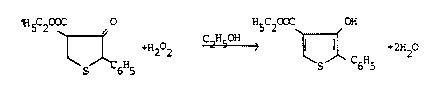Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1338108
Description
Process for the preparation of thiophene derivatives and
also new dihydrothiophene 1-oxides
It is known that thiophene derivatives can be prepared by
dehydrogenating dihydrothiophenes, examples of the de-
hydrogenating agents used being chlorides and bromides ofsulfuric or phosphoric acid, chlorine, bromine, N-halogen
compounds, tetrachloroquinone (chloranil), nitrosobenzene,
iodosobenzene, selenium or sulfur. Hydrogen peroxide H22
has also already been described as a dehydrogenating agent
for specific cases; cf., for example, A.P. Stoll and R.
Suess, Helvetica Chimica Acta volume 57, Fasc. 8 (1974)
No. 269-270, pp. 2487-2492. In this literature reference
4-ethoxycarbonyl-3-hydroxy-2-phenyldihydrothiophene is
dehydrogenated with H22 in ethanolic solution at
60-65C to give the corresponding thiophene derivative;
the reaction takes place in accordance with the following
equation:
~5 2 H5C2OX OH
~ ~ o C2H5H >
The yield for this reaction is quoted as 90 %.
Other dihydrothiophenes - namely those carrying, as sub-
stituents, electron-donating groups, such as, for example,
the amino group or a substituted amino group - do not
afford the corresponding thiophene derivatives uith H22
in glacial acetic acid, but instead the dihydrothiophene
sulfones; cf. T. Takaya et al., Bull. Chem. Soc. Japan,
volume 41, pp. 2086-2095 (1968). An example particularly
described in this literature reference is the reaction of
3-ethoxycarbonylamino-4-ethoxycarbonyl-2,5-dihydrothiophene
1338108
with H202/glacial acetic acid:
H5C200C~ NHCOX2H5 CH C~ H5C2~ 2 5
~ ~ +2H O ~
2
sulfone
It has now been found that it is also possible to dehy-
drogenate those dihydrothiophenes ~hich carry the amino group
or a substituted amino group as substituents by means of
H202 to give the corresponding thiophene derivatives,
if the dehydrogenation is carried out in two stages, name-
ly first reacting the dihydrothiophenes with H22 in aneutral medium, in the course of which the dihydrothiophene
1(= S)oxides are formed, and then adding an acid to the
dihydrothiophene 1-oxides - with or without isolation
of the latter - in the course of which the rearrangement
to give the corresponding thiophene derivatives takes
place.
The invention therefore relates to a process for the pre-
paration of thiophene derivatives of the formula I
~ 3
R \ N
C- C~ \ 2
R5 / S ~ R
in which R1 _ R5 independently of one another denote
hydrogen (H) or organic radicals by dehydrogenating di-
hydrothiophenes of the formulae IIa and/or IIb
S ~1~1 RS-- `5~ R
IIa I~
38108
-- 3
in ~hich the radicals R1 _ R5 have the same meaning
as in formula I, which comprises carrying out the dehy-
drogenation by means of H22 and in 2 stages,
a) by reacting the dihydrothiophenes of the formulae IIa
S and/or IIb ~ith an at least approximately equimolar
amount Of H22 in a neutral solvent or diluent in
the absence of acid(s), in the course of which the 1-
oxides of the initial dihydrothiophenes are formed,
and
b) by adding an acid to the dihydrothiophene 1-oxides
formed in stage a), with or without the isolation of
the latter, in the course of which the dihydrothiophene
1-oxides undergo rearrangement to give the thiophene
derivatives of the formula I.
In terms of formulae the process can be represented as
follows:
R4~ \ R2 R4 - c___r ~N ~ 2
RS--f~S--C~ n~
}~ H O
IIa TTT~
+ H2n~ ~ ~ H(~)
N ~ n4 ~R
RS--"~5~C~--Rl R5 `1--S ,"
1~ H o
,~;,, II
R4 ~R
HO ~ \ C C~ \ 2
~5 /c~s~c~R1
1338108
The invention also relates to the two individual process
stages a) and b) and to the dihydrothiophene 1-oxides
(of the formulae IIIa/IIIb) formed in stage a).
It could not have been expected and is, therefore, ex-
tremely surprising that the reaction of T. Takaya et al.
(loc. cit.) starting from dihydrothiophenes substituted
by amino groups and from H22 can be guided by the
modification according to the invention into a completely
different direction - namely to the formation of the cor-
responding thiophene derivatives (instead of the dihydro-
thiophene sulfones). The decisive factor for this dif-
ferent course is quite evidently that no acid is present
at the start of the reaction.
