Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 3375~3
Description
Process for purifying 2-t4-isobutylphenyl)-propionic acid
The present invention relates to a process for purifying
2-(4-isobutylphenyl)-propionic acid (called 2,4-acid in
the following text). The said compound is widely used,
for example as an analgesic, as an anti-inflammatory
agent or antirheumatic, and as an intermediate for many
further substances. In the form of amine salts, aqueous
solutions of 2,4-acid show good anti-corrosive behavior
in metal cutting.
There are numerous possibilities for synthesi 2 ing 2,4-
acid. One possibility (method A) of preparing the com-
pound is the carbonylation of 1-(4-isobutylphenyl)-eth-
anol with carbon monoxide in the presence of triphenyl-
phosphine and, if appropriate, in the presence of a trans-
ition metal halide, as is described, for example, in US
Patent Application Serial No. 28,514. With optimized
process operation, the resulting reaction mixture, which
has to be purified, contains about 85% to 93~ of 2,4-acid.
In addition to 2,4-acid, the reaction mixture also con-
tains about 40 organic impurities as well as triphenyl-
phosphine and triphenylphosphine oxide. In order to ob-
tain a purified 2,4-acid, which can be used as a pharma-
ceutical, from this reaction mixture which melts between65C and 70C, the organic impurities on the one hand and
the triphenylphosphine and triphenylphosphine oxide on
the other hand must be separated off or reduced to an
acceptable quantity, and the concentration of triphenyl-
phosphine and triphenylphosphine oxide in the purifiedproduct should be less than 10 ppm.
Just the separation of organic by-products from a 2,4-
acid reaction mixture without triphenylphosphine and
1 3~7553
-- 2 --
triphenylphosphine oxide is already very expensive and
requires several process steps, as is evident from
Romanian Patent 79,345. Thus, for example, the 2,4-acid
must first be converted into a water-soluble salt, for
example converted with sodium hydroxide solution into the
sodium salt, the latter must be freed of neutral
substances by extraction with methylene chloride, the
aqueous raffinate solution must be decolorized with
carbon, the carboxylic acid must then be liberated with a
suitable acid, i.e. hydrochloric acid, and finally
recrystallized from water/methanol and dried.
The invention will now be described in relation to the
drawings, in which:
Figure 1 is a graph illustrating the susceptibility of
2,4-acid to decomposition in which the particular melting
point, the depression of which is a measure of
impurities, is plotted as a function of the duration of
heating of 2,4-acid;
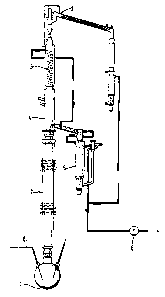
Figure 2 is a an illustration of a conventional
rectification apparatus (still rectification apparatus)
which may be used for the purification process according
to the invention;
Figure 3 is a gas chromatogram of all the impurities in a
2,4-acid mixture employed in a process according to the
invention; and
Figure 4 is a gas chromatogram of the main fraction
obtained after processing a 2,4-acid mixture using the
process according to the invention.
'
,~ ~
1 337553
- 2A -
In another patent specification (British Patent 971,700),
it is described that the 2,4-acid from a reaction mixture
is in the pure form only after having been recrystallized
3 times. Even in a more recent, very involved process,
such as described in EP-A 17û,147, recrystalli~ation is
chosen as the purification method for 2,4-acid.
~he separation problem becomes particularly acute if -
such as, for example, in method A described above - tri-
phenylphosphine is present in the reaction mixture. As
a base, triphenylphosPhine forms, ~ith the 2,4-acid and
other acids in the reaction mixture, an adduct, ~hich is
stable at room temperature, of the type of the following
formula
CO
~hich - as our experiments sho~ed - cannot be separated
off by extraction or by repeated recrystallization from
_ 3 _ 1 337553
-
solvents (see comparison example). Other separation pro-
cesses, such as adsorption on resins or ion exchangers,
also do not fulfil the aim of adequately separating off
all the impurities.
