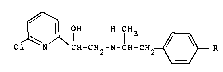Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~.
PB 53 165
Pyridylethanolamines
The present invention relates to new pyridylethanol-
amines, processes for thei~ preparation and pharmaceu-
tical compositions containing them.
We have found that certain novel pyridylethanolamines
exhibit valuable pharmacological properties, partic-
ularly an effect on metabolism, especially an effect
of reducing blood sugar and body fat, and the effect
of reducing levels of the atherogenic lipoproteins
VLDL and LD~,. Some of the compounds mentioned
also have an anabolic activity.
Thus according to one aspect the present invention
provides compounds of formula I
ClJ~CH-cH2_
(wherein
R represents a carboxymethoxy, carbomethoxymethoxy,
ethoxycarbonylmethoxy or 2-bydroxyethoxy group),
and the optical isomers, diastereoisomers and acid
addition salts thereof.
The compounds of ormula I may form salts, e.g. ~ -
wlth inorganic or organic acids, the particularly
preferred salts being those which are physiologically
acceptable.
1331 ~0~ -:
- 2 -
According to a further aspect, the present invention ;
also provides a process for the preparation of
compounds of the invention, said process comprising
at least one of the following steps: -
(a) reacting a compound of formula II
:' ',
H
Y - N - Y (II)
(wherein one of the moieties Y represents a hydrogen - :
atom and the other represents a group of formula ~ ~
~ OH , 3 ~ -
Cl N CH-CH2- - CH - CH2 ~ R ~ -~
' -
wherein R is as hereinbefore defined~
with a compound of formula III :
':, : - -
Zl U (III)
(wherein U represents a group of formula
,
CH
or - , 3 ~
-CH2-CH ~ N ~ Cl -cH-cH2 ~ R
o~r '~ ' ' ''
wherein R is as hereinbefore defined, and
.: ': ,' ','~,
Zl and V together represent a bond, or
;-- 133100$
!
Zl represents a nucleophilic leaving group such
as a halogen atom, e.g. a chlorine, bromine or
iodine atom, or a sulphonyloxy group, e.g. a methane-
sulphonyloxy, p-toluenesulphonyloxy or ethoxysulphonyl-
oxy group and
V represents a hydrogen atom)
(b) reductively aminating a carbonyl compound
of formula IV
- ,~
CH3-CO-cH2 ~ R ~
:,~: : : :'
Iwherein R i8 a~ hereinbefore defined)
~; with an amine of formula V
. . .~..
ON ~ X , . - :~.~;
Cl N CH-~H2-N
c) reducing a compound of formula VI
ClJ~CO-CN2-N-CX-CXz~R ~ ~
~:: :: :,.; ` . :
.,., . ..,,..,:,,- ~ .-:
= ~:
~ 3 3 1 0 a 3
(wherein R is as hereinbefore defined); -:
(d) reacting a compound of formula VII
-CHrN--CH-CH2~0H
with a compound of formula VIII
:
Z2 ~ Rl (VIII)
:, ; ..
(wherein .;: -
: Rl represents a carboxymethyl, methoxycarbonyl- ~ :
methyl, ethoxycarbonylmethyl or 2-hydroxy-ethyl -.
group, and
Z2 represents a nucleophilic ieaving group such
as a halogen atom or a sulphonyloxy group, e.g. ~ :
a chlorine, bromine or iodine atom or a methanesul-
phonyloxy, p-toluenesulphonyloxy or ethoxysulphonyloxy
~" : group, or " , :
Z2 together with a ~-hydrogen atom of the group
Rl represents an oxygen atom);
., - ~ ..
e) hydrolysing a resulting compound of formula - ..
I wherein R represents a methoxycarbonylmethoxy
or ethoxycarbonylmethoxy group to yield a corresponding . ::
compound of formula I wherein R represents a carboxy- .
methoxy group; : -:
:..: '
~) converting a compound of formula I into an
acid addition salt thereof, or an acid addition
,~,x,., ~,",~" ~
1 3 31 ~ 0 S
-- 5 --
salt of a compound of formula I into the free base;
and
(g) resolving a resulting compouna of formula
I or a salt thereof into its aiastereomers or enant- r
iomers.
