Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 1 - 1 3 ~ 3 775
28138-1
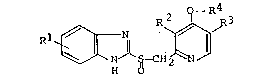
This invention relates to injectable solutions contain-
ing pyridine derivatives (hereinafter referred to, in some
instances, as "Compound (I)") of the below-described formula
which are useful as an anti-ulcer agent:
O -R
R ~ R3
N ~ S-CH ~ N /
o
[wherein R represents hydrogen, methoxy or trifluoromethyl; R
and R3, each being the same as, or different from, the other,
represent hydrogen or methyl; and R4 represents fluorinated C2
to C5 lower alkyl].
There have been known injectable solutions (refer to
the European Unexamined Patent Publication No. 124495), which
are obtained by dissolving omeprasole sodium salt having anti-
ulcer activity in sterilized water, followed by filtration and
lyophilization to give a lyophilized material, which is then
dissolved in a sterile-filtered mixed solution of polyethylene
glycol 400 for injection, sodium dihydrogenphosphate and
sterilized water.
In developing injectable solutions of Compound (I),
there are encountered some problems being attributable to its
characteristic properties.
Among the species of Compound (I), for example, 2-(3-
methyl-4-trifluoroethoxy-2-pyridyl)methylsulfinylbenzimidazole
1 333775
-- 2
28138-l
(hereinafter referred to briefly as "Compound (I-l)") shows a
certain degree of solubility in water but only in the strongly
alkaline region of pH 11 or more, with an extremely low
solubility in the pH range below pH 11 (33 mg/ml at pH 13, 1
mg/ml at pH 11 and 0.06 to 0.01 mg/ml at pH 9 to 3). Referring
to the stability in an aqueous solution., Compound (I-l) is
satisfactorily stable in an alkaline solution, but becomes less
stable as the pH of the aqueous solution is decreased to the
neutral to acid range, while the resulting solution turns in
appearance dark purple in a short period of time.
Thus, Compound (I), because of its characteristic
properties as described above, has been difficult to be processed
into the dosage form of injectable solution in a physiologically
allowable pH range using water alone as a solvent and without
employing a sophisticated processing technique, because injectable
solutions favorably exhibit a pH value not being far from
neutrality in terms of hemolysis, pain or local irritation.
In view of these circumstances described above, the
present inventors, after extensive investigation, found that
Compound (I) is very soluble in solvents, such as ethanol,
propylene glycol and polyethylene glycol, its stability in the
solvents being excellent, and that the powder produced by
lyophilizing:an alkaline solution of Compound (I) does not tend
to discolor with a length of time elapsed and is extremely
soluble in the above solvents, and the findings, coupled with
further research, have culminated into the present invention.
Thus, the present invention is directed to:
,i. ~
- 2a - 1 333775
~ 28138-1
(1) Injectable solutions co~taining (a) a compound of
the formula (I):
O -R
R2 ~ R3 (I)
N ~ S-CH ~ N
H 11 2
[wherein Rl represents hydrogen, methoxy or trifluoromethyl; R2
and R3, each being the same as or different from the other,
represent hydrogen or methyl; and R4 represents fluorinated C2
to C5 lower alkyl] and (b) at least one of ethanol, propylene
glycol and polyethylene glycol; and
(2) Injectable solutions consisting of a lyophilized
material of an alkaline solution of Compound (I) being
,~. -,
'w
_ 3 1 333775
dissolved in a mixed solution composed of (a) an acid
substance and (b) at least one of ethanol,
propylene glycol and polyethylene glycol.
In the above-described formula, examples of the
fluorinated C2 to C5 lower alkyl represented by R4
include 2,2,2-trifluoroethyl, 2,2,3,3,3--pentafluoropropyl,
2,2,3,3-tetrafluoropropyl, l,l,l-trifluoromethyl-
2,2,2-trifluoroethyl, 2,2,3,3,4,4,4-heptafluorobutyl,
2~2~3,3,4,4,5,5-octafluoropentyl and the like. In the
above formula, preferably, Rl and R3 are hydrogen, and
R2 is methyl, with R4 being 2,2,2-trifluoroethyl.
It is to be noted that the above-mentioned
Compound (I~ is a known compound as decribed in The
European Unexamined Patent Publication No. 174726.