In the search for a suitable separation process for puri-
fying reaction mixtures for isolating 2,4-acid, it has
now been found that this object can be achieved by a
vacuum rectification. This is extremely surprising be-
cause, until the said object was achieved by the presentinvention, purification of 2,4-acid, which melts at 74.8C,
by distillation or rectification was evidently regarded
as not feasible. Neither vapor pressure data nor the
boiling point of the oily-viscous liquid acid, existing
above the melting point of 74.8C, are known from the
literature. The relatively high melting point of the acid
makes it understandable that the chances for purification
were mainly sought in crystallization from various sol-
vents and - in the absence of triphenylphosphine - were
indeed found as described, for example, in the abovemen-
tioned patent specifications. Moreover, due to the in-
stability of 2,4-acid at high temperatures, purification
by rectification evidently appeared to be a priori not
very promising, in particular since other reactive com-
ponents such as triphenylphosphine, triphenylphosphineoxide, ketones, styrene derivatives, alcohols and bases,
which cause a deep-black discoloration going as far as the
formation of tar or resinification of the mixture, can also
be present in the crude acid mixture. Not last, it was
also to be expected in addition that 2,4-acid forms azeo-
tropic mixtures with one or more organic impurities.
Accordingly, the invention relates to a process for puri-
fying 2-(4-isobutylphenyl)-propionic acid from mixtures
such as are obtained in the preparation of 2-(4-isobutyl-
phenyl)-propionic acid, which comprises subjecting the
mixtures to a vacuum rectification. The process accord-
ing to the invention has the advantage over the processes
according to the state of the art that numerous,
_ 4 _ 1337553
~ simultaneosly present impurities, and in particular also
triphenylphosphine and triphenylphosphine oxide, can be
removed from the 2,4-acid in a single process engineering
- unit operation, with virtually no effluent and no waste
air arising.
Various types of column can be employed for carrying out
the process according to the invention. Columns with a metal
gauze packing and columns having a pressure drop compar-
able to that of columns with a metal gauze packing are particu-
larly suitable for the process according to the invention
because, in these columns, the pressure drop arising is
small as compared with other columns. In this connection,
pressure drop means the difference between the pressure
in the bottom region and in the top region of the column.
A small pressure drop causes a small temperature differ-
ence between the bottom and top of a column and is neces-
sary, because the bottom temperature in the rectification
of 2,4-acid should not exceed a certain upper limit, since
2,4-acid decomposes at high temperature. The suscepti-
bility of 2,4-acid to decomposition is illustrated by
Figure 1, in which the particular melting point - the
depression of which is a measure of impurities - is plot-
ted as a function of the duration of heating of 2,4-acid.
It can be seen that, even at 250C, the melting point
of the particular sample falls already after a short heat-
ing period, which is to be ascribed to decomposition of
the 2,4-acid. The susceptibility of 2,4-acid to decompo-
sition can be further increased by impurities. For this
reason, the maximum temperature, at which the process
according to the invention can still reasonably be used,
depends on the mixture composition. For purification of
the reaction mixture arising according to method A, the
bottom temperature in the rectification should therefore
preferably not be above about 250C, particularly prefer-
ably not above about 230C and especially not above
210C, when a conventional rectification apparatus
(still rectification apparatus) such as is illustrated,
for example, in Figure 2, is used.
_ _ 5 _ 1337553
When modern rectification apparatus is used, such as, for
example, of the type equipped with thin-layer evaporators,
or falling-film evaporators, very short residence times
of the material to be rectified can be achieved, and
higher bottom temperatures are thus possible in the case
of such rectification apparatus, without major product
losses being incurred. When the last mentioned recti-
fication apparatus is used, the bottom temperature should
preferably not exceed about 28ûC.