The reaction of step (a) may conveniently be carried
out in a solvent or mixture of solvents such as
methanol, ethanol, acetone, diethylether, methyl- - -
formamide, dimethylformamide, dimethylsulphoxide,
benzene, chlorobenzene, tetrahydrofuran, benzene/tetra~
hyarofuran, dioxan or in an excess of the compound ~ --
of formula III used. Optionally the reaction is -~
effected in the presence of an acid binding agent, -
e.g. an allcoxide such as potassium tert.butoxide,
an alkali metal hydroxide such as sodium or potassium
hydroxide, an alkali metal carbonate such as potassium
carbonate, an alkali metal amide such as soaium ~-
amide, an alkali metal hydride such as ~odium hydride,
a tertiary organic base~such as triethylamine,
N,N-diisopropylethylamine or pyridine, where the
latter may also be s1multaneously used as solvent,
or in the presence of a reaction accelerator such
potassium iodide. The reaotion is conveniently ` ~ -
effected at temperatures between O and 150-C, depending ;~ -
on the reactivity of the nucieophilically exchangeable ~`~
group, preferably at a temperature between SO u
and 120C, e.g. at the boiling temperature of the
solvent u~ied, The reaction may, howëver,~also be
carried out without a solvent. It is particularly
advantageous to carry out the reaction with a corres~
ponding epoxide of formula III without the addition ; ` ;`
of an acid binding agent. `-'~
The reductive amination of step ~b) may be carried `~
out in a ~olvent such as methanol, ethanol, tetra-
? ,`
~331~
- 6 -
hydrofuran, dioxan or acetonitrile in the presence
of a reducin~ agent such as a complex metal hydride,
but preferably in the presence of sodium cyanoboro-
hydride at a p~ of from 5 to 7, and conveniently
at temperatures of between 0 and 50C, but preerably
at ambient temperature.
The reduction of step (c) may preferably be carried
out in a solvent such as methanol, ethanol, diethylether
or tetrahydrofuran in the presence of a metal hydride
such as sodium borohydride, lithium aluminium hydride,
diborane, borane/dimethylsulphide or sodium cyanoboro-
hydride, but preferably with sodium borohydride
in methanol or ethanol and conveniently at temperatures
between 0 and 40C, but preferably at ambient témper-
ature. -
When the reduction of step ~c) is carried out with
a complex metal hydride such as lithium aluminium
hyaride, diborane or borane/dimethylsulphiae, a
carbonyl function present in the group R may be
reducea at the same time to yield a methylene group. ~ -
The reaction of step ~d) may conveniently be carried
out in a solvent such as diethylether, tetrahydrofuran,
dioxan, methanol, ethanol or dimethylformamide
ana preferably in the presence of an acid-binaing --~
agent such as soaium hydroxide or potassium tert.but-
oxide, but preferably in the presence of potassium
carbonate or soaium hydriae or pyridine, whilst
an organic base such as pyridine may also be use
as solvent, or, in order to prepare 2-hydroxy-ethoxy -~
compounas of formula I, with ethylene oxide. The
reaction conveniently is effected at temperatures
of between 0 and 100C, preferably at temperatures
of between 20 and 80C.
::
1331~0~
In the reactions of steps (aJ to (d) described
above, reactive groups may be protected during
the reaction with conventional protecting groups
which are then split off again by conventional
methods after the reaction.
Examples of suitable protective groups for a carboxy
group include benzyl, tert.butyl, tetrahydropyranyl,
trimethylsilyl, benzyloxymethyl, 2-chloroethyl
and methoxymethyl groups and a phenacyl group,
e.g. a benzoylmethyl group, suitable protecting ~ --
groups for an amino or alkylamino group include ~-
acetyl, benzoyl, tert.butoxycarbonyl, benzyloxycar~
bonyl, ethoxycarbonyl and benzyl groups and suitable ~ ~ -
protecting groups for a hydroxy group include,
for example, trityl, fluorenylmethyloxycarbonyl,
benzyloxycarbonyl and benzyl groups. --
. : ~, : ~,:
~, . .; ....
The optional subsequent splitting off of any protecting
group used is preferably carried out by hyarolysis
in an aqueous solvent, e.g. in water, isopropanol/
water, tetrahydrofuran/water or dioxan/water, in
the presence of an acid such as hydrochloric or
sulphuric acid or in the presence of an alkali ~ `;
metal base such as sodium hydroxide or potas~ium ;;-~
hydroxide, conveniently at temperatures of between
0 and 100C, preferably at the boiling temperature
of the reaction mixture. A benzyl or benzyloxycarbonyl
group, however, is preferably removed by hydroqenoly-
Si8, e.g. with hydroqen in the presence of-a catalyst
such as palladium/charcoal in a solvent such as -;
methanol, ethanol, etbyl acetate or glacial acetic
acid, optionally with the addition of an acid such
as hydrochloric acid, conveniently at temperatures
of between 0 and 50C, but preferably at ambient
temperature, under a hydrogen pressure of from
l to 7 bar, but preferably from 3 to S bar.
