depth, and rice seedlings of the 2.5-leaf stage were
transplanted therein, and the test plants were grown in a
greenhouse. Six days (at that time weeds began to germi-
nate) thereafter, a designed amount of the test compound
formulated in an emulsifiable concentrate~according to
Formulation Examle 2, 3 or 6 and diluted with water (5 ml?
was applied to ~he pots by perfusion. The test plants were
grown for further 20 days in the greenhouse, and the herbi-
cidai activity was examined. The results are shown in Table
.~
~-~ 8.
~:
. , " "
- 40 -
~33~qi~3
Table 8
- _ _ .
Compound DosageHerbicidal activity
No. tg/are) _ _ .
Rice Barnyard- Broad-leaved Arrow-
plant gxass wçed head
3 20 53 ~ 5 5 . 5
. 5 0 63 _ 5 5 5
12 2.5 _ 5 5 5
... . 0.63 0 . 5 5 5
. 16 0.63 _ 5 5 5
. 27 0 63 1 . 4 5 ~
37 0 63 1 5 5 5 ~ :
338 2 5 1 4 4 5
. 44 2.5 0 4 4 5
: 20 2.5 I 0 1 ¦ 3 1
1 0.63 0 1 0 l 0
~ ' _ ",'
Test Example 4
Vats (33 cm x 23 cm x ll cm) were filled with
upland field soil, and the seeds of soybean, peanut, cotton,
: 25 corn, tall morningglory, velvetleaf, redweed pigweed, black
nightshade, common lambsquarters, sic~lepod, barnyardgrass
: (Echinochloa crus-galli), johnsongrass and green foxtail
i~ were sowed therein to 1 to 2 cm depth. A designed amount of
the test compound formulated in an emulsifiable concentrate
30 according to Formulation Example 2, 3 or 6 was diluted with
: water, and the dilution was sprayed onto the soil surface by
,'r, ~ ~ means of a small hand sprayer at a spray volume of 10 liters
per are. The test plants were ~urther grown in a greenhouse
for 20 days, and the herbicidal activity was examined. The
- ~ . .
:~ 35 results are shown in Table 9.
~ ~ .
,
~ .
~.,, ," .,., .,,.,, " ,,.,.",,
133~3
.. ~ _
,~ u ulo 1'7 ~ O O
u7u~ oo
r o o
..
ff~}. ~r,, ~, ~u).ru~,,,, 1.., u~u~,, oo .
. ' _
' ~ U~ ~-~o
, , _, _ .
~ ~ u~ r~ O
e .
r I ~ O
~i Cr:g . . . ..
~ ~ ~5 u~ ~ O
.~ _
~ ~ u~ l I 00
~ ~ ~ .-
~ ,~oooooooooo,looooooo oo
. ~ ~ o_~ o,~o,-ooooo I r~O o oo ~,
.' ,~,,
,. ~ oIoooooooooooooooooo oo .,
. I _ ~ :
~ . or~ oooo_~o,ooooooooo oo ,'
~ _ _
~: 3 ~ ~ ~
1~ ~ t~ N ~ 1 ~ N ~ In t`i
~ . ~ :~
~ ' ~ ,~ N~.~I` r ~:
~:
~ .
- 42 -
13304~3
. Test Example 5
Vats (33 cm x 23 cm x 11 cm) were filled with
upland field soil, and the seeds of soybean, peanut, cotton,.
corn, sorghum, tall morningglory, common cocklebur, velvet-
leaf, sicklepod, black nightshade, prickly sida, hemp
sesbania, redroot pigweed, sun spurge, jimsonweed, common
lambsquarters, wild mustard, field bindweed, common
purslane, fall panicum, large crabgrass, green foxtail,
barnyardgrass (Echinochloa crus-qalli~ and johnsongrass were
sowed in 1 to 2 cm depth. A designed amount of the test
compound formulated in an emulsifiable concentrate according
to Formulation Example 2, 3 or 6 was diluted with water and
the dilution was sprayed to the surface of the soil by means
of a small hand sprayer at a spray volume of 10 liters per
are. The test plants were grown in outdoors for 20 days,
and the herbicidal activity was examined. The results are
shown in Table 10.