As the said polyethylene glycol, there can be
used polyethylene glycols having varied average molecular
weights, and favorably usable are those having preferably
an average molecular weight of 50 to 2000, more preferably
an average molecular weight of 100 to 600.
In the present invention, in cases where ethanol,
propylene glycol and polyethylene glycol are used as an
admixture of more than two kinds, their mutual mixing
ratio may be any proportions.
When water is contained in the solvent, an increased
proportion of water in the total volume of solvent results
in a lowered solubility of Compound (I); particularly in
the case of Compound (I) being dissolved in the solvent
composed of ethanol and water, the dissolved Compound (I)
in some instances separates out with a length of time
elapsed, and consequently, the proportion of water in the
total volume of solvent is desirably not more than 80 %
( V/V ) .
The injectable solutions according to the present
invention can be obtained by dissolving Compound (I) in
the form of amorphous powder or crystalline powder into
the above-mentioned solvent, but it is preferable to
- 28138-1
- 4 - ~ 3 3 37 7 5
dissolve a lyophilized material of an alkaline aqueous
solution of Compound (I) into a mixed solution composed
of an acidic substance and the above-mentioned solvent.
As the alkaline aqueous solution of Compound (I),
there may be mentioned, for example, an aqueous solution
produced by dissolving into water Compound (I) in conjunction
with a strongly basic substance such as sodium hydroxide,
potassium hydroxide, sodium carbonate and arginine, and
adjusting the resulting solution to a pH of not less
than 11, preferably not less than 12.
Among the strongly basic substances, sodium
hydroxide is particularly preferred.
The concentration of Compound (I) in the
alkaline aqueous solution may be of any concentration
only if it permits lyophilization, and is preferably
2 to 30 mg/ml, more pre~erably 5 to 30 mg/ml.
In order to provide the resulting lyophylized
material with improved solid-forming property, it is
preferred to add to the alkaline aqueous solution of
sucrose, and lactose, neutral amino acids, such as glycine,
alanine, proline, valine and methionine, inorganic salts,
such as sodium succinate, and the like. These additives can
be employed usually in an amount of 0.2 to 5 mg, preferably
0.5 to 3 mg, per mg of Compound (I).
Among these solid-forming agents, mannitol and
glycine arè preferable, with mannitol being particularly
preferred.
The said lyophilized material can be~ produced by
lyophylizing the alkaline aqueous solution of Compound (I)
by use of a method known per se, and in general,
lyophilization is preferably carried out by means of a
method which comprises freezing the solution at a
temperature of below -25C and drying the freezed material
by warming a tray up to 25C or 40C at a rate of 5 to
20C/hr while keeping the degree of vacuum in a dryer at
not more than about 0.1 Torr.
The lyophilized material obtained in this manner
~7
~ .
1 333715
_ - 28138-l
. . , . _
produces the appearance of white lump form or powder form, and
hardly varies in appearance with a length of time elapsed,
thereby offering the advantageo~s characteristic that
Compound (I) can be preserved stable for a prolonged
period of time.
The said lyophilized material contains a strongly
basic substance formulated, and when dissolved in the
above-mentioned solvent, produces a solution with a
strong alkalinity. In order to adjust the pH of the solution
to a physiologically allowable range, consequently, it is
preferable to incorporate into the a~ove-mentioned solvent
a specifically determined amount of an acidic substance
as a pH adjusting agent. Examples of the acidic substance
include inorganic acids, such as hydrochloric acid and
phosphoric acid, and organic acids, such as succinic acid
and tartaric acid, as well as sodium dihydrogenphosphate,
glycine, etc. Among others, hydrochloric acid or sodium
dihydrogen phosphate is preferable.
Also, it is desirable to formulate an acidic substance
in such a way that the pH of the injectable solutions of this
invention may be adjusted finally to 7 to 11.
The solvent having the above-mentioned acidic
substance admixed can permit the lyophilized material
containing Compound (I) to be disso~ved quickly, wherein
dissolution or dilution on the occasion of use is preferred.