',:'-'.,`,'..'
:` 133~
- 8 - 27169-150
The hydrolysis of step (e) is carried out either
in the presence of an acid such as hydrochloric,
sulphuric, phosphoric or trichloroacetic acid or
in the presence of a base such as sodium hydroxide
or potassium hydroxide in a suitable solvent such
as water, methanol, ethanol, ethanol/water, water/iso-
propanol or water/dioxan, conveniently at temperatures
of between -10C and 120C, e.g. at temperatures
of between ambient temperature and the boiling
temperature of the reaction mixture.
The compounds of formula I obtained may be converted
into the acid addition salts thereof, more particularly
for pharmaceutical use the physiologically acceptable
salts with inorganic or organic acids. Suitable
acids for salt formation include, for example,
hydrochloric, hydrobromic, sulphuric, phosphoric,
fumaric, succinic, lactic, citric, tartaric and
maleic acid.
As already mentioned hereinbefore, the compounds
of formula I may occur in the form of the enantiomers,
enantiomer mixtures or racemates thereof or in
the form of pairs of diastereoisomers or mixtures - -
of pairs of diastereoisomers.
Thus, the compounds of formula I obtained may be
separated into their diastereoisomers on the basis
of their physical/chemical differences by methods
known Per se, e.g. by chromatography and/or fractional
crystallisation. A pair of enantiomers thus obtained - ~-~
may then be separated into the optical antipodes
thereof by methods known Per se (see Allinger N.L.
and Eliel E.L. in "Topics in Stereochemistry",
Vol. 6, Wiley Interscience, 1971), e.g. by recrystal-
lisation from an optically active solvent or by
reaction with an optically active substance which
~33~ 00~
g
forms salts with the racemic compound, particularly
an acid, and separation of the salt mixture obtained ~ -
in this way, e.g. on the basis of differences in
solubility, into the diastereomeric salts, from
which the free antipodes can be liberated by the
action of suitable agents. Optically active acids
which are particularly useful include the D and ~ - -
L forms of tartaric acid, di-o-toluene tartaric
acid, malic acid, mandelic acid, camphor sulphonic
acid, glutamic acid, aspartic acid and quinic acid.
The compounds used as starting materials which
may, obviously, also be used in their optically
pure forms, may be obtained by methods known from
the literature (see ~Thiazole and its Derivativesn
in Heterocyclic Compounds, Vol. 34, and Advances - -~
in Heterocyclic Chemistry, Vol 17, page lOOff (1974)) p~ --
or are known from the literature. In some cases
these compounds are only present in the reaction
mixture and therefore cannot be isolated. ~ ~ ~
An intermediate compound of formula II, V, VI or - -
VII may be obtained by corresponding alkylation
of a suitable amine, e.g. as discussed in EP-A-239815.
An intermediate compound of formula III wherein
Zl represents a nucleophilic leaving group maybe ~ ~-
obtained by reduction of a corresponding acetyl
compouna, e.g. with a complex metal hydride, or -
by conversion of a corresponding hydroxy compound
into a reactive derivative tbereof.
''-''''.'- :
An epoxide of an intermediate compound of formula
III may be obtained for example by reacting a suitable
bromo- or tosylethanol with aqueous potassium or
sodium hydroxide ~olution.
' ' ' ~::
', ~
: 133100~
-- 10 --
AS already mentioned hereinbefore, the compounds of
formula I, the enantiomers, mixtures of enantiomers or
racemates thereof or, if they contain at least 2 asym-
metric carbon atoms, the diastereoisomers or mixtures
of diastereoisomers, and the acid addition salts
thereof, more particularly for pharmaceutical use the
physiologically acceptable acid addition salts thereof,
have valuable pharmacological properties, including,
in addition to the effect of inhibiting platelet aggre-
gation, an effect on the metabolism, especially an
effect of lowering blood sugar and reducing body fat,
and the effect of reducing the atherogenic ~-lipopro-
teins VLDL and LDL. Moreover, some of the compounds
mentioned above also have an anabolic activity.
The biological properties of the new compounds ~ -
were investigated as follows:
1. Antidiabetic activitv
.