.
- ~.
;~
~'
,.~ :
~:
- 43 -
133~43
- Table 10
.
Herbicidal activity on Compound No. 16
\ Dosage -~~-
~ re) 2.5 . 1.25
Weed ~
._
Soybean 1 O
Peanut 1 O
Cotton _
Corn 1 O
Sorghum _ 1
Tall morning- 5 5
glory
Common cocklebur 5 _
Velvetleaf . 5 5
15 Sicklepod 5 4
Black nightshade 5 5
Prickly sida 5 5
Hemp sesbania . 5 _
Redroot pigweed 5 5
Sun spurge 5 5
Jimsonweed 5 5
Common lambs- 5 5
quarters
. Wild mustard 5 5
Field bindweed 5 5
Common purslane 5 5
Fall panicum . 5 5
. Large crabgrass 5 5
Green foxtail 5 5
Barnyardgrass . 5 4
Johnsongrass 5 3
_ _ _
Test Example 6
m Vats (33 cm x 23 cm x ll cm) were filled with
upland field soil, and the seeds of wheat, pale smartweed,
35 catchweed bedstraw, common chickweed, persian speedwell and
blac~grass were sowed in 1 to 2 cm depth. A designed amount
of the test compound formulated in an emulsifiable concen-
trate according to Formulation Example 2, 3 or 6 was diluted
with water, and the dilution was sprayed to the surface of
the soil by means of a small hand sprayer at a spray volume
: .
~\ ~
- 44
~3304~3
, of 10 liters per are. The test plants were grown in a
greenhouse for 27 days, and the herbicidal activity was
examined. The results are shown in Table 11.
Table 11
S C~d Dosage Herbicidal activity i
No. (g/are) .
. . ~eat Pale Catch- Cnan Persian Black-
smart- weed chick- speed- grass
_ weed straw ~ weed well _
2 1 SS l S _ S _ 1 5
3 2.5 0 5 3 5 5 4
1.25 0 5 _ 5 4 3
4 2.5 1 5 _ 5 5 3
l 55 1 55 -5 55 3 _
1.25 0 5 4 5 5 3
12 2.5 1 5 5 S 5 3 . .
1.25 û 5 5 5 5 _
16 2.5 1 5 _ 5 5 4
1.2S _ 5 _ 5 5 3
17 2.5 _ 5 5 ~ 5 5 :
1.25 0 5 5 5 5 4
18 2.5 _ 5 5 S 5 3
27 2.5 1 _ 5 _ 5 _
28 2.5 _ 5 5 5 5 5
1.25 1 5 3 5 5 5
29 2.5 1 S 3 5 S 4
3~ 1.25 0 5 _ 5 3 _
. 31 2.5 _ S 5 5 5 5
.. 1.25 1 5 _ 5 4 4
. 37 2.5 1 _ 3 5 5 _
. A 2.5 0 0 j 0 ~ 0 0 0
1.25 0 0 0 1 0 0 0
I ~ .
Test ExamPle 7
Vats (33 cm x 23 cm x 11 cm) were filled with ~:
`upland field soil, and the seeds of corn, common cocklebur,
velvetleaf, tall morningglory, black nightshade and redroot
~"~; 40 pigweed were sowed therein and cultivated for 18 days in a
greenhouse. A designed amount of the test compol~nd formu-
.~' ' .
~ .