As the injectable solution o~ this invention,
there may be empioyed a liquid composition having Compound tI)
in conjunction with a strongly basic substance like the
previously mentioned ones dissolved in the above-described
solvent, which is to be diluted with a solvent being
admixed with the above-mentioned acidic substance on the
occasion of use to adjust its pH to the physiologically
allowable range (ca. 7 to 11).
The thus prepared injectable solution of this
invention desirably have a concentration of Cu--~o~l-d [I~
of O.l to 20 mg/ml, particularly 2 to lO mg/ml.
g_y~
1 333775
28138-l
In addition, it is preferable to incorporate
; the injectable solution according to the present invention
with additives, including buffer solutions for the
stablization of its pH, such as arginine, N-methylglucamine,
glycine, sodium dihydrogenphosphate, bisodium hydrogen-
phosphate, isotonizing agents for the adjustment of its
osmotic pressure, such as sodium chloride, stabilizers,
such as sodium hydrogensulfite, pain-relieving agents,
such as glucose, sorbitol, mannitol, benzyl alcohol,
mepivacaine hydrochloride and Xylocaine hydrochloride, and
preservatives, such as methyl p-oxybenzoate, propyl
p-oxybenzoate, thymelosal and chlorobutanol, as the case may
be. These substances can be added usually in an amount of
0.2 to 10 mg, preferably 0.5 to 5 mg, per mg of Compound (I).
In order to enhance the solubility of Compound (I),
also there can be formulated sodium chloride, magnesium
chloride and potassium chloride. The amount of these salts
in the formulation is usually 1 to 30 mg, preferably 3 to
18 mg, per mg of Compound (I).
The injectable solution of this invention is
preferably prepared-normally by means of the sterile
preparation method known per se.
The injectable solution according to the present
invention, preferably, is normally administered intravenously,
and its dosage amount is desirably selected in such a
way that it may be 5 to l~0 mg of Compound (I) daily for male
adults, preferably lO to 50 mg.
In the injectable solution of this invention, the
use as a soluent af at Ieast either one out of ethanol,
propylene glycol and polyethylene glycol renders Compound
(I) slightly soluble in water to solubilize and to be
~rovided with the desired degree of stability. Consequèntly,
it becomes possible to have Compound (I) demonstrate
its excellent anti-ulcer activity in an adequate manner.
Below described are the examples to illustrate
this invention specifically, but these are not to be
understood to limit the scope of this invention.
1 333775
-- 7
Example l
Compound (I-1 ) was dissolved in ethanol and propylene
glycol to the concentration of 2 mg/ml, respectively,
and the resulting solutions were sterile filtered and
5 filled in 5-ml portions into ampoules of a 5-ml capacity,
followed by sealing.
The test specimens as filled into the ampoules
were inves tigated for appearance, clarity and
content of Compound (I) immediately after preparation
10 and after storage at 25C for 24 hours, witl1 the results
being shown in Table l. l'he test specimens prepared by
dissolution in ethanol and propylene glycol were observed
to produce a slight change in appearance after storage
at 25C for 29 hours, but the cl1anges were judged to be
15 so slight that they might in no way influence the in jectable
solution. The test specimens were found to be stable in
terms of content of the active ingredient.
[l'able l] Stability of Compound (I-l) in the solution state:
Composition of test Item of l~fter ~fter storage
specimen ~in l ml) investigation preparationat 25C/24hrs.
Compound (I-l): 2mg ADPearance Colorless Yellowish
Clarity Clear Clear
Ethanol l Content* lO0. 0% 99. 2%
Compound ( I-l): 2mg Appearance Colorless Light red-purple
Clarity Clear Clear
Propylene glycol: lml Content* lO0. 0% 103 . 0%5 Note, *: As measured by use of high-performance liquid chroma-
tography ~ HPLC ), whereby the content determined
immediately af ter preparatlon was taken as 100 . 0 9~,
( the same method was employed for measurement in
examples to be described in the following).