The antidiabetic activity of the compounds according
to the invention can be measured as a blood sugar-
reducing effect in experimental animals. The test
substances were suspended in 1.5% methyl cellulose
and aaministered to laboratory-bred fema~e mice by
oesophageal tube. 30 minutes later, 1 g of glucose
per kg of body weight was dissolved in water and
administered subcutaneously. After another 30 minutes,
blood was taken from the retroorbital plexus venosus.
The level of glucose in the serum was determined by
the hexokinase method using an analytical photometer.
In the Table which follows, the reductions in blood
sugar observed in this experimental arrangement are
shown as the percentage of a parallel control group.
Statistical evaluation was carried out by the Students
t test taking p=0.05 as the limit of significance.
:~ 13310~
Compound ~i Change from the level of
(Example No. ) the control group
aosage [mg/kg]
O.l 0.3 l.O
l -66
2 -64 -
-66
2. Anti-adiPose activitY ~ ~
~ ,
The anti-adipose activity of the compounas according
to the invention was demonstratea by two experimental
arrangement
a) In the first experiment the increase in lipolysis ~ ~ ~
was measurea by the increase in glycerol in the serum. ~ ~;
The experimental procedure 1s identical to the experi-
mental arrangement described above for testing the
reauction in blood sugar. The glycerol level was
determined in a combined enzymatic/colorimetric test
using an analytical photometer. The results are shown
in the followlng Table as the percentage of a parallel
control group.
Compound~i Change from the level of
Example No.) the control group
aosage [mg/kg]
, O.l 0.3 l.O
l +882
2 1454 ` ~ -
3 +309
891
878 -~; ~
SA ;
",~
. . ,
',' ;'-',..' "; ,,-,
~` 1331~0~
- 12 -
b) In the second experimental arrangement for
determining the anti-adipose effect of the compounds
according to the invention, the reduction in fatty
tissue was measured on the fatty tissue of the ovary
by way of example. For this purpose, the compounds
were administered to mice once a day by oesophageal
tube in a 1.5% methylcellulose suspension. On the
fifth day the fatty tissue of the ovary was dissected
out and weighed. The following Table shows the results -
as a percentage of a parallel control group.
Compound % Change from the level of the con-
~Example No.) trol group
Dosage: 0.3 [mg/kg]
~ 5~ -54
3. Cardiac side effects
The compounds of the invention have been shown to have
no undesirable siae effects on the heart in the thera-
peutic aosage range which has an effect on the metabo-
lism. The evidence for this was provided by the
measurement of heart rate in mice during the tests for
the blood sugar reducing effect (see above). One hour
after oral administration of the compounas heart rate
was determined by means of an ECG-triggered tachograph.
The Table which follows shows the change in heart rate -~-
as a percentage of the control group.
Compound% Change from the level Dosage
~Example No.) of the control group
- ;
3 ~35 10 mg~kg
2 (+6.9) 0.3 mg/kg
27) 0.3 mg/kg
.
L;.. ~ ~ - , . . . : : . . : , -: . . ,: . . : .-
133~
- 13 -
The figures in brackets are not significant (p greater
than 0.05)
Furthermore, in the tests on the substances according
to the invention described above, no toxic side
effects were observed at the dosages used. The
substances are therefore well tolerated.
In view of their pharmacological properties, the
compounds of formula I and the physiologically --
acceptable acid addition salts thereof are therefore
suitable for the treatment both of diabetes mellitus
and also obesity, particularly for treating obese
diabetics. Moreover, the new compounds may be
used for the prophylaxis and treatment of athero- -
sclerotic changes in the blood vessels, which occur
particularly in diabetics and obese people. The -
dosage required can be matched entirely to the
metabolic physiological requirements of the individual
patients since the substances are free from any - -
effect on the heart or circulation over a wide -~
aosage range. In adults, therefore, the daily
dose may be between l and 3000 mg, but preferably
1 to 1000 mg, divided up into 1 to 4 doses per
day. For this purpose, the above-mentioned compounds
may be incorporated in conventional galenic prepa- ``
rations such as powders, tablets, coated tablets,
capsules, suppositories or suspensions, optionally
in conjunction with other active substances.
,:,, , . :Thus, in another aspect, the invention provides - -~
a pharmaceutical composition comprising a compound
of formula I or a physiologically acceptable acid ~-
addition salt thereof together with at least one ~ -~
pharmaceutical carrier or excipient. It will be
understood that the "pharmaceutical compositions~
,.. ::.- . - - . : , , , ~, .. , ,. ,, ,:: :. ., : . . , :,, , ,. ,, ,, . - , . . . .
1 3 ~
27169-150
of the invention include within their scope compositions suitable -~
for administration to non-human animals, i.e. veterinary composi-
tions.
