Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ 1 33635~
87- 3 56
BACKGROU~D OF THE INVENTION
The present invention is drawn to a process for
producing reformed gas for use in the direct reduction
of meta]. oxides containing iron to a metallized iron
product and a method for and apparatus for the direct
reduction of the metal oxides with the reformed gas.
The direct reduction of iron oxide, in forms such
as pellets or lump ore, to metallic iron in the solid
state has become a commercial reality throughout the
world in recent years. The combined annual capacity of
direct reduction plants currently in operation or under
construction is in excess of 15 million metric tons of
di.rect reduced iron product, which is used primarily for
feedstock in electric arc steelmaking furnaces. The
world demand for adaitional direct reduced iron is
projected to increase at a substantial rate for many
years to satisfy a growing world need for such
feedstock, as additional electric arc furnace
steelmaXing plants are constructea.
Known processes for the direct reduction of iron
oxide to metallic iron utilizes a reformed gas as the
reducing agent. ~atural gas is used as a source for
generating the reformed gas. The reformed gas for use
in the direct reduction process is generated in a unit
called a reformer by contacting the natural gas with an
.. - 1
~ ' 1 33635q
87-356
oxygen containing material in the presence of a
catalyst, usually a nickel catalyst, which activate~ the
. reformation reaction of the natural gas so as to yield a
reformed gas which is rich in ~2 and Co. The reformed
gas which is collected from the reformer is thereafter
fed to a reduction reactor containing the iron oxi.de
material wherein the direct reduction reaction is
carried out. Thus, direct reduction processes
heretofore known require two distinct reaction zones for
carrying out the process, namely, a first zone for
producing a reformea gas using a nickel catalyst and a
second zone for carrying out the actual direct reduction
process. In these conventional processes it i8 required
that the reformed gas product in the first zone be
-
treated prior to entering the reduction zone in order to
remove CO2 and/or water vapor.
Naturally, it would be highly de~irable to provide
a method for the direct reduction of iron oxide
.materials to metallic iron which would eliminate the
necessity of separate reaction zones and the use of
nickel catalysts.
Accordingly, the present invention seeks
to provide an improved method for the direct reduction
of metal oxides containing iron to a metallized iron
p~oduct.
~ .
_ ~ .
~ . 2
~ 1 336~59
In particular the present invention seeks to
provide a method as set forth above which is carried
out in the single reaction zone of a direct reduction
rea~tor.
Still further the present invention seeks to
provide a method as set forth above wherein direct
reduced iron (DRI) material is used as a catalyst to
produce a reformed gas directly in the reaction zone
of a direct reduction reactor.
Still further the present invention seeks to
provide an apparatus for carrying out the method of
the present invention.
Further desires and advantages of the
present invention will appear hereinbelow.
SUMMARY OF THE INVENTION
In accordance with the present invention the
desires and advantages are readily obtained.
The present invention is drawn to a process
for producing reformed gas ~or use in the direct
reduction of metal oxides containing iron to a
metallized iron product and a method for and apparatus
for the direct reduction of the metal oxides with the
reformed gas.
According to one aspect of the invention
there is provided a method for the direct reduction of
metal oxide containing iron to a metallized iron
product in a reduction reactor by reducing said oxide
--3--
~ 1 336359
with a reformed feeder gas which is rich in H2 and CO,
wherein reformation of the feeder gas is conducted
with direct reduced iron ( DRI ) which is heated to 850
to 950C and which is position in the reduction
reactor upstream of said oxide, with respect to flow
of said feeder gas, wherein prior to the reformation,
the feeder gas consists of a mixture of natural gas
and gaseous oxygen-containing material in which the
volume ratio o~ oxygen to natural gas is from about
0.75:1 to 1:1, and wherein the ratio of DRI to metal
oxide is from 0.25:1.00 to 0.50:1.00 by weight.
In particular the reformed feeder gas
comprises H2 and CO such that the re~ormed ~eeder gas
is suitable for the reduction of the metal oxide to
the metallized iron product; and the mixture of
natural gas and gaseous oxygen-containing material is
effective to produce the reformed feeder gas by the
reformation.
In a particular embodiment, the method
comprises providing a reduction reactor, positioning a
first layer of the DRI material in the reduction
reactor, introducing a metal oxide material layer into
the reactor above the DRI material, preheating the
reactor to reduction temperature and thereafter
feeding natural gas in the presence of an oxygen
containing material to the DRI material.