Chromatograpl-ic conditions of }~PLC:
Carrier; ~
Nucleosil 5 C1~3 (supplied l~y Gas-Chro Kogyo ~.K.
of Japan) 4 . 0 mm x 150 mm
Solven t;
Methanol:water:triethylamine (60:~10:1, pll 7)
De tec tion me thod;
UV spectrophotometry at 285 nm
~r~le - ~r~
_ - 8 - 13~3775
Example 2
Ethanol, polyethylene glycol 400, propylene glycol
and water were mixed at the composition ratios as shown
in Table 2 illustrated below, and 2 mg of Compound (I-l)
was dissolved in 1 ml each of the resulting solvents,
respectively, followed by adjustment to pH 9.0 with 5N-
aqueous sodium hydroxide solution. As a control, there
were prepared a suspension of 2 mg of Compound (I-l) in
1 ml of water being adjusted to pH 9.0 with 5N-~queous
sodium hydroxide solution and an aqueous solution of
2 mg of Compound (I-l) in 1 ml of O.OlN aqueous sodium
hydroxide solution. These solutions were sterile-filtered
by the conventional method and were filled in 5-ml
portions into ampoules of a 5-ml capacity, respectively,
followed by sealing (A control of the suspension was no~
subjected to sterile-filtration). The test specimens as
filled into ampoules were investigated for each item of
appearance, pH and content immediately after preparation
and after storage at 25C for 24 hours, with the results
being shown in Table 2.
As may be evident from Table 2, all of the test
specimens, except the controls, were observed to produce
a slight change in appearance after storage at 25C for
24 hours, but the changes were judged to be so slight
that they might in no way influence the injectable solution.
In addition, the test specimens were found to show slight
change in content. The control specimens, when adjusted
to the same pH value as other test specimens, failed to
allow adequate dissolution of Compound (I-l), and were
needed to he adjusted to a pH of not less than 11 in
order to secure dissolution.
.
- n - ! 333775
[Table 2] Stability of Compound (I--l) in the solution state
Composition of test ¦ Item After After storage
specimen (in 1 ml) ~nvestigation preparation at 25C/24 hrs.
Compound tI-1):2mg Appearance Colorless Slightly to light
PEG-400* :0.05m:. green-yellow
Ethanol :0.35m. pH . 9.0
Water :0.6ml. Content 100.0% 98.0~
Compound (I-1):2mg Appearance Colorless Slightly to light
PEG-400 :0.05m] greentoyellow
10 Propylene glycol:0.35nlpH 9.0
Water :0.6ml Content 100.0% 94.4%
Compound (I-1):2mg Appearance Colorless Slightly to light
green--yellow
PEG-400 :0.4ml pH 9.0
Water :0.6ml Content 100.0% 91.8%5 Compound (I-1):2mg Appearance Colorless Slightly to light
red-purple
Ethanol :0.5ml pH 9.0
Propyleneglycol:0,5m_ Content 100.0% 98.0%
Compound (I-l) :2mg Appearance White sus- Slightly to light
pension grey suspension
Water :lml pH 9.1
Content - -
Compound (I-1):2mg Appearance Colorless Colorless
pH 11.4
0 OlN aq.sodium . Content 100.0~ 92.5~
Note, *; Polyethylene glycol having an average molecular
weight of 400.
- I 33377 5
Example 3
Ethanol, polyethylene glycol 400, propylene glycol
and water were mixed at the composition ratios as shown
in Table 3 illustrated below, ~nd 5-mg of Compound (I-l)
was dissolved in 1 ml each of the resulting solvents,
respectively, followed by adjustment to pH 9.0 with
5N-aqueous sodium hydroxide sol~tion. ~s a control, there
were prepared a suspension of 5 mg of Compound (I-1) in
1 ml of water beillg adjusted to pll 9.0 with 5N-aqueous
sodium hydroxide solution and an aqueous solution of 5 mg
of Compound ( I-l) in 1 ml of 0.02N aqueous sodium
hydroxide solution. These solutions were sterile-filtered
by the conventional method and were filled in 5-ml
portions into ampoules of a 5-ml capacity, respectively,
Eollowed by sealing (~ control of the suspension was not
subjected to sterile-filtration).
These test specimens as filled into ampoules were
investigated for eacll item of appearance, pll and
c~rityimmediately a~ter preparation and after storage at
25C for 24 hours, witll the results being sllown in Table 3.