In a still further aspect, the invention provides a
method of treatment of the human or non-human body to combat
diabetes mellitus, obesity and athero6clerosis comprising
administering to said body a compound of formula I or a
physiologically acceptable salt thereof.
In a yet further aspect, the invention provides the use
of a compound of formula I or a physlologically acceptable salt
thereof for the preparation of a therapeutic agent for use in the
treatment of diabetes mellitus, obesity and atherosclerosis.
In a still further aspect of the invention there is
provided a commercial package containing as active pharmaceutical
ingredient a compound of formula I or a physiologically acceptable
salt thereof, together with instructions for the use thereof in
the treatment of diabetes mellitus, obesity and atherosclerosis. -
The compounds of the invention may thus also be used to
treat overwelght animals such as dogs and, as a consequence of
their body fat-reducing (lipolytic) effect, and they may be used
to reduce undesirable fat deposits in the fattening of animals,
i.e. in order to improve the quality of meat of fattened animals
! . such as pigs, cattle, sheep and poultry. In animals, the above-
mentioned compounds may be administered by oral or non-oral
routes, e.g. as a feed additive or by injection or by means of ;-
implanted minipumps. The daily dose is between 0.01 and 100
mg~kg, preferably between 0.1 and 10 mg/kg of body weight.
14
B ~ ~-
1331~û~
27169-150
In a still further aspect the invention thus also
provides an animal feed comprising a compound of formula I or a -~
physiologically acceptable salt thereof together with a feedstuff.
The Examples which follow are provided to illustrate the ~-~
invention without restricting its scope in any way.
Unless otherwise stated all percentages, parts and
ratios referred to herein are by weight.
, ~,. ... ..
-',. -:
,-.~;, . - .
. ,,, ~ , .
. . :.: -- :
~,: . ,,:
.,,.". ;~,:,,,
'''"~
-.,- .
: -~ .. , .-.
'.'.'' :".' '.: .,-''
~ ' ' '., . :- '
: '" ' ' '''.'
.: ~' ,~'.
, ,, , " ~:
.~
.:. .~ ,. .:~" ~:
~ " ~ ",
B 15 -
1331~û~
- 16 -
Example 1
N-[ 2-(4-CarbomethoxYmethoxyphenyl)-l-methylethyl]-
2-hydroxy-2-(6-chloro-pyridin-2-yl)ethanamine-hYdro-
chloride
.
0.23 g (0.00133 mol) of 2-hydroxy-2-(6-chloro-pyridin-
2-yl)ethanamine and 0.3 9 (0.00133 mol) of 1-(4-
carbomethoxymethoxyphenyl)propan-2-one are dissolved
in 15 ml of absolute methanol, O.Q8 9 (0.00133 mol)
of glacial acetic acid and 0.084 9 (0.00133 mol)
of sodium cyanoborohydride are added and the mixture
is stirred for 16 hours at ambient temperature.
It is then p~ured onto ice and acidified with hydro-
chloric acid. After 5 minutes it is made alkaline
with ammonia at 0 to 5C and extracted with methylene
chloride. The extract is dried over sodium sulphate
and concentrated by evaporation and purified over
a silica gel column using ethyl acetate/methanol
(9:1) as eluant. The base i8 converted into the
hydrochloride using ethereal hydrochloric acid.
Yield: 0.276 9 (46~ of theory),
Melting point: from 70C (decomposition).
Calculated: C 54.94 H 5.~2 N 6.74 Cl 17.07
Found: 54.60 6.00 7.00 17.40
According to l~-NMR spectrum (400 MHz, DMSO/CD30D)
an approximately 1:1 mixture of the pairs of aiastereo-
isomers is present.
delta = 1.17 ppm (d,~ CH-CH3)
delt~ - 1.20 pp~ (d,> 0 -~d3)
.- :'
-~ ~
~,~"~
~3310~
- 17 -
Example 2 ~ -
.: .