~ ~ " ~
. ~ i . r . : . ~ ~ ~
o ~. ,, :~ ~ ., . , ',, 1, -. ,.,. ', ~
- ~ 45 ~ ~33~ 4 ~
f- lated in an emulsifiable concentrate according to Formula-
tion Example 2, 3 or 6 was diluted with water containing a
spxeading agent, and the dilution was sprayed over the
foliage of the test plants by means of a small hand sprayer
at a spray volume of 5 liters per are. The test plants were
further grown in the greenhouse for 20 days, and the herbi-
cidal activity was examined. At the time of the applica-
tion, the test plants were generally at the 1 to 4 leaf
stage and in 2 to 12 cm height, although growing stage of
the test plants varied depending on their species. The
results are shown in Table 12.
Table 12
_
Go~x~nd Dosage Herbicidal activity
No. (g/are) _ -
Corn Tall ~0mon IVel~et- Black Redroot
morning- cocklebur¦ieaf night- pigweed
glory I _ d=de
2 0.10 5 _ 1 5 5 5
3 0.1_ 5 5 5 5 5
4 0.1_ 5 5 5 5 5
0.11 S 5 5 5 5
12 0.~0 5 5 5 5 5
16 0.11 5 5 5 5 5
27 0.1_ 5 5 5 5 5
28 0.1_ 5 5 5 5 5
29 0.10 _ 5 5 5 5
31 0.11 5 5 5 5 5
37 0.11 5 4 4 5 5
39 0.31 5 _ 5 5 5
0.10 5 _ 5 5 5
44 ~ 0.3_ 5 4 5 5 i 5
0.11 5 _ 4 5 5
~; 45 0.31 5 _ 4 5 5
0.10 5 _ _ 4 4
47 0.31 5 _ 4 4 4
A 0.3I O O O O O
~'~ I 0.1 I O O I O I O O O ,~
'
~ ~ .
I
~ ,~
~ ~ .
' .
- 46 -
1330~
~ Test Example 8
Vats (33 cm x 23 cm x 11 cm) were filled with
upland field soil, and the seeds of wheat, ladysthumb,
catchweed bedstraw, common chickweed and persian speedwell
were sowed therein and cultivated for 18 days in a green-
house. A designed amount or the test compound formulated in
an emulsifiable concentrate according to Formulation Example
2, 3 or 6 was diluted with water containing a spreading
agent, and the dilution was sprayed over the foliage of the
test plants by means of a small hand sprayer at a spray
volume of 5 liters per are. The test plants were further
grown in the greenhouse for 20 days, and the herbicidal
activity was examined. At the time of the application, the
test plants were generally at the 1 to 4 leaf stage and in 2
to l2 cm height, although growing stage of the test plants
varied depending on their species. The results are shown in
Table 13.
Table 13
Compound Dosage Herbicidal activity
No. (g/are)
Wheat Ladys- Catch- Common Persian
- thumb weed chick- speed- -
tdraw weed well
34 ~ 0 1 ' j l I S I SI S i ~ 55 ~ ;
S 0.1 1 S 5 5 5
12 0.1 _ 5 5 5 5
16 0.1 1 5 5 5 5
34 0 3 1 s5 54 4 45
42 0.. 31 1 5 4 _ 5
. .
A 0.3 0 0 0 0 0
_ 0.1 0 0 0 I 0 0
: :
:
~ .
_ 47 ~ 1 3 3 ~ 4 ~ 3
- Test Example 9
Seeds of corn, velvetleaf, redroot pigweed and
black nightshade were sowed in the field as previously laid
up in ridges and divided into plots of 3 m2. A designed
amount of the test compound formulated into an emulsifiable
concentrate according to Formulation Example 2 or 5 was
diluted with water, and the dilution was sprayed onto the
soil surface by means of a small hand sprayer at a spray
volume of 10 liters per are. The application was made with
three replications. After cultivation for 32 days, the
herbicidal activity were examined. The results are shown in
Table 14.
Table 14
Compound Dosage Herbicidal activity
No. (g/are)
Corn Velvetleaf Redroot Black
_ pigweed nightshade
i 1 68 I 55 5 55
16 1.6 0 5 5 5
0.8 0 5 5 5
B ¦ 4 1 0 ~ 4
.'
~:
:~
.
::
:
:~
,
.