The process affords high yields and purities of the products
and is extremely non-harmful in terms of the environment
and waste disposal. Hitherto it has only been possible to
convert dihydrothiophenes substituted by amino groups into
the corresponding thiophene derivatives using dehydrogen-
ating agents which are less harmless in terms of the
environment and waste disposal; the ability also to carry
out this conversion by means of H22 ~ from which no
byproducts other than ~ater are formed - constitutes an
outstanding advantage and advance.
The substituents R1 to R5 in the abovementioned formulae
I, IIa/IIb and IIIa/IIIb can - independently of one another -
be H or organic radicals.
Organic radicals preferred for R1, R4 and R5 are
optionally substituted aliphatic radicals, cycloaliphatic
and araliphatic radicals, optionally substituted aromatic
radicals, alkoxycarbonyl, carboxamido, acyl radicals or
CN; preferred organic radicals for R2 and R3 are op-
tionally substituted aliphatic radicals, cycloaliphatic
and araliphatic radicals, optionally substituted aromatic
radicals and acyl radicals; in addition one of the two
radicals R2 or R3 can also denote alkoxycarbonyl, or
-- 5 13~8108
R2 and R3, together ~ith the N atom to which they are
attached, can also form a 5-membered to 7-membered ring
which can also contain another heteroatom of the type
0, S or N as a ring member and can also be substituted.
s
The following organic radicals are particularly preferred
for R1 to R5
R1, R4 and R5: C1-C4-alkyl,
c5-c6-cycloalkyl~ CH2-c6H5~ C6H5~ COOCH3,
COOC2Hs, CONH2, COCH3, COC2Hs or CN;
R2 and R3: C1-C4-alkyl, optionally substituted by
COOCH3 or COOC2Hs, Cs-C6-cycloalkyl, (CH2)1_3-C6Hs,
C6Hs, optionally substituted by OH, OCH3, OC2Hs, F,
Cl or Br, or CO(C1-Cs-alkyl) in ~hich the alkyl radical
can be substituted by OH, F, Cl, ~r, (C1-C4)-alkoxy,
NH2 or (C1-Cg)-alkylamino groups, and one of the radicals
R2 or R3 can, in addition, also be COOCH3 or COOC2Hs,
or R2 and R3, together with the N atom to ~hich they
are attached, can form a pyrrolidino, piperidino, morpho-
lino, piperazino or homopiperazino ring.
The starting dihydrothiophenes of the formulae IIa and IIbare either kno~n from the literature or can be prepared
analogously to kno~n processes. Kno~n processes are,
for example, quoted in the abovementioned literature re-
ference of T. Takaya et al. (loc. cit.) - cf. especially
page 2090 - and in U.S. 3,855,243 (issued 17 December
1974). A summarizing recent survey is contained in the
book by Weissberger, The Chemistry of Heterocyclic
Compounds, A Series of Monographs, Arnold Weissberger and
Edward C. Taylor, volume 44/part 21 "Thiophene and Its
Derivatives", edited by S. Gronowitz (1986).
The starting dihydrothiophenes are reacted in stage a) of
the process according to the invention with an at least
approximately equimolar amount - preferably ~ith an ap-
proximately 1-molar to 2-molar amount ~ of H22 in a
neutral solvent or diluent and in the absence of acid(s).
J~ ~ ~
1~8108
_ - 6
Suitable neutral solvents or diluents are water and/or
neutral organic solvents. The follo~ing may be mentioned
as examples of neutral organic solvents:
alcohols, such as, for example, methanol, ethanol, 1-
propanol, isopropanol, 1-butanol, 2-butanol, tert.-butanol
or 2-methylpropanol;
aliphatic and cycloaliphatic hydrocarbons, such as, for
example, pentane, 2-methylbutane, hexane, 2,2-dimethyl-
butane, 2,3-dimethylbutane, 2-methylpentane, 3-methyl-
pentane, heptane, cyclohexane, methylcyclohexane, 1,2-
dimethylcyclohexane, 1,3-dimethylcyclohexane or 1,4-
dimethylcyclohexane;
aromatic hydrocarbons, such as, for example, toluene,
xylenes or isopropylbenzene;
aliphatic and aromatic halogenated hydrocarbons, such as,
for example, tetrachloroethylene, 1,1,2,2-tetrachloroethane,
1,1,1,2-tetrachloroethane, methylenechloride, dichloro-
propane, carbon tetrachloride, 1,1,2-trichloro-1,2,2-tri-
fluoroethane, trichlorofluoromethane, 1,2-dichloroethane,
1,1-dichloroethane or chlorobenzene;
ethers, such as, for example, diethyl ether, di-n-butyl
ether, diisopropyl ether, diisoamyl ether, methyl tert.-
butyl ether, ethylene glycol dimethyl ether, tetrahydro-
furan, dioxane or anisole;
esters, such as, for example, methyl acetate, ethyl ace-
tate or butyl acetate;
acid amides, such as, for example, dimethylformamide or
dimethylacetamide.