~ s may be evident from Table 3, all of the test
specimens, except the controls, were observed to produce
a sligllt cllange in appearance ~after storage at 25C for
24 hours, but tlle changes were judged to be so sligllt
tllat tltcy migllt in no way influelice tlle injecta~le solution.
In ad-lition, tl~ey were o~served to SllOWll no cl)allge in
clarity, The control specimens, ~llen adjusted to
tlle sallle pll value as otller test specimens, failed to
allow adequate dissolution of Compoulld (I-l), and were
needed to be adjusted to a pll of not less than 11 in
order to secure dissolution.
1 33377~
11 --
28138-1
[Table 3] Stability of Compound (I-l) in the solution state
Composition of test Item of After After storage
specimen (in 1 ml) investigation Preparation at 25C/24 hrs.
Compound (I-l): 5 mg Appearance Colorless Slightly to light
- green-yellow
Ethanol: 0.6 ml pH 9.0
Water: 0.4 ml Clarity Clear Clear
ompound (I-l): 5 mg Appearance Colorless Slightly to light
green-yellow
PEG-400*: 0.4 ml pH 9.0
Water: 0.6 ml Clarity Clear Clear
ompound (I-1): 5 mg Appearance Colorless Slightly to light
green-yellow
Ethanol: 0.3 ml pH 9.0
Propylene
glycol: 0.3 ml Clarity Clear Clear
Water: 0.4 ml
ompound (I-l): 5 mg Appearance Colorless Slightly to light
green-yellow
PEG-400: 0.3 ml pH 9.0
Ethanol: 0.3 ml Clarity Clear Clear
Water: 0.4 ml
Compound (I-l): 5 mg Appearance White Slightly to light
suspension grey suspension
pH 8.9
Water: 1 ml Clarity
Compound (I-l): 5 mg Appearance Colorless Colorless
pH 11.6
0.02N-aq. sodium
hydroxide solution:
1 ml Clarity Clear Clear
1 333775
- 12 -
28138-1
Example 4
A 1,000 mg quantity of Compound (I-l) was dispersed
in distilled water for injection, and 3 ml of lN-aqueous sodium
hydroxide solution was added to dissolve the Compound (I-l),
followed by addition of water to make up the total of 50 ml and
sterile filtration by the conventional method. The resulting
filtrate was filled in 1 ml portions into vials of a 12 cm3
capacity, followed by lyophilization-by means of the conventional
technique. The lyophilized powder as contained in vials was
dissolved in Solvent A (which was composed of 50 mg of N-methyl-
glucamine, 0.27 ml of lN-hydrochloric acid and 2 ml of propylene
glycol being admixed with ethanol to make up the total of 4 ml),
Solvent B (which was composed of 50 mg of N-methylglucamine, 0.27
ml of lN-hydrochloric acid, 1.2 ml of polyethylene glycol 400
and 1.2 ml of ethanol being admixed with distilled water for
injection to make up the total of 4 ml), Solvent C (which was
composed of 50 mg of N-methylglucamine, 0.27 ml of lN-hydrochloric
acid, 1.2 ml of ethanol and 1.2 ml of propylene glycol being
admixed with distilled water for injection to make up the total
of 4 ml) and Solvent D (which was composed of 50 mg of N-methyl-
glucamine, 0.27 ml of lN-hydrochloric acid and 2.5 ml of poly-
ethylene glycol 400 being admixed with distilled water for
injection to make up the total of 4 ml), respectively, to perform
inspection for their solubilities as well as to conduct
investigation into appearance, clarity and contents immediately
after dissolution and after storage at 25C for 24 hours.
The results are shown in Table 4. The lyophilized
powder showed excellent solubilities in all of these Solvents,
' - 13 - ~ 3337 7 5
~ 28138-1
and was able to be dissolved quickly. In addition to this, the
resulting solutions were observed to produce slight changes in
appearance immediately after dissolution and after storage for
24 hours, but the changes were found to be so slight that they
might in no way influence the injectable solution. The solutions
were found to show no change in the clarity while being observed
to decrease slightly in content of Compound (I-l).