The boiling temperature in the bottom and the vapor tem-
perature in the region of the top of the column are given
by the vapor pressure. For example at a vapor pressure
of 10 hPa in the column top and 13 hPa in the bottom, the
pure 2,4-acid passes over the top at 178C within a
temperature span of 0.1C, whereas in the bottom - due to
the higher pressure and the impurities content - the boil-
ing temperature varies between 190 and 220C. It is
advantageous to keep the vapor pressure in the bottom
region at lower than about 60 hPa, so that a boiling tem-
perature of 250C in the bottom is not exceeded.
The number of theoretical plates in the separation column
can be varied within a wide range. A number of theoret-
ical plates between about 10 and 150, particularly prefer-
ably between about 25 and 70, is to be preferred.
The rectification devices suitable for the purification
process according to the invention can be of different
structures. An example of a rectification device on the
laboratory scale is shown in Figure 2. The mixture con-
taining the 2,4-acid is heated in the still (1) of the
column. In place of the still, the device can also con-
tain, for example, a thin-layer evaporator or falling-
film evaporator. Low-boiling impurities can be distilled
off at the top (2) of the column. Below the condenser,
which can be, for example, a cooling coil (3) the conden-
sate is collected in the liquid divider (4), by means of
which the reflux ratio can also be adjusted. The
` - 6 - l 33755~ _ receiver (5~, in which various distillate fractions and
the pure product are collected, should advantageously be
controlled at a temperature of about 80C, so that the
product does not solidify. The vacuum required for the
rectification is generated by suitable vacuum pumps (6).
The separation column (7) is packed with a metal gauze packing
and produces a separation effect of 25 theoretical separ-
ation stages as a maximum.
1û It is advantageous to carry out the rectification under
inert gas which, in a preferred distillation device,
can be introduced, for example, via a gas leak capillary
tube (8) into the bottom of the column. Various inert
gases are suitable for this purpose. Nitrogen, argon and
carbon dioxide are preferred, and nitrogen is particu-
larly preferred. It can also be advantageous to extract
the crude 2,4-acid first with water before the rectifi-
cation, in order to remove water-soluble impurities such
as chlorides, phosphates, metal salts and the like.
2û
In principle, the rectification according to the inven-
tion can be carried out by means of one or more columns
either discontinuously, that is to say by taking off in-
dividual fractions, or continuously. If a continuous
one-column distillation is carried out, the column can
preferably be set up in such a way that the pure product
is taken off as a vapor side stream approximately in the
middle region of the column, while the column is prefer-
ably operated under total reflux. In continuous recti-
fication, both the low-boiling and the high-boiling sub-
stances can first be separated off before the 2,4-acid is
separated off, if for this purpose the temperature/sta-
bility limit critical for the particular reaction mixture
is not exceeded.
The process according to the invention is by itself suit-
able for processing any possible mixtures containing
2,4-acid. It is of particular importance, however, in
the processing of mixtures which are obtained in the
_ 7 _ l 337 553
~ synthesis of 2,4-acid, in particular those reaction mix-
tures which are formed in method A described above.
The process according to the invention is suitable for
removing all impurities from the crude acid, arising in
the production methods according to method A, without
prepurification. However, it may also be appropriate to
separate off only a part of the impurities by rectifica-
tion and to separate off the remaining impurities by one
or more purification methods. Crystallization from sol-
vents and melt crystallization without auxiliary materials
are particularly suitable for this purpose. In particular,
it can be appropriate to carry out at least one melt crys-
tallization before the rectification. In this case, the
crude acid melt is converted by cooling into blocks of
crystals, from which the heavily contaminated residual
melt is eliminated and the crystals are subjected, after
melting, to the rectification according to the invention.
As a result of the said process combinations, the recti-
fication according to the invention can be carried outvith a separation column having a smaller number of the-
oretical plates. The invention will be explained in more
detail by the illustrative examples vhich follow.
Example 1
500 9 of an approximately 90% 2,4-acid mixture of the
following composition are employed in a fractional rec-
tification apparatus as shown in Figure 2.