N-[2-(4-(2-Hydroxyethoxy~phenyl)-l-methylethyl]-2- , ' ~ ,
hydroxy-2-(6-chloro-pyridin-2-yl)ethanamine-hydro-
chloride
Prepared analogously to Example 1 by reacting 2- -
hydroxy-2-(6-chloro-pyridin-2-yl)ethanamine with - ~ - `
1-(4-(2-hydroxyethoxy)phenyl)propan-2-one and sodium ;
cyanoborohydride and subsequently purifying the -
product over a silica gel column using ethyl acetate/- ~ -
methanol (9:1) as eluant. The base is converted - --
into the hydrochloride using ethereal hydrochloric
acid. ;-
Yield: 65~ of theory,
Melting point: 68C ~decomposition) ~ ~-
Calculated: C 55.81 H 6.24 N 7.23 Cl 18.30
Found: 55.60 6.46 7.09 18.47 -~
According to H-NMR spectrum (400 M~z, d6-DMS0/CD30D) -
an approximately 1:1 mixture of the pairs of diastereo-
isomers i8 present.
delta = 1.121 ppm (d,> CH-CH3) ~-
delta = 1.136 ppm (d,~ CH-CH3) -~
ExamPle 3 -~
N-[2-(4-CarboxYmethoxYPheny~ -methylethyll-2
hyaroxy-2-~6-chloro-pvridin-2-Yl)ethanamine
,. . .
3.6 g (0.0095 mol) of N-12-(4-carbomethoxymethoxy-
phenyl)-l-methylethyl]-2-hydroxy-2-~6-chloro-pyridin-
2-yl)ethanamine are dissolved in 20 ml of methanol
and 50 ml tO.05 mol) of lN sodium hydroxide solution
are added. The solution iæ stirred for 30 minutes
at ambient temperature and then neutralised with
50 ml of lN hydrochloric acid (0.05 mol). The
solvent is distilled off in vacuo and the residue
- ;
1 3 3 1 0 ~ 5
- 18 - ;
is stirred with 20 ml of water and 20 ml of methanol
for 16 hours, suction filtered and washed with
a little methanol.
Yield: 1.8 g ~52% of theory),
Melting point: 218C ~decomposition).
Calculated: C 59.25 H 5 . 80 N 7.68
Found: 59.47 5.83 7.5s
According to 1H-NMR spectrum (400 MHz, D6-DMS0/CD30D)
there is an approximately 3:2 mixture of the pairs
of diastereoisomers.
delta = 4.92 ppm (d,~ CH-CH3)
delta z 4.98 ppm (d,~ CH-CH3) -
. :
ExamPle 4 ~-
- .
~-~ N-l2-~4-CarboxymethoxYphenyl)-l-methylethyl]-2-
hvaroxy- 2-~6-chloro-pyridin-2-Yl)ethanamine - ; -
. ., ~ . ~
~ Palr of di~stereolsomers A~
h''~ g.3 q of a 3:2 ~ixture of the pairs of dlastereoisomers
A and B obtained as describea in Example 3 are
m~ dis~olvea hot in ~mixturc of 600 ~1 of methanol
nd 900 l of water and crystallisea by cooling
in an ice b~th. This cry-tallisation prooess 1
- ~ repeated three times wlth 360 ml of meth~nol + -
450 Rl of ~ter ~nd 250 ml of methanol ~ 3~0 ml ~-
of water. The palr of diastereolsomers A ~re then
obtainea in a degree of purity of about 96%. ~ -
Ylela: l q (17.2~ of theory),
MeItlnq point: 234C
Calculated: C 59.26 H 5.80 N 7.67 Cl 9.71
Found: 59.07 5.69 7.66 9.97
1H-NMR spectrum ~400 MHZ, CD30D/D6-DMS0)
delta ~ 4.949 ppm (dd~ CH-OH)
~. .. ~ ~ , ..
133~0~
",
- 19 - 27169-150
Example S
N-12-(4-Carboxymethoxyphenyl~-l-methylethyl~
2-hYdroxy-2-(6-chloro-pyridin-2-Yl)ethanamine-hvdro- '' .. , ',
chloride
Pair of diastereoisomees B
3.6 9 (0.0095 mol) of a 1:1 mixture of the pairs
of diastereoisomers A and B of N-[2-(4-carbomethoxy-
methoxyphenyl)-l-methylethyl]-2-hydroxy-2-(6-chloro-
pyridin-2-yl)ethanamine obtained as described in
Example 1, are stirred in 20 ml of methanol and
50 ml t0.05 mol) of lN sodium hydroxide solution
at ambient temperature for 45 minutes. The solution
is mixed with 50 ml of lN hydrochloric acid and
brought to dryness. The residue is stirred overnight
with 20 ml of methanol and 20 ml of water and the
solid product precipitated is suction filtered.