1 336359
In accordance with the present invention,
the reformed gas is produced in the reduction zone of
a direct reduction reactor wherein it immediately
contacts the iron oxide material to be reduced. In
accordance with the present invention, an apparatus is
provided for the direct reduction of the metal oxides
containing iron to be metallized iron product wherein
a feeder gas is first contacted with the DRI material
so as to form a reformed gas which thereafter contacts
the metal oxides for the direct reduction thereof.
The method of the present invention allows
for a single reaction zone of a direct reduction
reactor to be employed for both the production of the
reformed gas for use in the reduction process and for
the actual direct reduction of the iron oxide
material.
In accordance with another aspect of the
invention, there is provided an apparatus for carrying
out the direct reduction of a metal oxide containing
iron to be metallized iron product comprising a
reduction reactor de~ining a reaction zone, a feeder
means for feeding a feeder gas into the reaction zone,
and a flow path for gas in the reactor, such that the
feeder gas contacts a direct reduced iron (DRI) and is
reformed prior to the contact with the metal oxide, a
first support means on which a layer of direct reduced
iron is located within the reaction zone at a first
location, and a second support means on which the
metal oxide containing iron is located in the reaction
zone at a second location close to the first location,
the ratio of DRI to metal oxide being from 0.25:1.00
to 0.50:1.00 by weight, the first support means being
upstream of thé second support means in the flow path.
r~
-
1 336359
87-356
BRIEF DESCRIPTION OF THE DRA~INGS
Fig. 1 is a schematic illustration showing a
reduction reactor in accordance with the present
invention.
Figs. 2a, 2b and 2c are schematic illustrations of
the test equipment used in the comparative examples of
the instant disclosure.
Fig. 3 is a graph comparin~ the results of direct
reduction in accordance with the present invention and
that of ~nown processes.
DETAILED DESCRIPTIO~
The method for the direct reduction of metal oxides
containing iron to a metallized iron product comprises
providing a reduction reactor, positioning a first layer
of DRI material in the reduction reactor, introducing a
metal oxide material layer into the reactor above the
DRI material, preheating the reactor to reduction
temperature and thereafter feeding natural gas in the
presence of an oxygen containing material to the DRI
material.
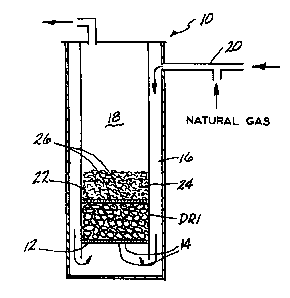
Fig. 1 i8 a schematic illustration of an apparatus
for carrying out the method of the present invention.
l 33635q 87-356
The apparatus comprises a reduction reactor 10 having a
first support surface 12 for supporting direct reduced
iron (DRI) which acts as a catalyst in the method of the
present invention for producing reformed gas rich in
S H2 and C0 from natural gas. The direct reduced iron
(DRI) is a product of the direct reduction process of
the present invention. The first support surface 12 is
provided with a plurality of apertures 14 through which
gas is fed from an annular preheating zone 16 which
defines a reaction zone 18. Inlet 20 is provided for
feeding a feeder gas (to be described in detail
hereinafter) to the annular preheating zone 16. The
reactor includes a second support element 22 positioned
above the first support surface 12 so as to define a
space therebetween which is occupied by the D~I
material. The second support surface 22 supports the
iron oxide particles 24 which are to be reduced to
metallic iron in the direct reduction process of the
present invention. The second support surface 22 is
provided with a plurality of orifices 26 in the same
manner as first support surface 12.
In accordance with the method of the present
invention a feeder gas is fed to the annular preheating
zone and from there up through the DRI material wherein
~ 1 336359
87-356
the feeder gas i5 reformed to a gas rich in H2 and
Co. The feeder gas consists of natura~ gas mixed with
an oxygen containing material. The oxygen containing
material may be air, ~0~, H20, pure oæygen or any
other process gas having oxygen as a component thereof.
In accordance with the present invention, the oxygen
should be present in an amount with respect to the
natural gas of about 0.75:1.0 to 1.0:1Ø In accordance
with the further feature of the present invention,
nitrogen can be fed to the reactor during the preheating
thereof or with the natural gas in oxygen mixture. The
purpose of the nitrogen is to avoid reoxidation of the
DRI material. In accordance with the method of the
present invention, the feeder gas first contacts the DRI
material in the reduction reactor and is reformed so as
to form a reformed gas which i8 rich in H2 and CO.