_ - 8 ~ 133755~
Table 1
Compound % by weight
2-(4-lsobutylphenyl)-propionic acid 88.77
5 Triphenylphosphine 0.18
Isobutylphenylethane 1.90
4-Isobutylbenzene 0.02
4-Isobutylstyrene 0.15
4-Isobutylacetophenone 0.78
10 1-(4-Isobutylphenyl)-ethanol 0.08
1-(4-Isobutylphenyl)-chloroethane 0.59
Ethyl 2-(4-isobutylphenyl)-propionate 0.05
2-(3-Isobutylphenyl)-propionic acid 1.10
3-(4-Isobutylphenyl)-propionic acid 1.90
15 Light ends of average molecular weight 178 1.80
Heavy ends of average molecular weight 320 1.20
Methyl ethyl ketone 0.48
Remaining unidentified impurities about 1.0
Figure 3 shows a gas chromatogram of all the impurities
in the mixture.
The mixture containing the said impurities was fraction-
ally distilled in the presence of nitrogen under a pres-
sure of 10 hPa and at a bottom temperature from initially
150 to finally 230C with varying reflux ratios. The
following distillate fractions were here taken off:
1) 20 9 of light ends as a yellow liquid
2) 158 9 of intermediate fraction with a 2,4-acid content
of 94 - 98%
3) 300 9 of main fraction.
22 9 remained as residue in the flask. The main fraction
had the following composition:
1 3~7 553
-
Compounds % by weight
2-(4-Isobutylphenyl)-propionic acid 99.5
Triphenylphosphine O.OOOOS
Other non-phosphororganic impurities 0.5
Figure 4 shows a gas chromatogram of the main fraction.
Example 2
Using the rectification apparatus described in Example 1,
the approximately 90% 2,4-acid mixture described therein
was subjected to a two-stage rectification. In this case,
in the first distillation pass after the light ends have
been separated off, emphasis was placed only on separat-
ing off the bottoms, in which the critical triphenylphos-
phine impurity concentrates. In a second rectification
pass, the main fraction, containing approximately 97% of
2,4-acid, from the first rectification was rectified once
more, the bottom temperature being about 200C. In this
case, the following fractions were obtained from 942 9 of
feed product:
1) 245 9 of intermediate fraction with a 2,4-acid con-
tent of up to 98.8%
2) 640 9 of main fraction
3) 57 9 of distillation residue.
The main fraction had the following compostion:
Compounds % by weight
2-(4-lsobutylphenyl)-propionic acid 99.5
Triphenylphosphine 0.00005
Other non-phosphororganic impurities 0.5
Example 3
The approximately 90% 2,4-acid mixture of a composition
as in Example 1 was introduced as a melt into a tubular
crystallizer, the cooling/heating jacket of which is
connected to a thermostat. The melt having a
- 10 - 1 337553
solidification point of 58C was cooled to 40C within
10 hours, compact crystals depositing in the apparatus. The
dark-brown liquid fraction remaining in the crystallizer
was then drained off, and the light crystals thus obtained
S were melted and subjected to the rectification according
to the invention. 440 9 of crystals having an acid purity
of 98.5% were obtained from 500 9 of 90% feed product.
The 2,4-acid prepurified in this way was then subjected
to the rectification according to the invention up to a
bottom temperature of 220C. About 370 9 of a main
fraction of the following composition were obtained in
this case:
15 Compounds % by weight
2-(4-Isobutylphenyl)-propionic acid 99.6
Triphenylphosphine 0.00005
Other non-phosphororganic impurities 0.4
Comparison example 1
20 9 of 2,4-acid, which contained 0.4% of triphenylphos-
phine, were dissolved in 50 ml of n-hexane at the reflux
temperature of the solvent in a 250 ml glass flask with
reflux condenser. The solution was then cooled to room
temperature, and the acid which had precipitated was
filtered off, washed with 20 ml of cold n-hexane and dried
in an exsiccator. Analysis of the recrystallized acid
showed that the triphenylphosphine content had remained
unchanged. The 2,4-acid thus obtained was then recrys-
tallized again for a second, third and fourth time from
n-hexane, without it being possible to change the tri-
phenylphosphine content.