The mother liquor is concentrated by evaporation
and the residue is triturated with 25 ml of water,
then twice with 50 ml and 30 ml, respectively,
of acetone and suction filtered. The pair of diastereo- ~- -
isomers B is then present in the form of the hydro-
chloride with a degree of purity of 90%. - - -
Yield: 1 9 (56% of theory),
Melting point: about 80C ~decomposition)
Calculated: C 53.87 H 5.52 N 6.97 Cl 17.66
Found: 53.82 5.66 6.86 17.50
H-NMR spectrum ~400 MHz, CD3OD/D6-DMSO)
delta = 4.907 ppm (dd,> CH-OH)
~ - .
~ ~'
- ' .
~ .~
:
,:
.
~ -20- 27169-150
1331~
Example 6
N-[2-(4-(2-Hydroxyethoxy)phenyl-l-methylethyl]-2-hydroxy-2-(6-
chloro-pyridin-2-yl)ethanamine
Pair of diastereoisomers A:
48.6 g of a 1:1 mixture of the pairs of diastereoisomers
of N-[2-(4-(2-Hydroxyethoxy)phenyl)-l-methylethyl~-2-hydroxy-2-
(6-chloro-pyridin-2-yl)ethanamine (Example 2) are dissolved in
500 ml of hot acetone and brought to crystallization by cooling in
an ice bath, whereby an approximately 8:2 mixture of pairs of
diastereoisomers B and A is obtained (yield 15.5 g, melting point:
134C. The volume of mother liquor is reduced to 200 ml and this
is cooled overnight in a refrigerator. The product precipitated ~ ~-
is suction filtered, dissolved in 150 ml of hot acetone and
. . .
crystallized by cooling in an ice bath. This procedure is -
: ..., .. ~ .
repeated with 100 ml of acetone and with 70 ml of acetone, yielding -
6 g of a product with a melting point of 108C. These 6 g were ~ -
-~ . . -
recrystallized twice more from 60 ml and 40 ml, respectively, of - -
acetone, to give the pure pair of diastereoisomers A.
. ~, . .-~:, : .
Yield: 4 g (16.5% of theory), ~-
Melting point: 109-110C
Calculated: C 61.62 H 6.60 N 7.98 Cl 10.10
Found: 61.55 6.64 7.86 10.30 - -
H-NMR spectrum (400 MHz, CDCl )
delta = 4.620 ppm (dd, ~ CH-OH)
Pair of diastereoisomers B:
15.5 g of a mixture of pairs of diastereoisomers N-~2-(4-
(2-Hydroxyethoxy)phenyl)-l-methylethyl]-2 hydroxy-2-(6-chloro-
.. .:: :.
-20a- 27169-150
~. ~ 3 1 ~
pyridin-2-yl)ethanamine (Example 2) (B:A = about 8:2) were re-
crystallized four times from 600 ml, 500 ml, 400 ml and 350 ml,
respectively, of acetone. The pair of diastereoisomers B is
obtained with a degree of purity of 96 to 98%. -~
,. i ,.,~ -' ;~
- 13310~
- 21 -
Yield: 7 9 ~56.5~ of theory),
Melting point: 142-143C
Calculated: C 61.62 H 7.98 N 7.98 Cl 10.10
Found: 61.45 8.01 7.689.98
~ -NMR spectrum (400 MHz, CDC13)
delta = 4.686 ppm ~dd, CH-0H)
Example I
Coated tablet containinq 10 mq of N-12-(4-(2-hYdroxY-
ethoxY)Phenvl)-l-methYlethyl]-2-hyaroxy-2-(6-chloro- --
Pyridin-2-Yl)ethanamine
ComPositiOn
1 coated tablet contains~
(1) Active ~ubstance 10.0 mg
(2) Lactose 69.0 mg -~
(3) Corn starch 35.0 mg
(4) Polyvinylpyrrolidone5.0 mg -~
(5) Magnesium stearate 1.0 mq
'20.0 mg
PreDaration ;" ~,~ ~:, .'' ',:',,
Components (1), (2) and (3) are mixed together ;~
and moistened with component (4) in an aqueous -;~
solution. The moist mass is passed through a screen
with a mesh size of 1.6 mm and dried at 45C in
a circulating air dryer. The dry granules are
passed through a screen with a mesh size of 1 mm -
and mixed with component (5). The finished mixture
is compressed to form tablet cores. ; `~
~.,-',.'".;'-,'"''`.'
Weight of core: 120 mg
Diameter: 7.0 mm
Radius of curvature: 6.0 mm
.`''''' ~
~.':' :' -
::- :: .
- :'`"'
~33100~
.
- 22 -
The tablet cores thus produced are coated in known
manner with a coating consisting essentially of
sugar and talc. This coating may also contain
dye extracts. The finished coated tablets are
polished with wax.