The DRI material should be present in an amount with
respect to said iron oxide material of from about
0.25:1.0 to 0.50:1.0 in order to assure enough reformed
gas for the direct reduction process. The reactor is
operated under the following conditions during the gas
,reforming-direct reduction process: Temperature: 850
to 950C; Pressure: 1.1 to 1.2 BAR; GAS FLOW RATE: 5
to 20 LT/min. The reformed gas produced by contacting
1 33b359
87-356
the feeder gas with the DRI material flows up through
the iron oxide particles and acts as the reducing agent
for the direct reduction of the metal oxides to a
metallized iron product. In order to have an effective
reduction process, the reformed gas should be present in
an amount of from about 800 to 1400 Nm3/ton,
preferably from about 1000 to 1200 Nm3/ton with
respect to the iron o~ide material.
The method and apparatus of the present invention
allows for an efficient direct reduction process which
is superior to processes heretofore known.
Further advantages of the present invention will be
obvious from the following exemplificative examples.
EXAMPLE 1
In order to demonstrate the advantages of the
method and apparatus of the present invention compared
to prior art direct reduction processes, a three step
program was conducted. Figs. 2a, 2b and 2c are
schematic illustrations representing each of the three
steps.
Fig. 2a represents the direct reduction processes
heretofore known. In accordance with these known
processes a reactor 100 defining a reaction zone 102
contains a mineral sample 104 of a metal oxide
containing iron. The reaction zone is selectively fed
_q
1 336359
87-356
via line 106 with nitrogen from reservoir 108 an~ a
reducing gas mixture comprising 72~ ~, 14~ C0, 9%
C02, 5~ CH4 from reservoir 110. The reduction
process was carried out under standard direct xeduction
conditions. After completion of the reduction process
the weight loss of the material was measured
continuously by means of a thermobalance and a reduction
curve generated using the following formula:
%R = initial weight - final weight x 100
initial weight
The reduction curve as shown in Fig. 3 indicates that
90~ reduction was obtained for the iron oxide material
employing the prior art direct reduction process.
EXAMPLE 2
In order to demonstrate the utility of DRI as a
catalyst in the generation of a reformed gas, a reaction
zone identical to that of Example 1 was employed. With
reference to Fig. 2b the reaction zone contained a
charge of DRI material. The reaction zone was
selectively fed with nitrogen (solely for heating and
cooling purposes), C02 and natural gas from reservoirs
112, 114, and 116 respectively at the following flow
rates: 5 lts/min., 4 lts/min. and 6 Its/min. The
~)
-~- 133635~
87-356
reformation reaction was conducted under the following
conditions: 200 gr. DRI material; Temperature of 900C
with a feeder gas of 10 lts/min. (4 lts/min. CO2 and 6
lts/min. CH~). At different times during the
reformation reaction exit gases were sampled in order to
determine composition of same. Table 1 hereinbelow sets
forth the time exposure in the reaction chamber and the
reformed gas produced using DRI as a catalyst. As can
be seen, the reformed gas is rich in H2 and CO.
~\
- ` ~ 3363~9
87-356
TABLE I
Exposure Reactor Reformed Gas With DRI
Time Feeder Natural
. Gas H2 C0 C02 gas
(mln. )
Mixture of C02 49.5 36 7,5 7,0
Natural Gas
Mixture of C02 49.5 36 7.5 7,0
~atural Gas
100 Mixture of Co2 49.5 36 7.5 7.0
Natural Gas
120 Mixture of CO2 49.5 36 7.5 7.0
Natural Gas
/~
1 336359
~7-356
EXAMPLE 3
In order to determine the effectiveness of the
direct reduction process of the present invention, iron
oxide was reacted in the reactor described in Fig. 1 in
S the following manner. The DRI material 200 grams was
positioned in the reactor and the iron oxide material
500 grams was placed on top of the DRI material. The
reaction zone was preheated to the reduction temperature
of 900C. Thereafter a mixture of Co2 and natural gas
40% CO2 and 60% CH4 was fed to the reaction zone at
a rate of 10 lts/min. from the bottom of the reaction
zone so as to insure contact with the DRI material prior
to contact with the metal oxide. After completion of
the reduction process weight loss was measured and a
second re~uction curve generated. As can be seen from
Fig. 3, the grade of reduction obtained in accordance
with the process of the present invention is virtually
identical to that obtained in the known reduction
process of Example 1 thereby demonstrating the
effectiveness of the method of the present invention.
This invention may be embodied in other forms or
carried out in other ways without departing from the
spirit or e~sential characteristics thereof. The
1 33~359
87-356
present embodiment is therefore to be considered as in
all respects illustrative and not restrictive, the scope
of the invention being indicated by the appended claims,
and all changes which come within the meaning and range
of equivalency are intended to be embraced therein.