Solvents or diluents which are particularly preferred are
water and C1-C4-alkanols (in particular ethanol and
isopropanol), on their own or mixed with one another.
- 1338108
The reaction temperature in stage a) can vary within a
fairly wide range. In general, the reaction temperature
here is between about -20 and about +100C, preferably
between about -15 and about +70C.
In order to carry out the reaction, the appropriate di-
hydrothiophenes of the formulae IIa and/or IIb are first
dissolved or suspended in an appropriate neutral solvent
or diluent. The corresponding amount of H22 - pre-
ferably also mixed with one or more of the solvents ordiluents mentioned above - is then added dropwise in a
normal manner and the mixture is kept at the reaction
temperature until starting material can no longer be de-
tected; the detection is carried out in a customary
manner, preferably by taking a sample and carrying out a
thin layer chromatogram.
The dihydrothiophene 1-oxides of the formulae IIIa or IIIb
formed can be isolated either by cooling and filtering off
with suction or by concentrating the solution. They can in
some cases be formed as mixtures of diastereomers, and these
can be separated by chromatography. The dihydrothiophene
1-oxides of the formulae IIIa and IIIb are new compounds.
In the course of the rearrangement according to stage b)
of the process according to the invention the dihydro-
thiophene 1-oxides of the formulae IIIa and lIlb afford
the equivalent thiophene derivatives. For this rearrange-
ment it is possible to redissolve or resuspend the di-
hydrothiophene 1-oxides which have been isolated in one
of the abovementioned solvents or diluents, or the re-
action mixture obtained in stage a) can be processed fur-
ther directly as such.
An acid is added dropwise or introduced in the form of
gas (HCl or HBr) into the solution or suspension. De-
pending on the starting compounds, catalytic or larger
amounts of acid are preferable or necessary for this.
1~38108
_ -- 8
The acids used can be inorganic and/or organic acids.
Aqueous, alcoholic or ethereal solutions of hydrogen chlor-
ide or hydrogen bromide or sulfuric acid are preferred.
The reaction temperature possible embraces the range be-
tween the solidification point and the boiling point of
the solvent or diluent used; room temperature is pre-
ferred for this reaction.
The mixture is worked up in a customary manner. The free
aminothiophenes can be obtained in a known manner from
the aminothiophene salts formed in a given case.
The new dihydrothiophene 1-oxides and also the known thio-
phene derivatives formed by the rearrangement thereof inaccordance with the process of the invention are valuable
starting materials or intermediates for the preparation
of, in particular, plant protection agents and pharma-
ceuticals. Examples of pharmaceuticals which may be
mentioned here are the substituted 3-aminoacylaminothio-
phenes of the general formula IV below, which are de-
scribed in DE-C 1,643,325 mentioned above and which are
suitable, above all, as local anesthetics:
R'~
R' ~H - C-A- ~
~ \R2' (IV)
R' ~ S R'
in which R1 is H or an organic radical, R2 is an
organic radical or R1 and R2 can also - together
with the N atom to which they are attached - form a ring,
R3 - R5 are H or organic radicals and A is a C1-C4-
alkylene group.
The compounds are prepared, for example, in accordancewith variant b) of the process of the DE-C text, as
follo~s:
- 1~3810~
q
R
R NH2 R R3 ' {~O-A-N/
~ + HOOC-A-N / ~ ~H R
R4 5--R5 ' R2R4 '~S ~R5 '
(V) (Vl) (IV)
The starting materials (V) can be prepared more advan-
tageously and in a manner less harmful to the environment
than hitherto by the process according to the invention via
the new intermediates IIIa/IIIb.
The following examples serve to illustrate the invention
without, however, limiting the latter. The melting points
and decomposition points quoted are not corrected and de-
pend on mixtures of diastereomers which may be formed.
Example 1
a) 3-Amino-4-methoxycarbonyl-2,5-dihydrothiophene 1-oxide
3.2 9 (O.OZ mol) of 3-amino-4-methoxycarbonyl-2,5-dihydro-
thiophene are suspended in 30 ml of isopropanol. 2.2 ml
of 35 % strength hydrogen peroxide are added, with cool-
ing. The mixture is then stirred at an internal temperature
of 50C until starting material can no longer be de-
tected in a thin layer chromatogram (approx. 5 hours).