Weight of coated tablet: 180.0 mg
Example II
Coated tablet containinq 50 mq of N-12-(4-(2-hydroxy-
ethoxv)Phenyl)-l-methvlethyl~-2-hydroxv-2-(6-chloro-
P~Yridin-2-yl)ethanamine
comPOSition:
1 coated tablet contains:
'.,
(1) Active substance 50.0 mg ~ ~-
(2) Lactose 110.8 mg
(3) Corn starch 50.0 mg
(4) Polyvinylpyrrolidone8.0 mg
(5) Magnesium stearate 1.2 mq
220.0 mg
.,
PreParation:
The tablets are prepared analogously to Example I.
Weight of core: 220.0 mg
Diameter: 9.0 mm
Radius of curvature: 8.0 mm
Weight of coated tablet:300.0 mg ~ -
Example III
Tablets containinq 150 mq of N-[2-(4-(2-hYdroxYethoxY)-
phenYl)-l-methylethYl]-2-hYdroxY-2-(6-chloro-pyridin
2-yl)ethanamine
-` 1331~
Composition~
1 tablet contains:
(1) Active substance 150.0 mg
~2~ Lactose 86.0 mg
(3) Corn starch 50.8 mg
(4) Microcrystalline cellulose2S.0 mg
(5) Polyvinylpyrrolidone 7.0 mg
(6) Magnesium stearate 1.2 mq
320.0 mg
. ,~
PreParation- ' ''' ~
- : -. :-
Components (1), (21, (3), (4) and (S) are mixea ;~
together and moistened with water. The moist mass
is passed through a screen with a mesh size of
1.6 mm and dried at 45C. The dried granules are ;- ;~
passed through the same screen again and mixed
with component (6). Tablets are compressed from
the finished mixture. - -
.,
Weight of tablet: 320.0 mg ~-
Diameter: 10.0 mm
, ~ ,-.. ,-,
The tablets are provided with a divlding notch -
to make it easier to break them in half.
ExamPle IV "",~
Hard aelatin caPsules containinq 100 mq of N-[2-
4-(2-hYdroxyethyl)phenyl)-l-methylethyl]-2-hydroxv- '~
2-~6-chloro-PYridin-2-yl)ethanamine
ComPosition
1 capsule comprises~
. ' '~'~','~` .
Capsule casing: hard gelatin capsules, size 3 ~ ~
,.- ~. .,~, ..
",.:,.
~ ~3310~5
- 24 -
Capsule contents:
(1) Active substance 100.0 mg
(2) Lactose x lH2o 38.0 mg
(3) Dried corn starch 60.0 mg
(4) Magnesium stearate 2.0 mq
Weight of capsule filling: 200.0 mg
(5) Distilled water q.s.
PreParation:
A small amount of lactose is dissolved to about
10% in distillea water ~granulating liquid~. The
active substance, the remaining lactose and corn
starch are mixed together and moistened with the
granulating liquid. The mass is screened, dried,
re-screened and homogeneously mixed with magnesium
stearate. The fine granules are packed into capsules
in a suitable machine.
ExamPle V
Hard qelatin caPsules containinq 200 mq of N-[2-
(4-~2-hYdroxYethoxY)Phenyl)-l-methylethvl]-2-hydr
2-~6-chloro-pYridin-2-Yl)ethanamine
'~1`, ,. ~ .
ComPosition~
~. . ..
Capsule casing: hard gelatin capsules, size 1
Capsule contents:
i ' ' ! ~ 1) Active substance200.0 mg
~2) Lactose x lH2O 47.0 mg
~3) Dried corn starch70.0 mg
~4) Magnesium stearate3.0 mq
Weight of capsule filling: 320.0 mg
(5) Di~tilled water q.s.
:" '
t331~0~ j
- 25 -
. ., :
PreParation: ;
A small amount of lactose is dissolved, to about -
10% in distilled water ~granulating liquid!. The
active substance, the remaining lactose and corn ~ ~ .
starch are mixed together and moistened with the
granulating liquid. The mass is screened, dried,
re-screened and homogeneously mixed with magnesium
stearate. The fine granules are packed into capsules
in a suitable machine.
-., ~
1 ~ ' ~ ' ' " " ~,: ,' ` . ';
'''`~ ' .
':
~,
'`'., "' '"'': ~,~
,: ~ ,.,.. :,
.'..: '' ~ -:
; -: . '':, .-: '-
' ~` ~ ' , .