[Table 4] Stability of the lyophilized Compound (I-l) after
being dissolved in vials:
-_____ Solvent A B C D
Item ~
Solubility Good Good Good Good
pH after
dissolution 8.7 9.0 9.0 9.0
After dissolution:
Appearance Colorless Colorless Colorles~ Colorless
Clarity Clear Clear Clear Clear
Content 100% 100% 100% 100%
After storage at
25C for 24 hrs.:
Appearance Slightly to lightly green-yellow
Clarity Clear Clear Clear Clear
Content 97.0% 96.5~ 96.7% 96.1%
Example 5
There were prepared 100 ml each of three kinds of
aqueous solutions containing Compound (I-l), mannitol and sodium
1 333775
- 13a -
28138-1
hydroxide at the individually different compositions per ml as
shown in Table 5. The resulting aqueous solutions were sterile-
filtered by the conventional method, and the filtrates were
filled in 2 ml portions into vials of a 18 cm3 capacity,
respectively, followed by lyophilization by the conventional
technique to give three kinds of lyophilized vials (Vl, V2 and
V3) having the formulations as shown in Table 6.
Then, there were prepared 500 ml each of five kinds
of aqueous solutions containing PEG-400, sodium chloride,
N-methylglucamine, sodium dihydrogenphosphate, hydrochloric acid
and sodium hydroxide at the individually different compositions
per each 10 ml as shown in Table 7. The aqueous solutions were
sterile-filtered by the conventional method, and the resulting
filtrates were filled in 10 ml
1 333775
- 14 -
portions into ampoules of a lO ml capacity, respectively.
The am~oules were sealed and sterilized by high-pressure
steam at 115C for 30 minutes to give five kinds of the
solutio~s for dissolution (Sl, S2, S3, S4 and S5) having
the individual formulations as shown in Table 7.
Following the combinations as described in Table 8,
then, the lyophilized vials were dissolved a~in in the
solutions for dissolution, and the resul.ting drug solutions
were investigated for pll and clarity immediately
after preparation and after storage a~ 25c for 24 hours.
~s may be evident from the results shown in Table 8, the
dxug solutions resulting from any of the combinations
were found to be stable in pH and good in clarity
whereby the lyophilized vials after being dissolved by any
of the combinations exhibited good solubility.
[Table 5~ Composition of the aqueous solution to be
filled into vials (in l ml)
Ingredie~ ~ . ........ 1 ........... 2.. ......... 3.
Compound (I-l) 15 mg ls50 mg 15 mg
5~ Mannitol 15 mg ~ mg - 15 mg
Sodium hydroxide 2.4 mg 2.4 mg 4.8 mg
Distilled water Suitable volume to make up to the
for injection total of 1 ml.
vf~
[Table 6] Composition of the lyophilized vi~ls (per vila)
Ingredie~t~N Vl - V2 V3
Compound (I-l) 30 mg 30 mg 30 mg
Mannitol 30 mg 100 mg 30 mg
Sodium hydroxide 4.8 mg ~.8 mg 9.6 mg
- 15 - . ~ 1333~75
[Table 7] Compositions after bein-g dissolved (per 10 ~1)
~. . . ...................................................
- Ingred ~ Sl ~: S-2 S3 S4 S5
PEG-400 3000mg 3000 mg3000 mg 3000 mg 3000mg
Sodium chloride 90 m.g 90 mg 90 mg 90 mg 90 mg
N-methylglucamine 10 mg 40 mg 40 mg
NaH2PO4 2H2o 13 mg 50 mg
Hydrochloric acid 1.1 mg 7.7 mg 8,4 mg - -
Sodium hydroxide - - - 2~8 mg 11 mg
10 pH 9.0 3.1 8~5 8.5 7.0
[Table 8] Stability a-fter dissolution of lyophilized
vial-s containing Co~pound (I--l~
~ ~ l t - ImmediatelyAfter storage
Combin-atibo~ ~after preparation at 25~C for 24 hrs.
Vial Solution pH Clarity 1. ..pH.. . Clarity
-fordiss'll 1
Vl Sl9.8 Clear 9.8 Clear
V2 Sl9.9 Clear 9.8 Clear
V3 S210.1 Clear 9.9 Clear
Vl S49.9 Clear 10.0 Clear
V2 S410.2 Clear 10.0 Clear
V3 S5 10.0 Clear 9.9 Clear