The mixture is then cooled in an ice bath and the preci-
pitated crystals are filtered off with suction and washed
with ice-cold isopropanol and with diethyl ether. Yield:
3.5 9 (91 % of theory). ~hen recrystallized from methanol,
the crystals melt at 137 - 140C.
A number of other dihydrothiophene 1-oxides were prepared
analogously; the details can be seen from Table 1.
13:~8108
- - 10 -
Table 1
H /R /R3
R 7~5~Rl R~\
H ~ or H ~ H
O O
No. Rl R2 R3 R4 R5 m.~.(C~
la H H H COOCH3 H 137-140
lb COOCH3 H H CH3 H 212-218
lc H H H CN H 165-167
ld H -(CH2)2c6H5 H COOCH3 H il
le COOCH3 -COCH3 H CH3 H 93- 98
lf H -COCH3 H -COOCH3 H 119-122
lg H -COOC2H5 H -COOCH3 H il
lh COOCH3 -COOC H H CH3 H 132-135
11 COOCH3 -C6H4-4-OCH3 H CH3 H 158-165
lk COOCH3 -CH3 H CH3 H oil
11 H CH3 C6H5 CN H oiL
lm H CH3 CH3 CN H 88- 93
ln CH3 H H COOCH3 H 131-138
lo COCH3 H H CH3 H 171-175
lp H COCH3 H CN H oil
lq COOCH3 CH2-COOC2H5 H CH3 H 93- 98
lr COOCH3 CH2-COOCH3 H CH3 H oil
~ 3
ls COOCH3 OC-CH-NHC3H7 H CH3 H oil
81 0 8
Example 2
3-Amino-4-methoxycarbonylthiophene
3.8 9 (0.02 mo() of 3-amino-4-methoxycarbonyl-2,5-dihydro-
thiophene 1-oxide are suspended in 20 ml of isopropanol.
5 ml of a S N solution of hydrogen chloride in isopropanol
are added dropwise, with ice cooling, and the mixture is
then stirred for approx. 1 hour at a bath temperature of
60C. ~hen the thin layer chromatogram indicates complete
reaction, the suspension is evaporated to dryness on a
rotary evaporator, and the residue is triturated with a
little ethyl acetate and the product is filtered off with
suction and dried. Yield: 2.8 9 (89 % of theory);
melting point 194-201C).
The thiophene derivatives in Table 2 were prepared ana-
l ogous l y .
Table 2
R4 N ~ R3
~ R
R ~ R
No. R R2 R3 R4 R5m.p.(C~
2a H H H C00CH3 H 194-201
(HCl)
2b COOCH3 H H CH3 H 128-131
2c H H H CN H 214-216
(HCl)
2d H -(CH2)3C6H5 H COOCH3 H 145-148
(HCl)
- 12 - 1338108
Table 2 (continued)
No. Rl R2 R3 R4 R5~,~,(C)
2e COOCH3 COCH3 H CH3 H 117
2f H COCH3 H COOCH3 H 58- 60
2g H -COOC2H5 H COOCH3 H 49- 52
2h COOCH3 -COOC2H5 H CH3 H 80- 83
21 COOCH3 -C6H4-4-ocH3 H CH3 H 97-100
2k COOCH3 CH3 H CH3 H 151-154
(HCl)
21 H C6H5 CH3 CN H 52- 53
2m H CH3 CH3 CN H 142-144
(HCl)
2n CH3 H H COOCH3 H 154-159
(HCl)
COCH3 H H CH3 H 161-162
(HCl)
2p H -COCH3 H CN H 162-167
2q COOCH3 -CH2COOC2H5 H CH3 H 48- 50
2r COOCH3 -CH2-COOCH3 H CH3 H 72- 74
CH
2s COOCH3 OC-CH-NHC3H7 H CH3 H 175-176
Example 3
3-Amino-2-methoxycarbonyl-4-methylthiophene
3.46 9 ~0.02 mol) of 3-amino-2-carbomethoxy-4-methyl-4,5-
dihydrothiophene are dissolved in 25 ml of isopropanol,and 2.2 ml of 35 % strength hydrogen peroxide are added
dropwise, with ice cooling. Stirring is continued for
30 minutes at room temperature. The mixture is then
cooled with ice water and 4 ml of 5 N hydrochloric acid
in isopropanol are added. The mixture is then heated to
60C and is stirred at this temperature for approx. 90
minutes. It is worked up by distilling off the isopropanol,
stirring the crystalline residue thoroughly in a little
ethyl acetate and filtering off the product with suction
-
. - 13 - 1~38108
and drying it.
Yield of hydrochloride: 3.86 9 (= 93 % of theory), melt-
ing point 128-130C.
Melting point of free base: 85C.
The compounds described in Example 2 can also be prepared
analogously in a one-pot process.